Introduction
Giant cell tumors (GCTs) of the bone are aggressive
benign primary bone neoplasms, which present mostly at the
meta-epiphysis of long bones, causing extensive lytic lesions with
an estimated incidence of 1.3 per million per year (1,2). GCTs of
the spine reportedly account for 2.7–6.5% of all GCTs in bone
(3). Resection at an early stage
remains the best strategy for treatment with a low recurrence rate
(4,5).
Although denosumab has been reported to induce
effective clinical results with respect to tumor shrinkage in a
short-term follow-up clinical study, total spondylectomy is
recognized as the treatment of choice for eradicating GCTs of the
spine (6). The present study reports
a case involving a GCT in the eleventh thoracic vertebra,
complicated by idiopathic scoliosis and treated using total en bloc
spondylectomy (TES) following preoperative denosumab therapy for 8
months. Surgery was performed using a computed tomography
(CT)-based navigation system that optimized accuracy by recognizing
the area of the detached parietal pleura, the irregular border of
the collapsed vertebra and the adjacent vertebra. Although recent
studies have reported that preoperative denosumab treatment induces
marked regression of GCTs of the spine, which subsequently
permitted surgical resection that may otherwise have been
unresectable had it not been for tumor shrinkage (7–10), there
are no reports involving GCTs of the thoracic spine in a patient
with idiopathic scoliosis treated by a posterior one-stage TES
following preoperative denosumab therapy. In this case, analysis of
the CT images after 8 months of preoperative denosumab therapy
revealed the border between the vertebral body and soft tissue,
indicating consolidation of the vertebral cortex. A CT-navigation
system facilitated the safe use of the TES that followed.
Case report
A 35-year-old woman was referred to the Outpatient
Department of Kitasato University Hospital (Kanagawa, Japan) for
the evaluation of severe back pain, which started 4 months prior to
her first visit to the hospital on 4th March 2015. The pain had
gradually increased and persisted regardless of motion. The patient
had been diagnosed with adolescent idiopathic scoliosis at 14 years
old and the condition had never been treated. Although a
neurological examination was negative, percussion tenderness around
the thoracolumbar junction was noted.
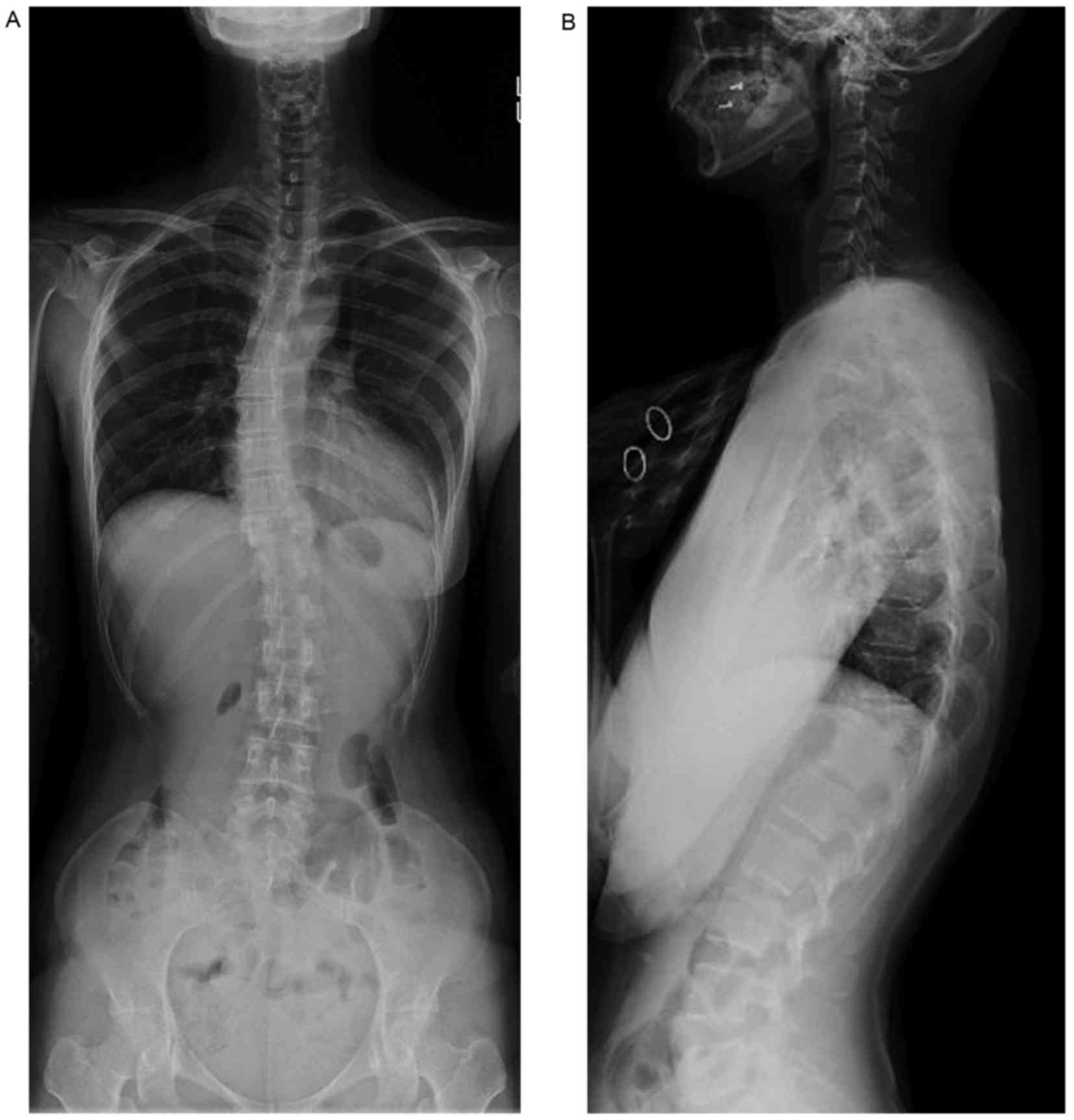
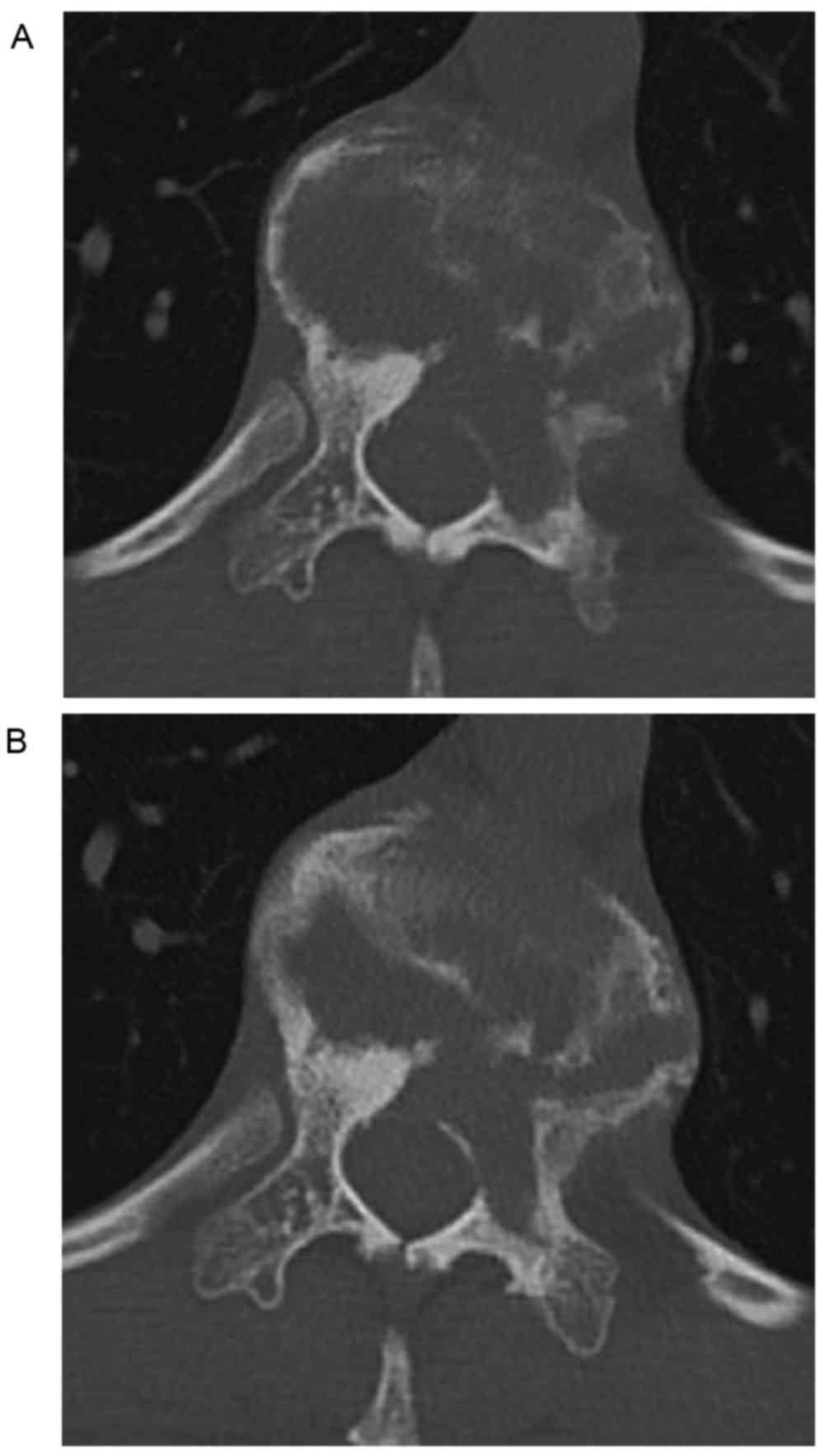
Radiographic analysis revealed the collapse of the
T11 vertebral body and idiopathic scoliosis with a Cobb angle
measurement of 21° (Fig. 1). The
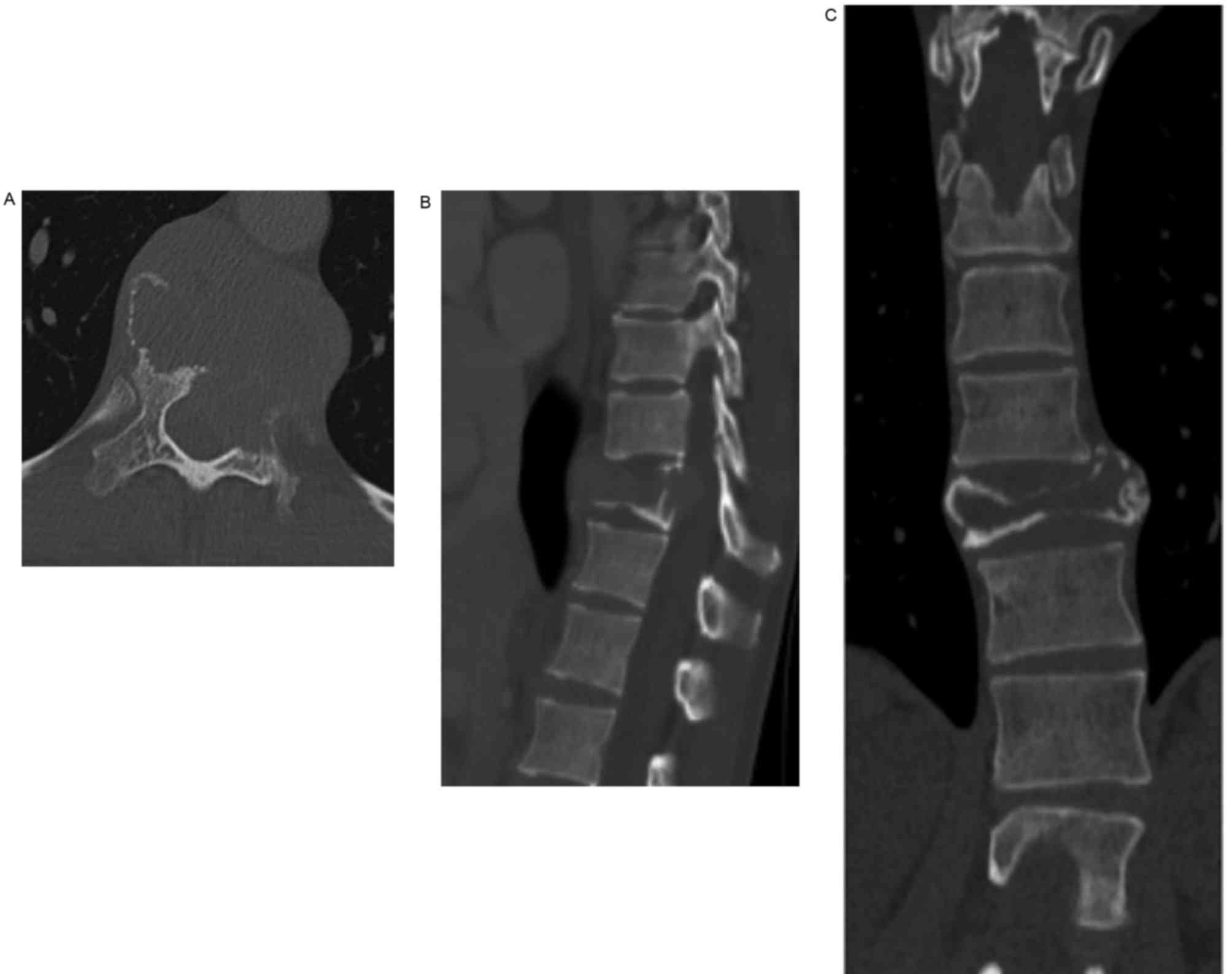
spinal CT revealed an osteolytic lesion involving the T11 vertebral
body and the surrounding soft tissue, which had resulted in
collapse of the vertebral body (Fig.
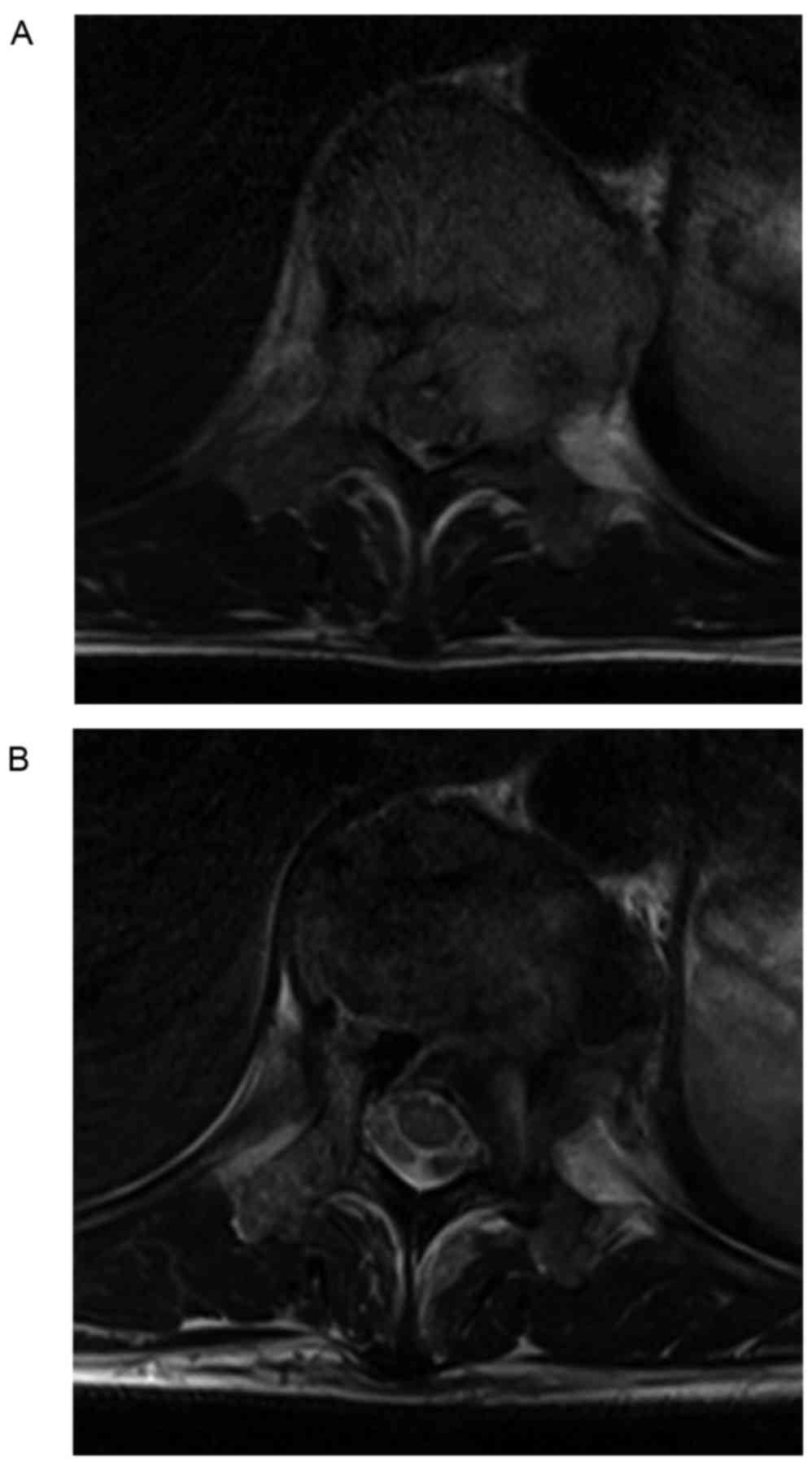
2). Magnetic resonance imaging (MRI) of the thoracolumbar spine
showed the tumor extended toward the paravertebral soft tissue and
into the left pedicle resulting in compression of the spinal cord
(Fig. 3A). No additional sites of
neoplasm were noted in the whole body. A needle biopsy was
immediately performed to collect a sample that could be used to
verify the diagnosis. Following fixation with 20% neural buffered
formalin for 24 h at room temperature, paraffin-embedded,
4-µm-thick tissue sections from the biopsy specimen were stained
with antibodies against vimentin (V9, Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA; cat. no. M6725), CD68 (PGM-1, Dako;
Agilent Technologies, Inc.; cat. no. M0876), p53 (DO-7, Dako;
Agilent Technologies, Inc.; cat. no. M7001) and MIB-1 (Dako;
Agilent Technologies, Inc.; cat. no. M7240) antibodies for
immunohistological analysis. For vimentin and MIB-1, slides were
deparaffinized using PT-Link (Dako; Agilent Technologies, Inc.;
cat. no. PT109) at 98°C for 20 min, and then blocked with
peroxidase-blocking reagent included in Envision FLEX Package High
PH (Dako; Agilent Technologies, Inc.; cat. no. k8010) for 5 min.
Dako AutoStainer plus (cat. no. S3400) was used with secondary
antibody and visualization reagent included in Envision FLEX
Package High PH (Dako; Agilent Technologies, Inc.; cat. no. k8010).
For CD68 and p53, slides were deparaffinized with EZ Prep 10x
(Ventana Medical Systems, Inc.; cat. no. 950-102), and then blocked
with inhibitor reagent with 3% H2O2 included
in the i-View DAB Universal kit (Roche Diagnostics, Basel,
Switzerland, 760-041). Autostainer Ventana BenchMark XT (Ventana
Medical Systems, Inc., Tucson, AZ, USA) was used with the i-View
DAB Universal kit. An Olympus BX51 polarizing microscope (Olympus
Corporation, Tokyo, Japan) was used to observe the
immunohistological staining results at a magnification of ×40-400.
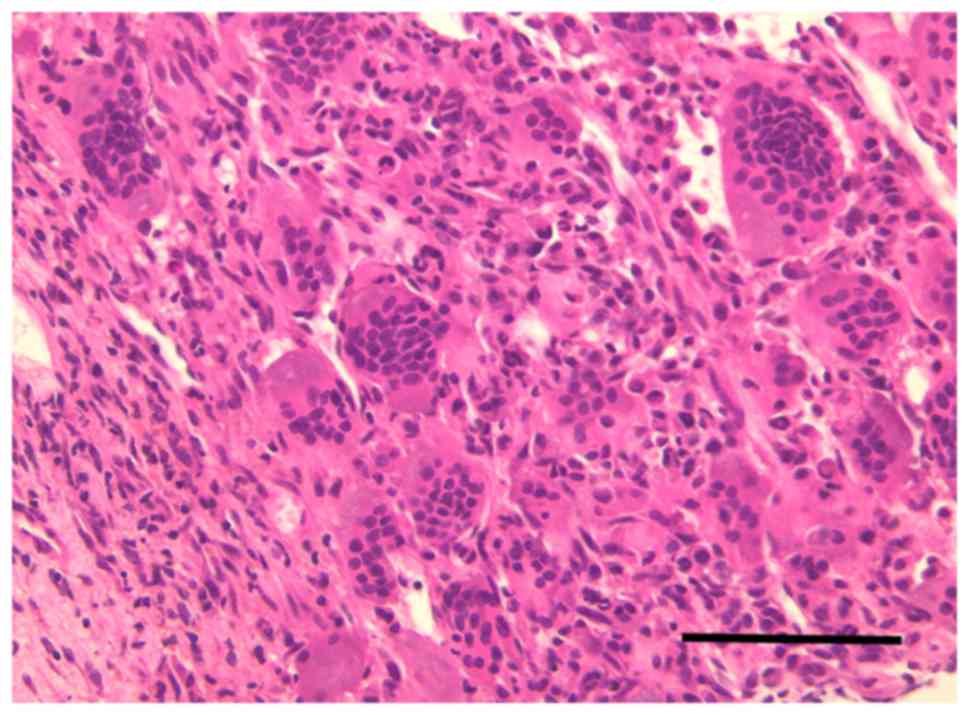
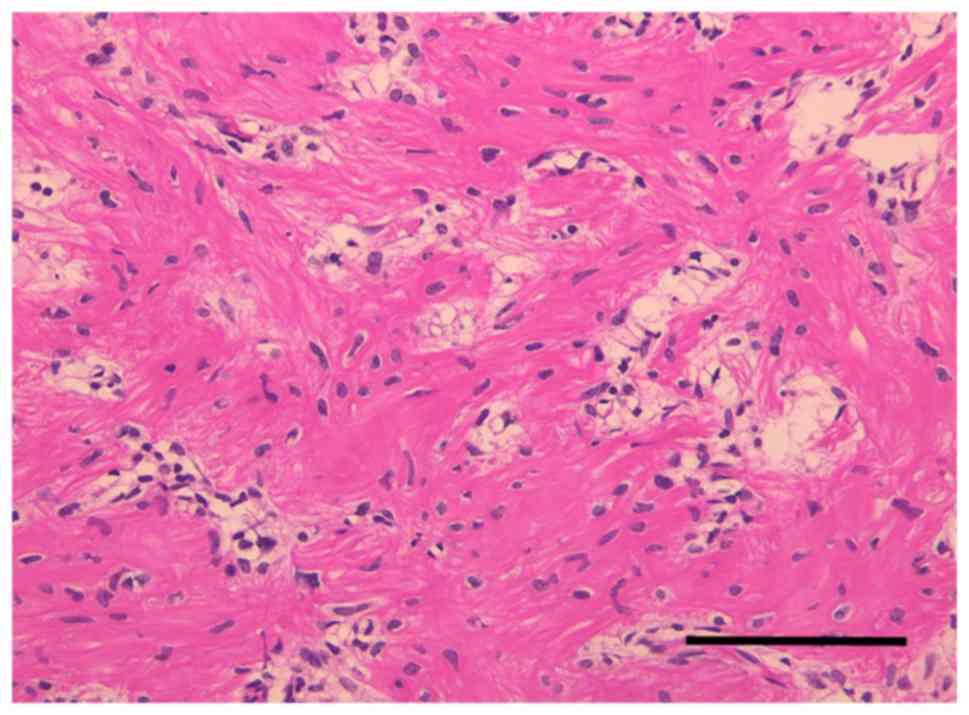
Pathological and immunohistochemical analyses confirmed a GCT
characterized by multinucleate giant cells surrounded by neoplastic
stromal cells (Fig. 4).
Based on evidence reported in a phase 2 clinical
study, the patient was prescribed denosumab in weekly 120-mg
subcutaneous injections for 3 weeks, followed by monthly 120-mg
subcutaneous injections for 7 months (11). No adverse effects were observed. At 3
and 7 months after the start of denosumab therapy, thoracolumbar CT
scans were performed and the imaging series showed the border of
the vertebral body and soft tissue, including the spinal canal,
indicating vertebral cortex consolidation (Fig. 5). The MRI showed that the intensity of
vertebral body decreased to a level similar to normal vertebrae
upon T2-weighted imaging, and compression of the spinal cord
decreased owing to tumor shrinkage (Fig.
3B). The patient was scheduled for a TES of the T11 vertebra
following 8 months of denosumab treatment. Preoperative angiography
and embolization of the segmental artery from T10 to T12 was
performed the day prior to surgery to reduce intraoperative
bleeding (12). The TES for the
resection of the T11 vertebra involved surgery via the posterior
approach using transcranial electrical motor-evoked potentials for
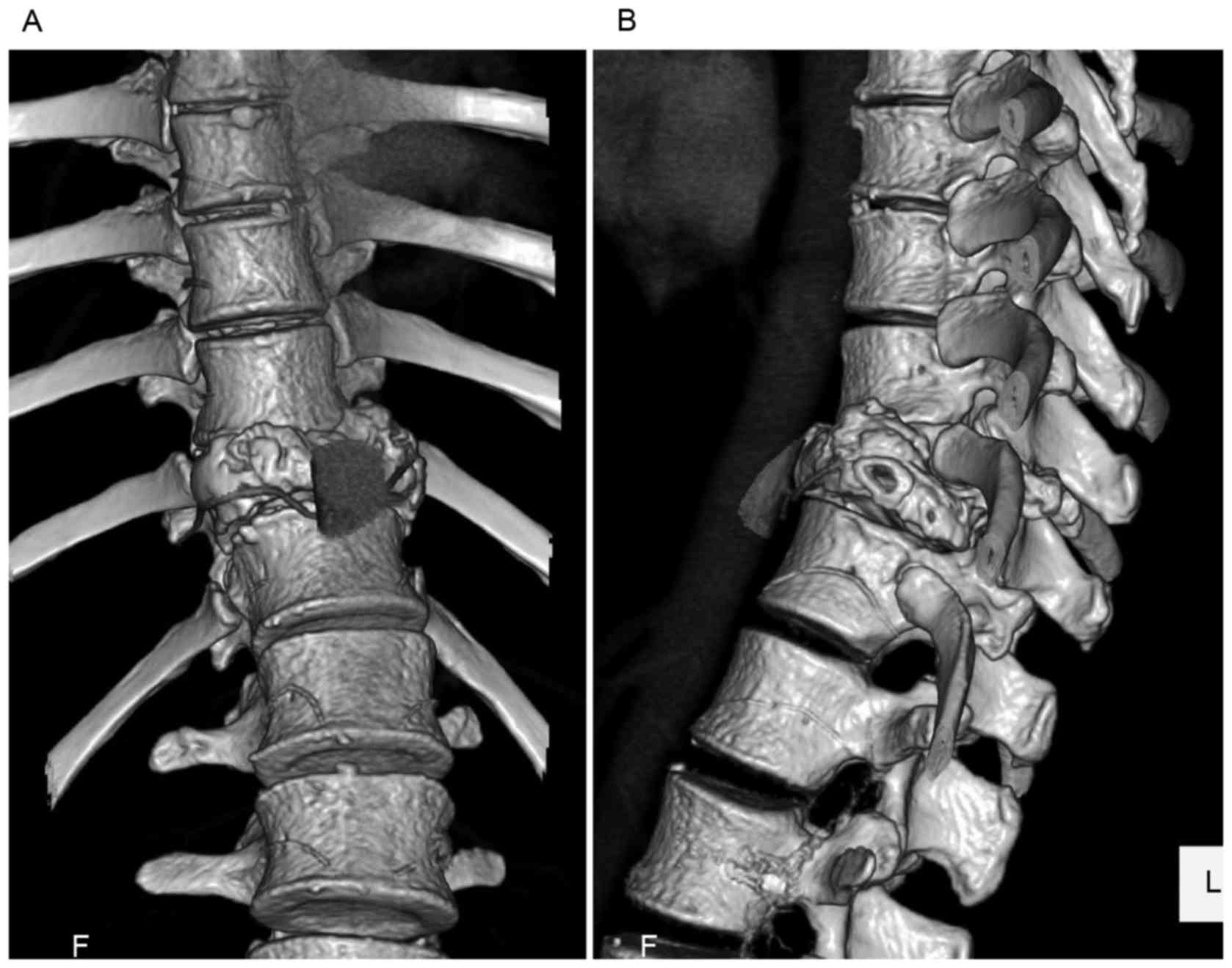
spinal cord neuromonitoring purposes. Immediately prior to surgery,
a three-dimensional CT angiography image revealed that the anterior
wall of the GCT had collapsed and the edge of the anterior wall was
overlapping the adjacent vertebrae, which made the resection line
irregular (Fig. 6). A CT-based
navigation system (StealthStation; Medtronic Ltd., Memphis, TN,
USA) was used for correct screw placement and to ensure the
completion of an en bloc spondylectomy. Release of the border of
the T11 vertebra was confirmed using the tip of the navigation
probe. The TES was performed without any unexpected perioperative
events (Fig. 7). Pathological
analysis of the T11 vertebra demonstrated an absence of giant cells
and stromal cells (Fig. 8). The
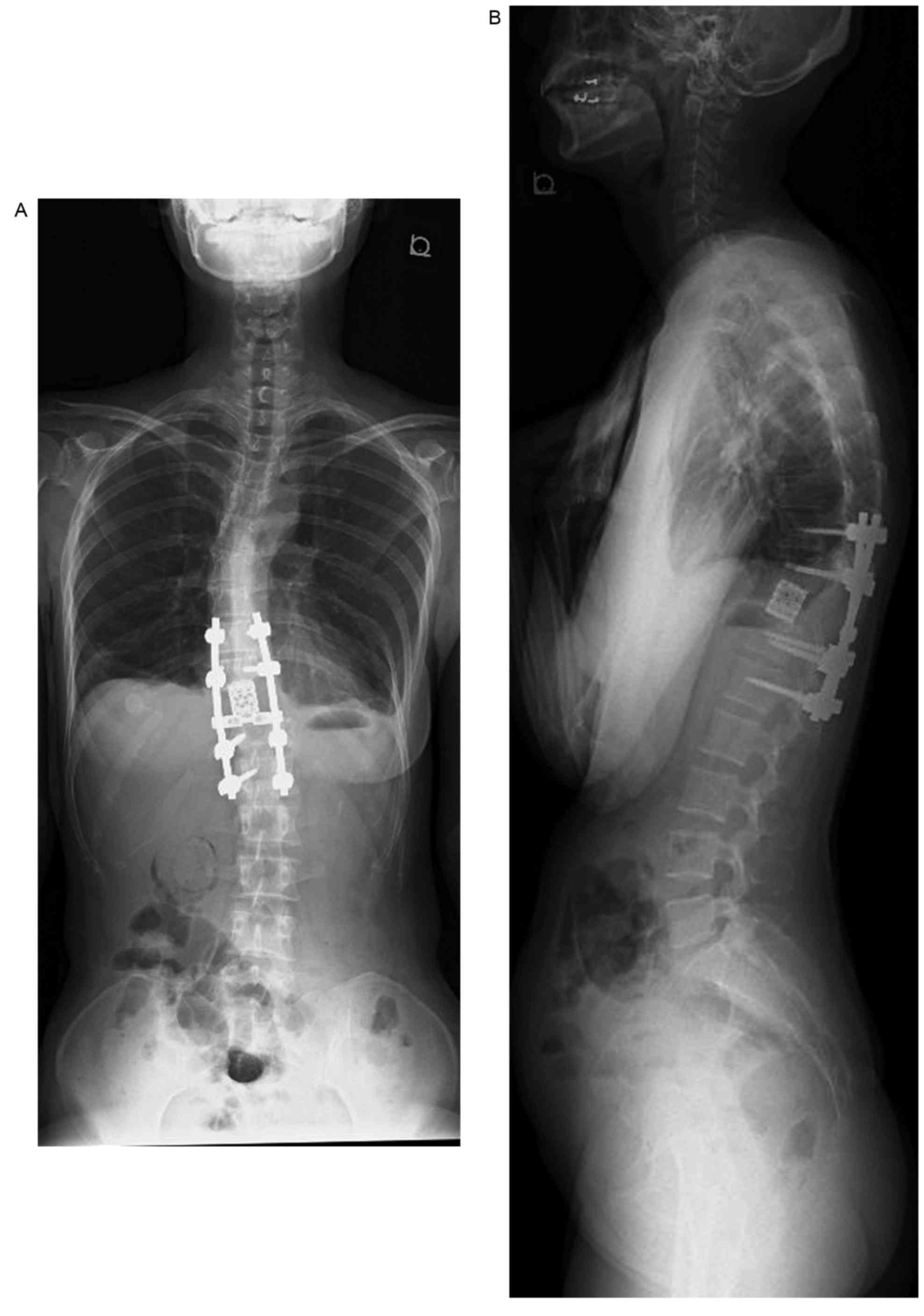
patient is followed-up every 3 months and continues on monthly
denosumab treatment without any complications or evidence of
recurrence (Fig. 9).
Written informed patient consent was obtained for
the publication of this study.
Discussion
A GCT of the spine is a rare entity, accounting for
2.7 to 6.5% of all types of GCTs in the bone (3), and its treatment remains a challenge.
Currently, there is no consensus regarding the optimal treatment,
which can be surgical or conservative (non-invasive) in nature,
with or without adjuvant therapy (e.g., radiotherapy, arterial
embolization, argon beam coagulation, cryotherapy, bisphosphonates
or interferon). A treatment strategy should be decided on by taking
into consideration multiple factors, including the age of the
patient, lesion location, degree of tumor involvement, neurological
status, feasibility of a wide resection and the presence of
metastases or fractures (13). A
complete surgical resection of the tumor is recognized as the
ultimate goal when treating a GCT of the spine, as when it is
performed efficiently, it can result in oncological control, a
negated risk of local recurrence and the obviation of comorbidities
associated with repeat surgery (13–15).
Intralesional curettage with adjuvant treatment is
also an option, depending on the status of the patient, as it can
provide good functional results. However, as high recurrence rates
ranging from 36 to 49% have been reported (15), reoperation and radiotherapy treatment
may be necessary if the treatment is not performed meticulously
(13).
Recently, denosumab has been commonly used for the
treatment of GCTs. In an open-label, phase 2 study, 86% of patients
treated with denosumab therapy for 6 months were identified with an
objective response, defined as >90% elimination of giant cells
on histological evaluation or no radiographic progression of the
lesion (16). Another phase 2 study
reported no disease progression in 96% of patients after a median
follow-up time of 13 months (11).
Based on these results, the U.S. Food and Drug Administration
approved denosumab for the treatment of adults and skeletally
mature adolescents with GCTs of the bone. In the present case, the
vertebral cortex consolidation that was observed via CT imaging,
following treatment for 8 months with denosumab, was marked. Recent
studies have reported that preoperative denosumab treatment induced
marked regression of GCTs of the spine, which subsequently
permitted surgical resection on tumors that may otherwise have been
unresectable had it not been for tumor shrinkage (7,8,17). Agarwal et al (17) reported that a GCT at T6 had markedly
shrunk after 13 months of denosumab therapy followed by a wide
resection that included the lower lobe of the lung. Goldschlager
et al (7) reported a
multicenter, prospective series of 5 cases of GCT of the spine
treated preoperatively with denosumab; following a mean treatment
period of 6 months, denosumab reduced the tumor size by 10–40%
compared with the size before treatment (7). de Carvalho Cavalcante et al
(8) reported a case of TES for L4,
following preoperative denosumab treatment for 6 months, which
showed tumor regression of ~90% with vertebral body calcification.
Therefore, total resection subsequent to preoperative denosumab
therapy may be one therapeutic option available for the management
of aggressive and locally advanced GCTs of the spine.
The duration of denosumab treatment is not only
controversial prior to surgery, but also postoperatively. Following
surgery, the present patient continued to receive denosumab, even
after a histological evaluation of the removed vertebra, which did
not contain any giant cells or stromal cells, similar to the case
reported by de Carvalho Cavalcante et al (8). The aforementioned case involved the
continuation of denosumab once every 3 months after surgery without
any evidence of tumor recurrence (17), whereas other studies were of patients
who stopped denosumab therapy prior to surgery (7). Xu et al (6) reviewed 102 patients who underwent TES
and reported that long-term postoperative bisphosphonate treatment
significantly reduced tumor recurrence rate, as assessed by
multivariate analysis (6). After TES,
and in the absence of postoperative bisphosphonate treatment,
>50% of patients experienced tumoral relapse within a mean
follow-up period of 39.9 months, suggesting that spinal GCTs can
indeed recur after TES. By contrast, Müller et al (9) reported 18 cases of GCTs in the
extremities or sacrum of patients who were treated postoperatively
with monthly denosumab for 6 months and who remained free of
recurrent disease after a mean follow-up period of 22 months
(9). Based on this evidence, and with
the informed consent of all patients, the authors now use denosumab
prophylactically after TES. Furthermore, the treatment plan
involves discontinuing denosumab 6 months after surgery, and
follow-up spinal radiographs and CT are performed every 3 months
and 1 year after surgery, respectively, to evaluate for potential
disease recurrence. However, a longer follow-up period is
necessary.
Recently, 3 cases of high-grade sarcoma arising in
GCTs of the bone in patients treated with denosumab have been
reported (18,19). The potential association between
sarcomatous transformations of GCT and osteosarcoma in patients
receiving denosumab therapy is unclear, owing at least in part to
the limited published data for this population. Long-term follow-up
is therefore necessary. The clinical outcome, following completion
of an adequate duration of denosumab treatment, remains uncertain.
Further evidence to support how to use denosumab after the
resection of spinal GCT is definitely required.
In terms of the present case, a CT-based navigation
system was used as the patient presented with idiopathic scoliosis,
the affected vertebra was rotated and collapsed, and the edge of
the tumor had overlapped the adjacent vertebrae. Recently, clinical
studies have demonstrated that CT-based navigation systems are
useful as an assistance device to optimize the accuracy of pedicle
screw placement during surgery in patients with scoliosis (20–22).
Muscloskeletal oncologists also use CT-based navigation systems for
pelvic and sacral tumor resection, suggesting its potential to
increase the accuracy of tumor resections of anatomical and/or
surgical complexity (23,24). For malignant bone tumors of the
metaphyses of the long bones, or in iliac bones, CT-based
navigation is reported to facilitate precise planning and execution
of joint-preserving tumor resections and reconstructions, resulting
in good functional and oncological outcomes (25–27). Tian
et al (28) used CT-based
navigation in posterior decompression surgery for thoracic
ossification of the posterior longitudinal ligament (OPLL) in order
to identify the border of the vertebrae and part of the OPLL
(28). In the present case, by
positioning the tip of the navigation probe at the surface of the
vertebra or osteotomy line, the surgeons were able to recognize the
area of detached parietal pleura, the irregular border of the
collapsed T11 vertebra and the adjacent vertebrae, making it
possible to insert the chisel at an angle and in the necessary
direction for optimizing accuracy. As a result, the TES was
performed in a safe manner, particularly considering the collapsed
and expanded GCT, and the accuracy of screw insertion was
optimized.
In conclusion, the present study reports a case of a
GCT in the spine of a patient with idiopathic scoliosis who was
treated using a TES with the aid of CT-based navigation following 8
months of denosumab treatment. Denosumab can be an effective
adjuvant therapy and it can reduce the complexity of TES, a major
surgical procedure used for the effective treatment of GCTs of the
spine.
Acknowledgements
The authors would like to thank Mr. Tatsuru Kuba,
(Department of Pathology, Kitasato University Hospital) and
Professor Hiroyuki Takahashi, (Kitasato University School of Allied
Health Sciences) for their technical support in pathological
evaluations.
References
|
1
|
Rockberg J, Bach BA, Amelio J, Hernandez
RK, Sobocki P, Engellau J, Bauer HC and Liede A: Incidence Trends
in the diagnosis of giant cell tumor of bone in Sweden since 1958.
J Bone Joint Surg Am. 97:1756–1766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong L, Liu W, Sun X, Sajdik C, Tian X,
Niu X and Huang X: Histological and clinical characteristics of
malignant giant cell tumor of bone. Virchows Arch. 460:327–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimada Y, Hongo M, Miyakoshi N, Kasukawa
Y, Ando S, Itoi E and Abe E: Giant cell tumor of fifth lumbar
vertebrae: Two case reports and review of the literature. Spine J.
7:499–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niu X, Zhang Q, Hao L, Ding Y, Li Y, Xu H
and Liu W: Giant cell tumor of the extremity: Retrospective
analysis of 621 Chinese patients from one institution. J Bone Joint
Surg Am. 94:461–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang K, Zhu B, Yang S, Liu Z, Yu M and Liu
X: Primary diffuse-type tenosynovial giant cell tumor of the spine:
A report of 3 cases and systemic review of the literature. Turk
Neurosurg. 24:804–813. 2014.PubMed/NCBI
|
|
6
|
Xu W, Li X, Huang W, Wang Y, Han S, Chen
S, Xu L, Yang X, Liu T and Xiao J: Factors affecting prognosis of
patients with giant cell tumors of the mobile spine: Retrospective
analysis of 102 patients in a single center. Ann Surg Oncol.
20:804–810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldschlager T, Dea N, Boyd M, Reynolds J,
Patel S, Rhines LD, Mendel E, Pacheco M, Ramos E, Mattei TA and
Fisher CG: Giant cell tumors of the spine: Has denosumab changed
the treatment paradigm? J Neurosurg Spine. 22:526–533. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Carvalho Cavalcante RA, Silva Marques
RA, dos Santos VG, Sabino E, Fraga AC Jr, Zaccariotti VA, Arruda JB
and Fernandes YB: Lumbar spondylectomy for giant cell tumor after
denosumab therapy. Spine (Phila Pa 1976). 41:E178–E182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller DA, Beltrami G, Scoccianti G,
Campanacci DA, Franchi A and Capanna R: Risks and benefits of
combining denosumab and surgery in giant cell tumor of bone-a case
series. World J Surg Oncol. 14:2812016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deveci MA, Paydaş S, Gönlüşen G, Özkan C,
Biçer ÖS and Tekin M: Clinical and pathological results of
denosumab treatment for giant cell tumors of bone: Prospective
study of 14 cases. Acta Orthop Traumatol Turc. 51:1–6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomita K, Kawahara N, Murakami H and
Demura S: Total en bloc spondylectomy for spinal tumors:
Improvement of the technique and its associated basic background. J
Orthop Sci. 11:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fisher CG, Saravanja DD, Dvorak MF,
Rampersaud YR, Clarkson PW, Hurlbert J, Fox R, Zhang H, Lewis S,
Riaz S, et al: Surgical management of primary bone tumors of the
spine: Validation of an approach to enhance cure and reduce local
recurrence. Spine (Phila Pa 1976). 36:830–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bandiera S, Boriani S, Donthineni R,
Amendola L, Cappuccio M and Gasbarrini A: Complications of en bloc
resections in the spine. Orthop Clin North Am. 40:125–131, vii.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arbeitsgemeinschaft Knochentumoren, .
Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy
L, Matejovsky Z, Szendroi M, et al: Local recurrence of giant cell
tumor of bone after intralesional treatment with and without
adjuvant therapy. J Bone Joint Surg Am. 90:1060–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal A, Larsen BT, Buadu LD, Dunn J,
Crawford R, Daniel J and Bishop MC: Denosumab chemotherapy for
recurrent giant-cell tumor of bone: A case report of neoadjuvant
use enabling complete surgical resection. Case Rep Oncol Med.
2013:4963512013.PubMed/NCBI
|
|
18
|
Aponte-Tinao LA, Piuzzi NS, Roitman P and
Farfalli GL: A high-grade sarcoma arising in a patient with
recurrent benign giant cell tumor of the proximal tibia while
receiving treatment with denosumab. Clin Orthop Relat Res.
473:3050–3055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Broehm CJ, Garbrecht EL, Wood J and
Bocklage T: Two cases of sarcoma arising in giant cell tumor of
bone treated with denosumab. Case Rep Med. 2015:7671982015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kotani Y, Abumi K, Ito M, Takahata M, Sudo
H, Ohshima S and Minami A: Accuracy analysis of pedicle screw
placement in posterior scoliosis surgery: Comparison between
conventional fluoroscopic and computer-assisted technique. Spine
(Phila Pa 1976). 32:1543–1550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai Y, Matsuyama Y, Nakamura H, Katayama
Y, Imagama S, Ito Z and Ishiguro N: Segmental pedicle screwing for
idiopathic scoliosis using computer-assisted surgery. J Spinal
Disord Tech. 21:181–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi J, Hirabayashi H, Hashidate H,
Ogihara N and Kato H: Accuracy of multilevel registration in
image-guided pedicle screw insertion for adolescent idiopathic
scoliosis. Spine (Phila PA 1976). 35:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krettek C, Geerling J, Bastian L, Citak M,
Rücker F, Kendoff D and Hüfner T: Computer aided tumor resection in
the pelvis. Injury. 35:S-A79–S-A83. 2004. View Article : Google Scholar
|
|
24
|
Hüfner T, Kfuri M Jr, Galanski M, Bastian
L, Loss M, Pohlemann T and Krettek C: New indications for
computer-assisted surgery: Tumor resection in the pelvis. Clin
Orthop Relat Res. 426:219–225. 2004. View Article : Google Scholar
|
|
25
|
Wong KC and Kumta SM: Joint-preserving
tumor resection and reconstruction using image-guided computer
navigation. Clin Orthop Relat Res. 471:762–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho HS, Oh JH, Han I and Kim HS:
Joint-preserving limb salvage surgery under navigation guidance. J
Surg Oncol. 100:227–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Wang Z, Guo Z, Chen GJ, Yang M and
Pei GX: Irregular osteotomy in limb salvage for juxta-articular
osteosarcoma under computer-assisted navigation. J Surg Oncol.
106:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian W, Weng C, Liu B, Li Q, Sun YQ, Yuan
Q, Zhang B, Wang YQ and He D: Intraoperative 3-dimensional
navigation and ultrasonography during posterior decompression with
instrumented fusion for ossification of the posterior longitudinal
ligament in the thoracic spine. J Spinal Disord Tech. 26:E227–E234.
2013. View Article : Google Scholar : PubMed/NCBI
|