Introduction
Ulcerative colitis (UC) is a chronic relapsing
disorder associated with uncontrolled inflammation within the
gastrointestinal tract (1). Patients
with longstanding UC have an increased risk of colorectal cancer
(2,3).
The molecular pathway that induces cancer in UC appears to differ
from the well-known ‘adenoma-carcinoma sequence’, as these types of
cancer often develop in flat or mildly elevated lesions and are
distributed multifocally within an area of intestinal inflammation,
called the ‘inflammation-dysplasia-carcinoma sequence’ (4–6).
Therefore, the timely colonoscopic detection and diagnosis of
neoplasia during early phase is crucially important for
treatment.
Previously, chromoendoscopy with dye-spraying, which
provides a more detailed visualization of the mucosa by enhancing
its morphology, was developed to improve upon the accuracy afforded
by conventional endoscopy (7–9). The authors of the present study
(10,11) and other studies (12–16) have
previously demonstrated that this imaging technique facilitates the
detection of early-stage neoplastic lesions in UC.
Animal models of colitis-associated tumors for the
study of cancer prevention and early detection have been reported
(17,18). The oral administration of dextran
sulfate sodium (DSS) to mice has been revealed to induce colonic
inflammation that is clinically and histologically similar to UC
(19). Furthermore, the repeated
administration of DSS may induce dysplastic/cancerous lesions via
the ‘inflammation-dysplasia-carcinoma sequence’ (20). If a detailed analysis of focal mucosal
lesions is possible with the present model, it may be useful for
identifying microscopic lesions, which would result in an improved
understanding of the mechanisms underlying tumor formation and the
development of novel strategies for prevention and
intervention.
The present study induced colitis-associated tumors
in mice and subsequently determined whether stereomicroscopic
observation with dye-application may be used to detect and
discriminate the tumors.
Materials and methods
Ethical approval
Prior to the initiation of this study, the
experimental protocol was examined and approved by the Animal
Research Committee of Kurume University (Kurume, Japan). The
present study was undertaken with strict adherence to the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institute of Health (21) and extra care was taken to avoid animal
suffering.
Mice and treatment
C57BL/6 7–8-week-old female mice (n=67; mean weight,
19.1 g) were purchased from SLC Co., Ltd., Shizuoka, Japan. Mice
were housed in standard wire-mesh cages and provided with drinking
water with or without 0.7% or 1.5% DSS (molecular weight, 40,000;
ICN Biomedicals, Aurora, OH, USA) for 7 days, followed by water
without any additives for the next 10 days. The mice were fed a
standard basal diet (AIN-76; for its composition, see http://www.testdiet.com) or a basal diet enriched
2-fold with iron (final iron concentration, 90 mg/kg) (22,23). These
mice were allowed free access to water and rodent chow. When mice
exhibited moribund symptoms, including i) lack of responsiveness to
manual stimulation, ii) immobility or iii) an inability to eat or
drink, they reached the humane endpoints (24). Following 15 treatment cycles, the
surviving mice were weighed and sacrificed with carbon dioxide
asphyxiation and their colons were investigated morphologically and
histologically.
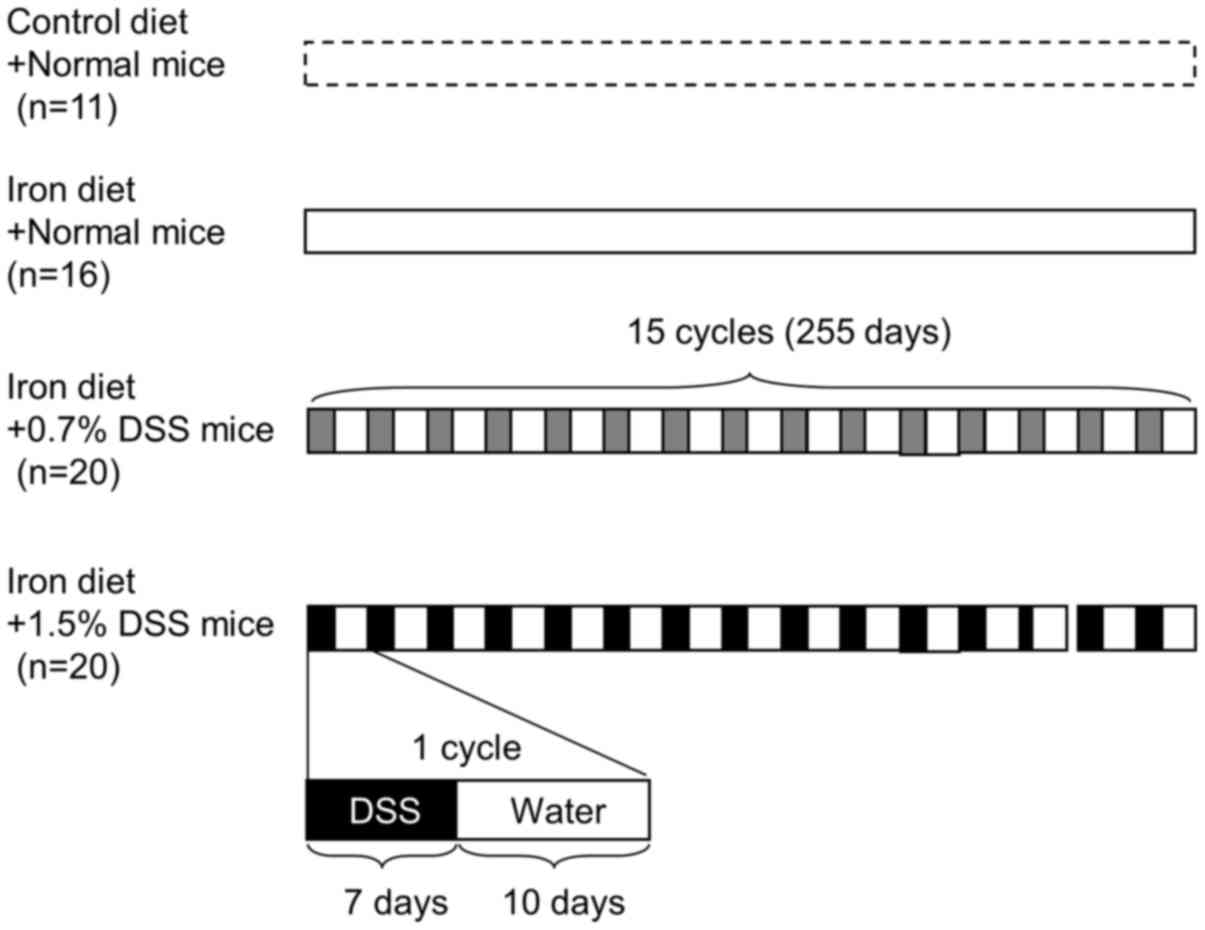
The mice were divided into 4 groups: Normal mice fed
a control diet; normal mice fed an iron-supplemented diet; 0.7% DSS
mice fed an iron diet; and 1.5% DSS mice fed an iron diet (Fig. 1). The mean weight change after each
treatment was 136.9, 137.7, 139.3 and 119.1%, respectively.
Assessment of colitis
A clinical score was generated based on a 0–4 rating
of the following factors: Change in body weight, stool consistency
and intestinal bleeding (25,26). Each variable was allocated equal
weight, with the overall clinical activity score ranging from 0–12.
These parameters were determined by an investigator who was blinded
to the treatment group. Following randomization, a histologic score
was assigned by two pathologists who were also group-blinded.
Sections stained with hematoxylin and eosin were
histopathologically evaluated for the severity of colitis in a
blinded manner, as described previously (27). The histologic score for each segment
(cecum, proximal colon, middle colon and distal colon) ranged from
0–9 and represented the sum of the scores for the severity of
inflammation, damage/necrosis and regeneration. The total
histologic score ranged from 0–12 and consisted of the sum of the
score for the distal colon and the score for disease extent.
The severity of colitis was also determined based on
the colonic length from the ceco-colonic junction to the anal
verge, as this evaluation is an established inflammatory parameter
for DSS-induced colitis (19).
Tumor morphology
The macroscopic features of the tumors were
classified according to size, shape and location. The size of each
lesion was determined by determining the largest diameter of the
lesion using an ocular micrometer. The shape of each lesion was
classified based on the Paris endoscopic classification (28) used in human pathology studies of
colorectal cancer (Fig. 2).
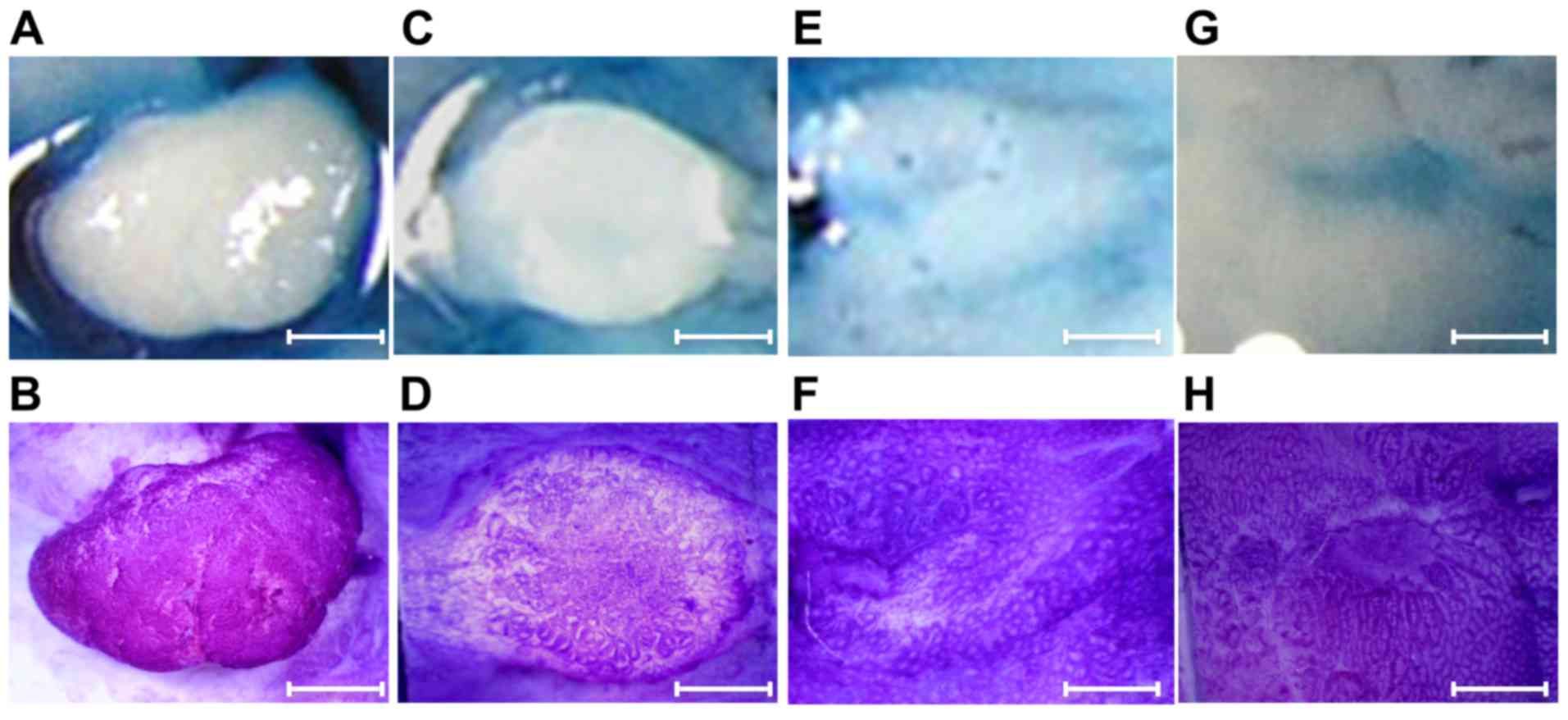
Pit pattern diagnosis
Ex vivo observations using Nikon SMZ-1000
stereomicroscope (Nikon Corporation, Tokyo, Japan) were performed
after the topical application of 0.06% crystal violet or 0.2%
indigo carmine for 30–40 sec at room temperature to enhance mucosal
details. Crystal violet stains the circumferential convex portions,
but not the grooves, whereas indigo carmine does not stain the
colonic mucosa but accumulates inside the grooves, highlighting
subtle mucosal irregularities (29).
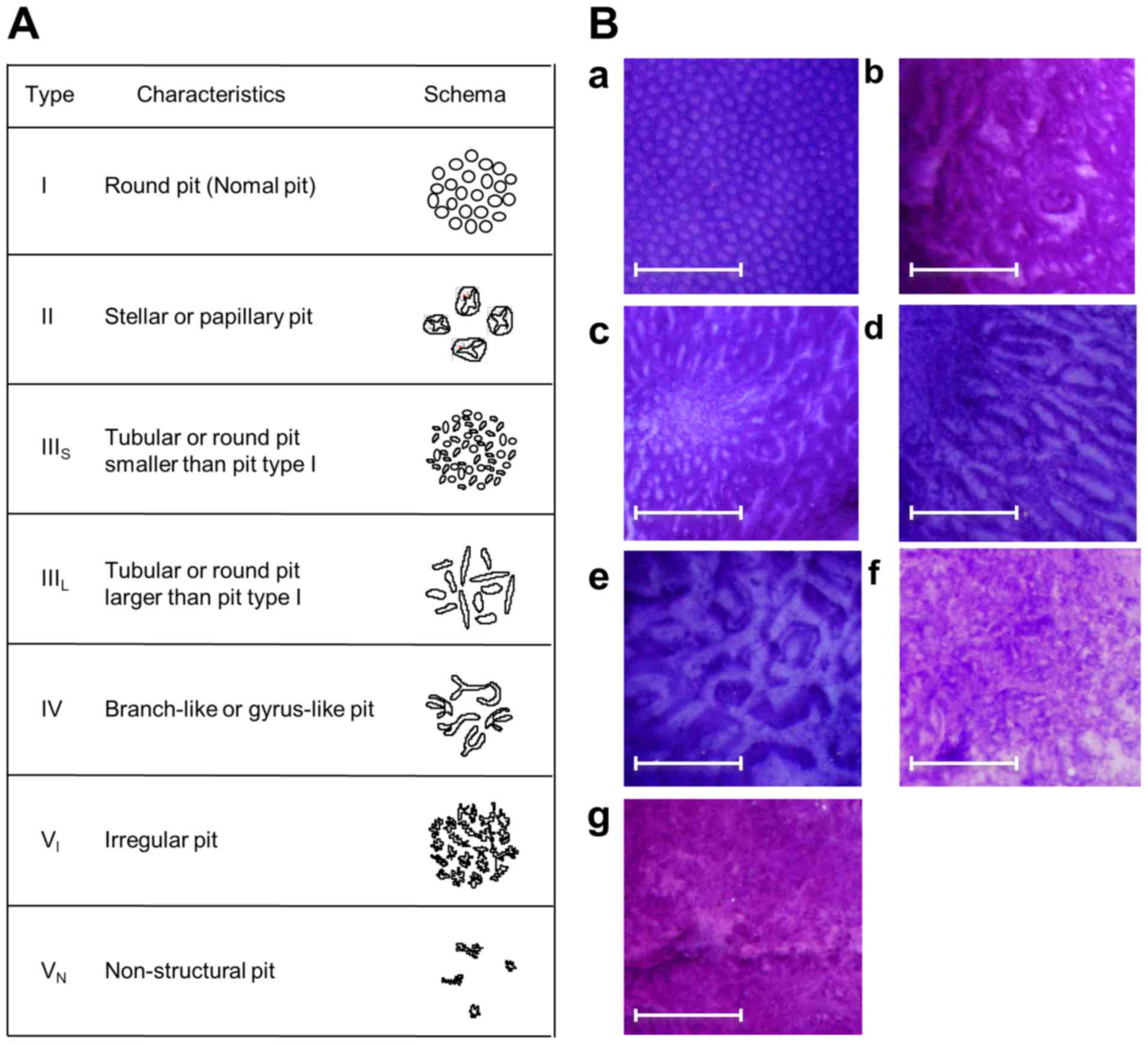
The pit pattern classification by Kudo et al
(30,31) divides colorectal lesions into 5
classes (Fig. 3). Only focal mucosal
lesions that were distinguished clearly from the surrounding mucosa
were classified according to the pit pattern classification, due to
inflammatory alterations often displaying mucosal irregularities.
Type I pit pattern represents regular round crypts, type II pattern
represents stellar or papillary crypts, type III pattern represents
small tubular or roundish crypts (IIIS) or large tubular
or roundish crypts (IIIL), type IV pattern consists of
branch- or gyrus-like crypts and type V pattern consists of
irregular crypts (VI) or non-structural crypts
(VN). For lesions exhibiting multiple pit patterns, the
pit pattern of the most atypical area was used.
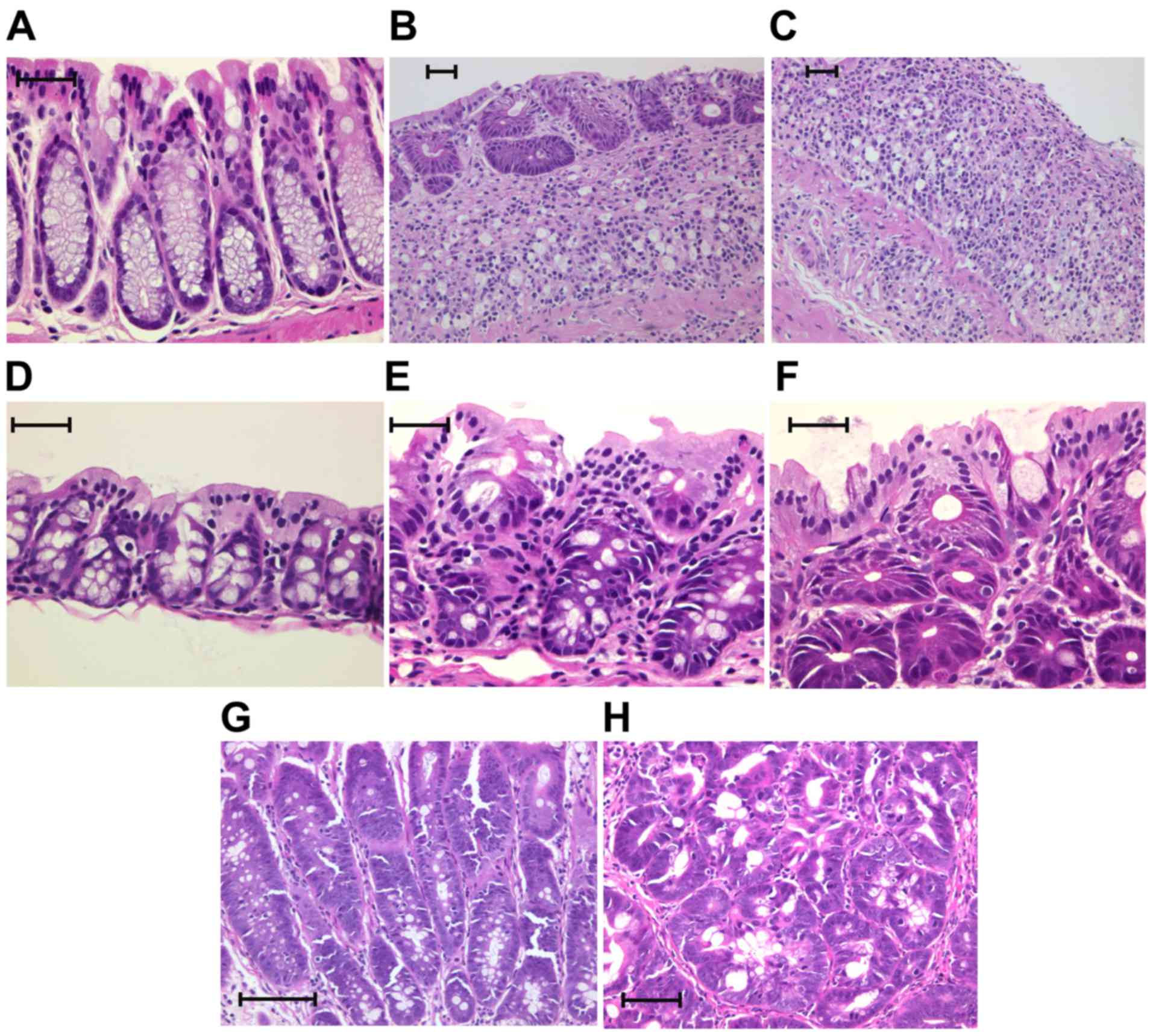
Histopathology
Each specimen was treated with 10% neutral-buffered
formalin and sectioned at a 4-µm thickness and stained using
hematoxylin and eosin (H&E). Two gastrointestinal pathologists
who were blinded as to the stereomicroscopic diagnosis examined all
the specimens by using a Nikon Optiphot microscope (Nikon
Corporation). Based on the classification by Riddell et al
(32), the tumor histology was
categorized as negative, indefinite or positive for dysplasia. The
positive category was divided into two subcategories: Low-grade
dysplasia (LGD) and high-grade dysplasia (HGD; Table I; Fig.
4) (32,33).
 | Table I.The histological classification of
dysplasia by Riddell et al (30). |
Table I.
The histological classification of
dysplasia by Riddell et al (30).
| Negative | Normal mucosa | Inactive
colitis | Active colitis |
|---|
| Indefinite | Probably negative
(probably inflammatory) | Unknown | Probably positive
(probably dysplastic) |
| Positive | Low-grade
dysplasia | High-grade
dysplasia | – |
Statistical analysis
Where the data were normally distributed, a single
analysis of variance was used to identify regional differences and
the differences between groups was analyzed using the Tukey-Kramer
honestly significant difference test (JMP statistical package
version 12; SAS Institute, Cary, NC, USA). Where the data was not
normally distributed, the differences were analyzed using the
nonparametric Wilcoxon/Kruskal-Wallis test (rank sums). The
probability of survival analysis was estimated using the
Kaplan-Meier method. The statistical significance of each
comparison was determined using the log-rank test. The results are
presented as the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference.
Results
Time course for DSS-induced
colitis
Food consumption remained unchanged among the
controls and iron-supplemented mice at the end of the experiment
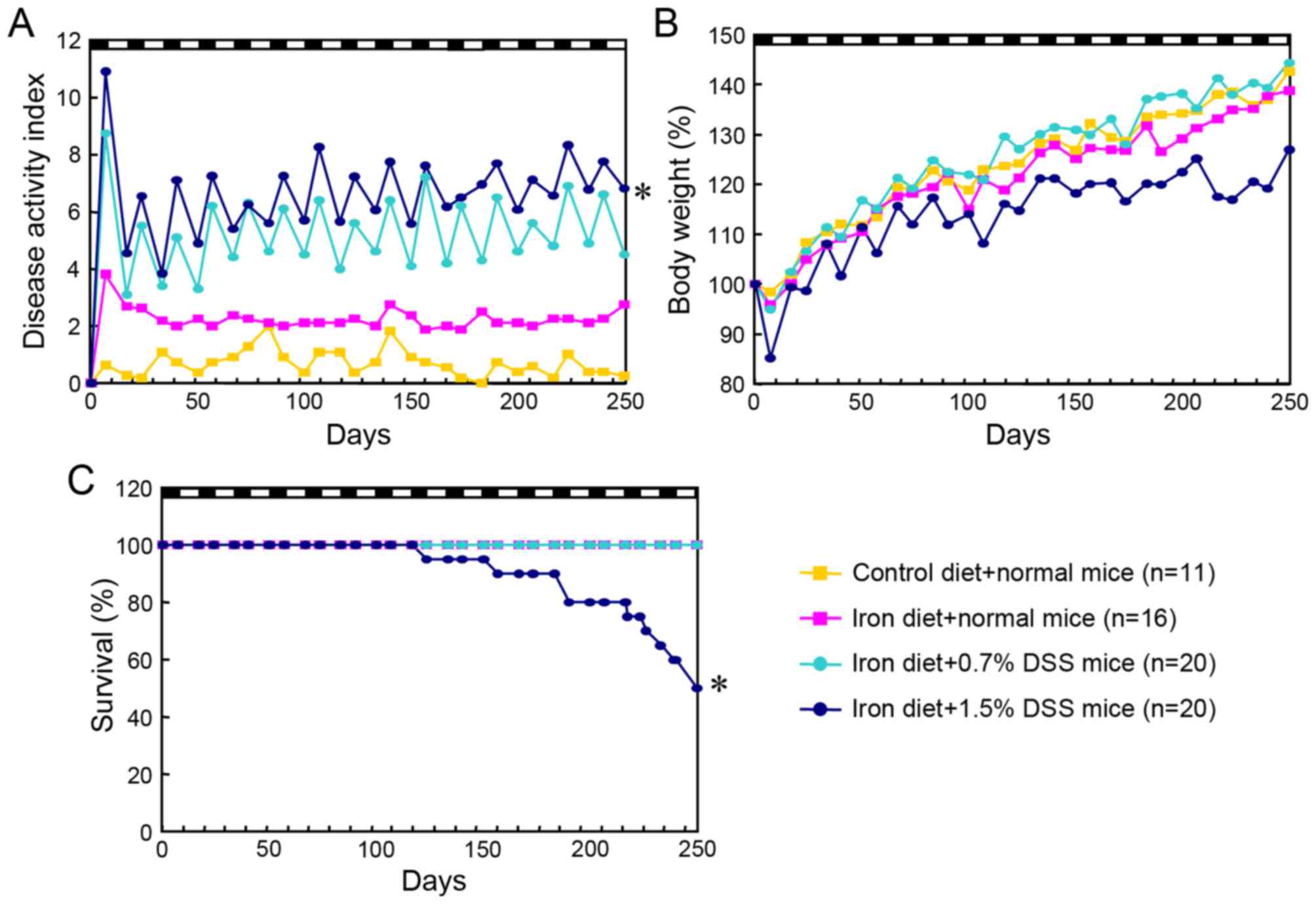
(data not shown). Higher disease activity scores were evident in
the 1.5% DSS mice fed an iron-enriched diet, compared with the
normal mice fed a control diet (P<0.0001), the normal mice fed
an iron-enriched diet (P<0.0001) and the 0.7% DSS mice fed an
iron diet (P=0.004; Fig. 5A). The
1.5% DSS mice (mean ± standard deviation, 22.8±3.1 g; minimum
weight, 18.5 g; maximum weight, 27.0 g) had a lower body weight
than the control mice (26.1±1.3 g; minimum weight, 24.0 g; maximum
weight, 28.0 g) at the end of the experiment, although the
difference was not statistically significant (Fig. 5B). A total of 10 mice from the 1.5%
DSS mice fed an iron diet succumbed prior to completion of 15 DSS
cycles; these mice exhibited significant weight loss, severe rectal
bleeding and diarrhea and extensive ulceration of the colon.
However, no colon tumors were identified, indicating that the
probable cause of mortality was not cancer development but severe
colonic inflammation (Fig. 5C).
Colonic damage in DSS-induced
colitis
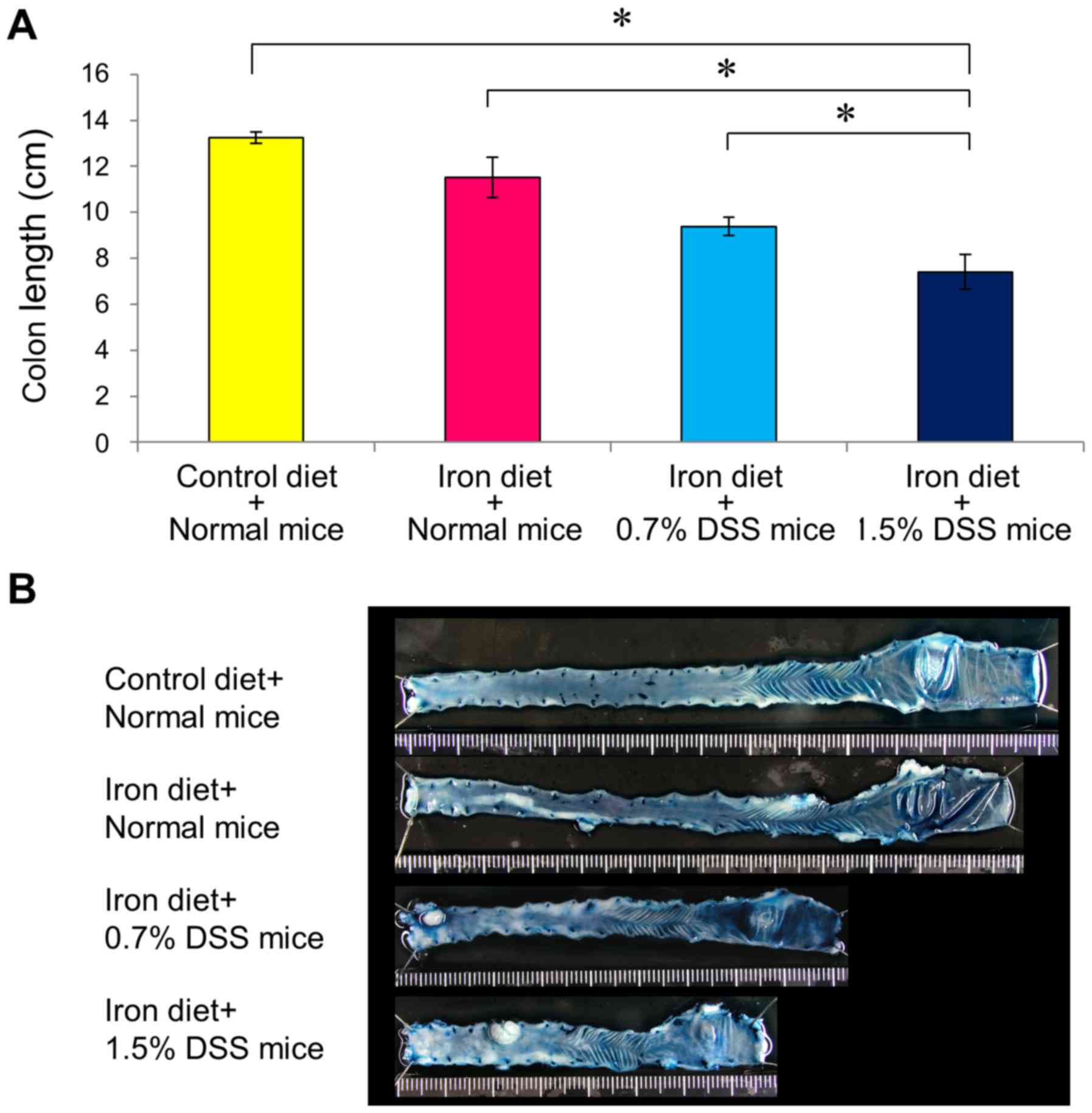
Colonic damage was assessed according to the colonic
length and histologic scores. Drinking water containing DSS
resulted in colon injury, as determined according to the colon
length; the colon injury was more severe in the 1.5% DSS mice fed
an iron-enriched diet compared with in the 0.7% DSS mice fed an
iron-enriched diet (Fig. 6). Mice
that underwent the repeated administration of DSS exhibited
colitis, particularly in the distal colon. Higher pathological
scores were demonstrated in the 1.5% DSS mice fed an iron-enriched
diet, compared with 0.7% DSS mice fed an iron-enriched diet
(Fig. 7). Notably, mild inflammation
was revealed in the normal mice fed an iron-enriched diet compared
with the normal mice on the control diet (P<0.0001).
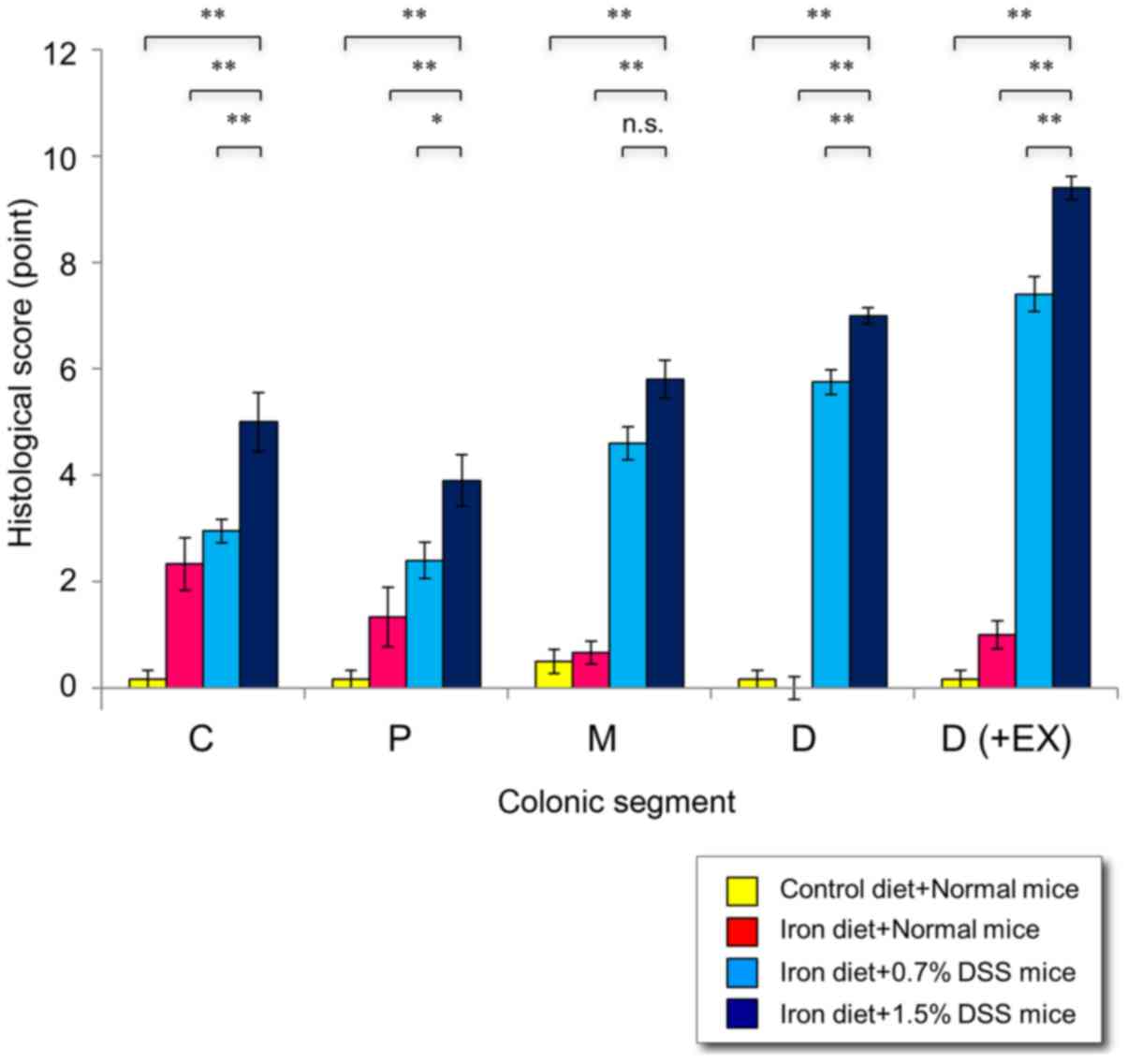 | Figure 7.Histological scores in mice with
chronic DSS-induced colitis. Following 15 cycles of DSS
administration, the mice were sacrificed and the colons were
examined histologically. The histologic score for each segment
(cecum, proximal colon, middle colon and distal colon) ranged from
0–9 and represented the sum of the scores for the severity of
inflammation, damage/necrosis and regeneration. The total
histologic score ranged from 0–12 and consisted of the sum of the
score for the distal colon and the score for disease extent.
*P<0.05 and **P<0.01 vs. in the 1.5% DSS mice fed an iron
diet. C, cecum; P, proximal colon; M, middle colon; D, distal
colon; D (+Ex), distal colon plus disease extent; DSS, dextran
sulfate sodium. |
Incidence of dysplasia in DSS-induced
colitis
The results for tumor incidence are presented in
Table II. LGD, but not HGD, was
observed in one normal mouse fed the iron-enriched diet, suggesting
that dietary iron supplementation did not significantly affect
tumor development. A total of 9/10 (90.0%) mice treated with 1.5%
DSS plus an iron-enriched diet developed dysplasia, with a mean
tumor multiplicity of 1.7 tumors per tumor-bearing mouse (15/9). A
total of 7/20 mice (35%) treated with 0.7% DSS plus iron-enriched
diet developed dysplasia, with a mean tumor multiplicity of 1.3
tumors per tumor-bearing mouse (9/7). The incidences and the
multiplicities of dysplasia were significantly higher in the 1.5%
DSS mice fed the iron-enriched diet compared with in the 0.7% DSS
mice fed the iron-enriched diet. The colonic tumors were confirmed
using a histopathological analysis and were classified as
indefinite, LGD and HGD. Of the 15 areas of dysplasia in the 1.5%
DSS mice fed the iron-enriched diet, HGD was observed in 46.7%
(7/15) and LGD was observed in 53.3% (8/15). Of the 9 areas in the
0.7% DSS mice fed the iron-enriched diet, HGD was observed in 44.4%
(4/9) and LGD was observed in 55.6% (5/9).
 | Table II.Incidence of colonic tumors induced
by chronic DSS exposure. |
Table II.
Incidence of colonic tumors induced
by chronic DSS exposure.
| Experimental
group | Negative, n | Indefinite, n | LGD tumor/mouse,
n | HGD tumor/mouse,
n | LGD+HGD
tumor/mouse, n |
|---|
| Control diet +
normal mice (n=11) | 6 | 0 | 0 | 0 | 0 |
| Iron diet + normal
mice (n=16) | 4 | 1 | 1/1 | 0 | 1/1 |
| Iron diet + 0.7%
DSS mice (n=20) | 0 | 13 | 5/4 | 4/4 | 9/7 |
| Iron diet + 1.5%
DSS mice (n=10) | 0 | 1 | 8/5 | 7/6 | 15/9 |
Analysis of dysplasia in DSS-induced
colitis
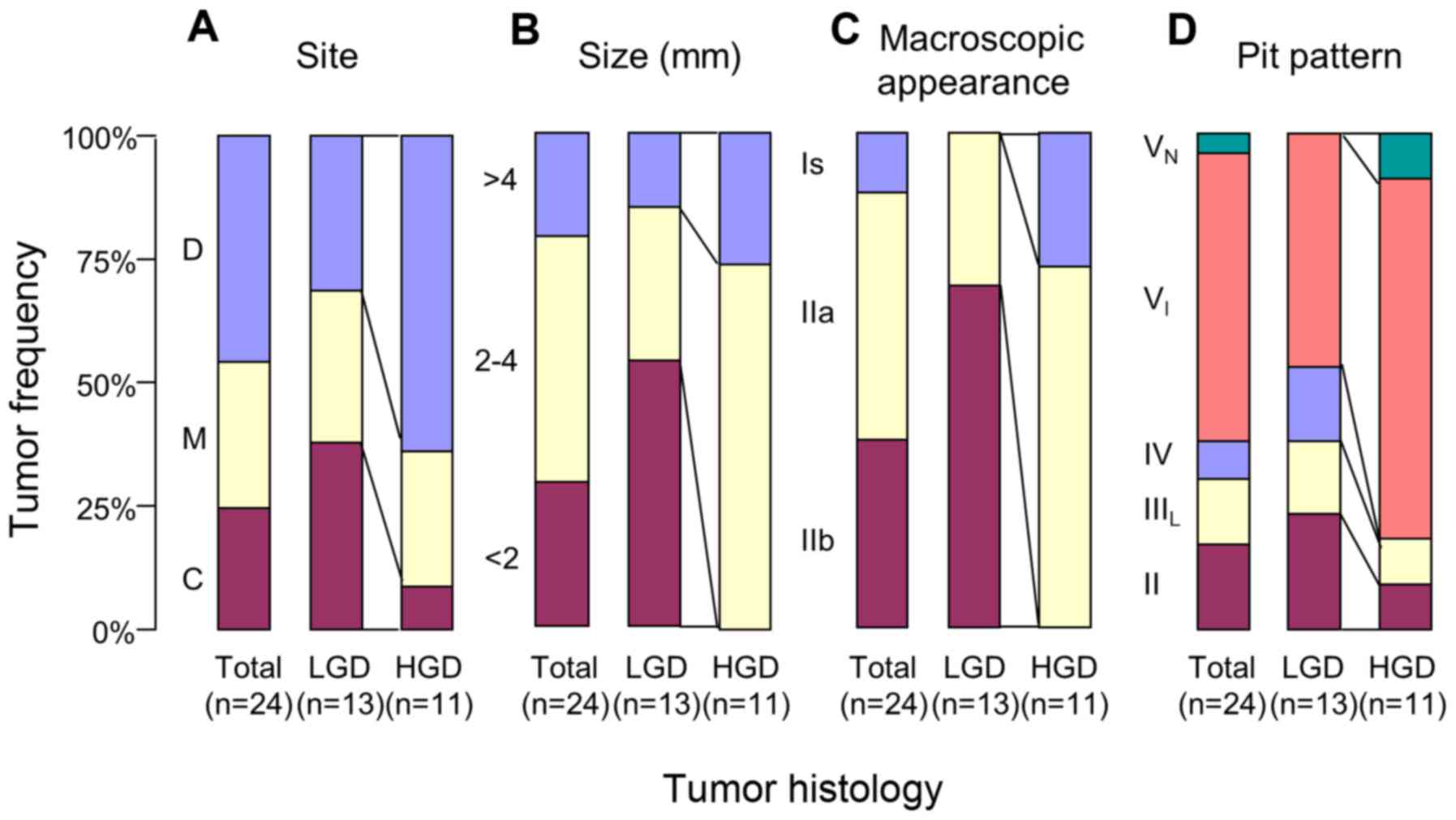
The areas of dysplasia in the DSS mice were further
characterized according to pathological and morphological
variables. HGD was predominantly located in the distal colon
(Fig. 8A). The lesion size was larger
for HGD compared with for LGD (Fig.
8B).
Finally, the histopathological diagnosis was
compared with the pit pattern assessment. When compared with LGD,
HGD exhibited a higher frequency of elevated lesions (Is and IIa)
and a lower frequency of flat lesions (IIb) based on the
macroscopic features (Fig. 8C);
furthermore, VI pits were more numerous and IV,
IIIL and II pits were less numerous (Fig. 8D). Fig.
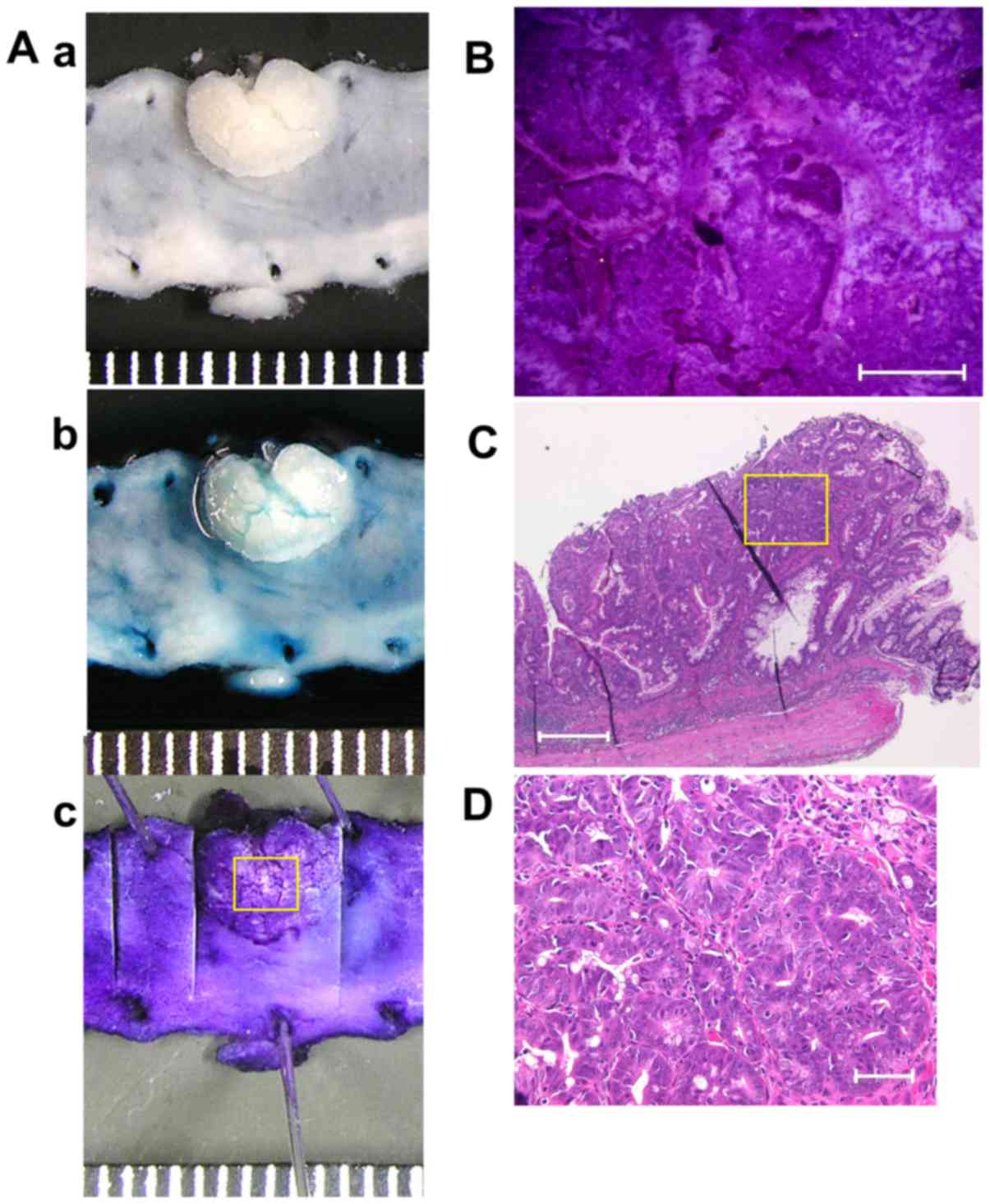
9 exemplifies the stereomicroscopic and histological pictures
of colonic tumor in a mouse with DSS colitis.
Discussion
UC patients are well-known to have a higher risk of
developing colorectal cancer compared with the general population.
The early detection of premalignant and malignant lesions remains
the best means of reducing the risk of mortality from colorectal
cancer (2,3). The use of animal models allows the
design of novel methods to screen for early signs of colon cancer
(17,18). These models will expand our
understanding of the mechanisms underlying tumor formation and may
be useful to identify novel response indicators that are correlated
with the early stages of tumorigenesis. Finally, these animal
models allow preclinical testing of novel treatment strategies for
prevention and intervention.
Long-lasting active inflammation may be an important
factor during the earlier stage of the initiation of dysplasia and
cancer. A previous study demonstrated that the simple repeated
administration of DSS induced dysplasia in mice with a low tumor
incidence (20). Subsequently, Seril
et al (22) revealed that a
2-fold dietary iron supplementation enhanced the development of
DSS-induced dysplasia resulting in a tumor incidence of >70%
tumor, partly due to the augmentation of oxidative and nitrosative
stress (22,23). Using this modified model, the present
study characterized colonic tumors with respect to their morphology
and histology.
The present study demonstrated a higher incidence
and multiplicity of dysplastic lesions in the 1.5% DSS mice
compared with in the 0.7% DSS mice. This finding suggested that the
incidence of colitis-induced tumors increased with the severity of
inflammation, similar to data for UC in humans (3,34,35).
When compared with LGD, HGD was predominantly
located in the distal colon, was larger in size and had a higher
incidence of elevated lesions (Is and IIa) and a lower incidence of
flat lesions (IIb), according to its macroscopic features. Although
invasive cancer was not observed in the present study, these
findings agree with data for UC in humans that revealed that
dysplasia and early cancer are predominantly located in the distal
colon and exhibit protruded or flat-elevated features (3,34,35). Furthermore, long-term studies using
the presently reported DSS colitis model may be useful for studying
the sequence of HGD and colitis-associated cancer.
The most important aim of the present study was to
investigate whether the pit pattern classification in humans is
applicable to the murine model used. The pit pattern classification
reported by Kudo et al (30,31)
divides colonic lesions into 5 classes: Lesions classified as
having a I–II pit pattern are generally considered to have benign
histology, whereas type III–V pit patterns tend to predict
neoplastic lesions (30,31). Previous studies in patients with UC
have revealed that the dysplastic lesions and early cancer had type
IIIS-IIIL or type IV pits (12). A
previous study demonstrated that 13/15 (86.7%) dysplasia/cancer
lesions in UC exhibited a neoplastic pit pattern (10), suggesting that a pit pattern analysis
may be useful for detecting and discriminating neoplastic lesions
in UC, although coexisting inflammatory changes may modify the
mucosal details.
The present study used stereomicroscopy with
dye-application to improve the detection of epithelial changes
representing neoplastic or dysplastic alterations. To the best of
our knowledge, using the pit pattern classification, the present
study demonstrated for the first time that HGD exhibited an
increased incidence of type VI pits and a decreased
incidence of type IV, IIIL and II pits. This allowed the
present study to classify the colonic tumors with regards to their
pit pattern even in colitis-induced tumors in mice. Therefore,
consistent with studies examining UC in humans (10–16),
chromoendoscopy has emerged as a useful approach for detecting and
discriminating neoplastic changes in colitis-associated tumors in
mice.
Previously, a safe method for performing endoscopy
in mice was developed, permitting long-term studies in living mice
(36). Using this method, further
study may aid investigators to determine the success of their
experiment in vivo at an early stage and to reduce the
number of animals required for experiments examining prevention or
interventions.
In conclusion, the repeated administration of DSS
with iron-supplementation induced dysplastic lesions with a high
incidence. Pit pattern evaluations using stereomicroscopy with
dye-application were useful for detecting and discriminating
neoplastic changes in DSS mice and may be useful for furthering our
understanding of the mechanisms underlying tumor formation in UC
patients and the characterization of pharmaceutical responses.
Acknowledgements
The present study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Culture and
Science (grant no. 25460964) and the Health and Labour Sciences
Research Grants for research on intractable diseases from the
Ministry of Health, Labour and Welfare of Japan.
References
|
1
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Invest.
117:514–521. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morson BC and Pang LS: Rectal biopsy as an
aid to cancer control in ulcerative colitis. Gut. 8:423–434. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernstein CN, Shanahan F and Weinstein WM:
Are we telling patients the truth about surveillance colonoscopy in
ulcerative colitis? Lancet. 343:71–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blackstone MO, Riddell RH, Rogers BH and
Levin B: Dysplasia-associated lesion or mass (DALM) detected by
colonoscopy in long-standing ulcerative colitis: An indication for
colectomy. Gastroenterology. 80:366–374. 1981.PubMed/NCBI
|
|
7
|
Basu S, Torigian D and Alavi A: The role
of modern molecular imaging techniques in gastroenterology.
Gastroenterology. 135:1055–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Axelrad AM, Fleischer DE, Geller AJ,
Nguyen CC, Lewis JH, Al-Kawas FH, Avigan MI, Montgomery EA and
Benjamin SB: High-resolution chromoendoscopy for the diagnosis of
diminutive colon polyps: Implications for colon cancer screening.
Gastroenterology. 110:1253–1258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu KI, Sano Y, Kato S, Fujii T, Nagashima
F, Yoshino T, Okuno T, Yoshida S and Fujimori T: Chromoendoscopy
using indigo carmine dye spraying with magnifying observation is
the most reliable method for differential diagnosis between
non-neoplastic and neoplastic colorectal lesions: A prospective
study. Endoscopy. 36:1089–1093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshioka S, Mitsuyama K, Takedatsu H,
Kuwaki K, Yamauchi R, Yamasaki H, Fukunaga S, Akiba J, Kinugasa T,
Akagi Y, et al: Advanced endoscopic features of ulcerative
colitis-associated neoplasias: Quantification of autofluorescence
imaging. Int J Oncol. 48:551–558. 2016.PubMed/NCBI
|
|
11
|
Watanabe T, Ajioka Y, Mitsuyama K,
Watanabe K, Hanai H, Nakase H, Kunisaki R, Matsuda K, Iwakiri R,
Hida N, et al: Comparison of targeted vs random biopsies for
surveillance of ulcerative colitis-associated colorectal cancer.
Gastroenterology. 151:1122–1130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sada M, Igarashi M, Yoshizawa S, Kobayashi
K, Katsumata T, Saigenji K, Otani Y, Okayasu I and Mitomi H: Dye
spraying and magnifying endoscopy for dysplasia and cancer
surveillance in ulcerative colitis. Dis Colon Rectum. 47:1816–1823.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hata K, Watanabe T, Kazama S, Suzuki K,
Shinozaki M, Yokoyama T, Matsuda K, Muto T and Nagawa H: Earlier
surveillance colonoscopy programme improves survival in patients
with ulcerative colitis associated colorectal cancer: Results of a
23-year surveillance programme in the Japanese population. Br J
Cancer. 89:1232–1236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumoto T, Nakamura S, Jo Y, Yao T and
Iida M: Chromoscopy might improve diagnostic accuracy in cancer
surveillance for ulcerative colitis. Am J Gastroenterol.
98:1827–1833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiesslich R, Goetz M, Lammersdorf K,
Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR and
Neurath MF: Chromoscopy-guided endomicroscopy increases the
diagnostic yield of intraepithelial neoplasia in ulcerative
colitis. Gastroenterology. 132:874–882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiesslich R, Fritsch J, Holtmann M,
Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR and
Neurath MF: Methylene blue-aided chromoendoscopy for the detection
of intraepithelial neoplasia and colon cancer in ulcerative
colitis. Gastroenterology. 124:880–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kiesler P, Fuss IJ and Strober W:
Experimental models of inflammatory bowel diseases. Cell Mol
Gastroenterol Hepatol. 1:154–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanneganti M, Mino Kenudson M and
Mizoguchi E: Animal models of colitis-associated carcinogenesis. J
Biomed Biotechnol. 2011:3426372011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okayasu I, Yamada M, Mikami T, Yoshida T,
Kanno J and Ohkusa T: Dysplasia and carcinoma development in a
repeated dextran sulfate sodium-induced colitis model. J
Gastroenterol Hepatol. 17:1078–1083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Committee for the Update of the Guide for
the Care and Use of Laboratory A and National Research Council:
Guide for the Care and Use of Laboratory Animals. 8th. National
Academies Press; Washington, DC: 2011
|
|
22
|
Seril DN, Liao J, Yang CS and Yang GY:
Systemic iron supplementation replenishes iron stores without
enhancing colon carcinogenesis in murine models of ulcerative
colitis: Comparison with iron-enriched diet. Dig Dis Sci.
50:696–707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao J, Seril DN, Yang AL, Lu GG and Yang
GY: Inhibition of chronic ulcerative colitis associated
adenocarcinoma development in mice by inositol compounds.
Carcinogenesis. 28:446–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tardif SD, Coleman K, Hobbs TR and Lutz C:
IACUC review of nonhuman primate research. ILAR J. 54:234–245.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
26
|
Takaki K, Mitsuyama K, Tsuruta O, Toyonaga
A and Sata M: Attenuation of experimental colonic injury by
thiazolidinedione agents. Inflamm Res. 55:10–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dieleman LA, Ridwan BU, Tennyson GS,
Beagley KW, Bucy RP and Elson CO: Dextran sulfate sodium-induced
colitis occurs in severe combined immunodeficient mice.
Gastroenterology. 107:1643–1652. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
The Paris endoscopic classification of
superficial neoplastic lesions: Esophagus, stomach, and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58:(6 Suppl).
3–43. 2003. View Article : Google Scholar
|
|
29
|
Hata K, Watanabe T, Shinozaki M, Kojima T
and Nagawa H: To dye or not to dye? That is beyond question!
Optimising surveillance colonoscopy is indispensable for detecting
dysplasia in ulcerative colitis. Gut. 53:17222004.PubMed/NCBI
|
|
30
|
Kudo S, Hirota S, Nakajima T, Hosobe S,
Kusaka H, Kobayashi T, Himori M and Yagyuu A: Colorectal tumours
and pit pattern. J Clin Pathol. 47:880–885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Riddell RH, Goldman H, Ransohoff DF,
Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton
SR, Morson BC, et al: Dysplasia in inflammatory bowel disease:
Standardized classification with provisional clinical applications.
Hum Pathol. 14:931–968. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schlemper RJ, Riddell RH, Kato Y, Borchard
F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF,
Geboes K, et al: The Vienna classification of gastrointestinal
epithelial neoplasia. Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barral M, Dohan A, Allez M, Boudiaf M,
Camus M, Laurent V, Hoeffel C and Soyer P: Gastrointestinal cancers
in inflammatory bowel disease: An update with emphasis on imaging
findings. Crit Rev Oncol Hematol. 97:30–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matkowskyj KA, Chen ZE, Rao MS and Yang
GY: Dysplastic lesions in inflammatory bowel disease: Molecular
pathogenesis to morphology. Arch Pathol Lab Med. 137:338–350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Becker C, Fantini MC, Wirtz S, Nikolaev A,
Kiesslich R, Lehr HA, Galle PR and Neurath MF: In vivo imaging of
colitis and colon cancer development in mice using high resolution
chromoendoscopy. Gut. 54:950–954. 2005. View Article : Google Scholar : PubMed/NCBI
|