Introduction
Intrahepatic cholangiocarcinoma (ICC) originating
from biliary epithelial cells is the second most common primary
intrahepatic malignancy after hepatocellular carcinoma (HCC)
(1). ICC can be classified into three
types on the basis of the morphology of the tumour: Mass forming,
periductal infiltrating, and intraductal growing. Among which, the
intrahepatic mass-forming cholangiocarcinoma (IMCC) is the most
common form (2,3). In recent decades, the incidence and
mortality of ICC and HCC are rising markedly (4). As is known, in cirrhotic patients, the
most frequent hepatic malignant tumor is HCC (5). However, evidences suggest that cirrhosis
is an important risk factor for ICC (6,7). It is of
great importance to distinguish ICC from HCC because of the dismal
prognosis of the former and different treatment options between
them. For HCC, surgical resection, liver transplantation, and
percutaneous ablation are all available. As for ICC, surgical
resection is the only curative treatment option (5,8).
Contrast-enhanced multiple-phase (CEMP) computed
tomography (CT) and magnetic resonance imaging (MRI) are widely
used in investigating ICC and HCC in clinical practice. As the high
incidence and various enhancement patterns of HCC in cirrhotic
liver, any focal lesion in cirrhotic liver can be misdiagnosed as
HCC by many radiologists, which may lead to inappropriate
treatments (9). Therefore, its
necessary to assess and summarize the CEMP CT and MRI features of
IMCC and HCC in the setting of cirrhotic liver. However, the CEMP
CT and MRI findings of IMCC with hepatic cirrhosis have not been
well addressed. Until now, there are only five studies (4,6,9–11)
describing CT or MRI characteristics of IMCC with hepatic
cirrhosis. And the comparison between IMCC and HCC in cirrhotic
liver is fewer. Further, no one that discusses the imaging features
of both CEMP CT and MRI findings has been published. In this study,
we highlight the predominant CEMP CT and MRI features of IMCC and
HCC in cirrhotic liver, combining the accompanying characteristics
as well as serum tests to distinguish IMCC from HCC in cirrhotic
liver. To the best of our knowledge, this is the first report
describing both CT and MRI data for patients with IMCC in cirrhotic
liver and comparison with HCC.
Materials and methods
Patients
Our institutional review board approved this
retrospective study and waived the requirement for informed
consent. A retrospective case-control study was conducted. The
study included 22 patients (16 men, 6 women; mean age, 58.32 years;
range, 31–69 years) with IMCC and cirrhosis consecutively
registered in our hospital between January 2010 and December 2015.
The enrolled criteria were as follows: i) Pathologically proven
diagnosis of IMCC excluding patients with mixed
hepatocellular-cholangiocarcinoma and multiple lesions; ii)
availability of an abdominal CEMP CT and/or MR scans; iii) patients
with cirrhosis diagnosed by imaging, pathology, or clinical
criteria. CT (n=20), MRI (n=15) and serum tests (n=21) features
were retrospectively reviewed. The underlying causes of liver
cirrhosis included hepatitis B (n=13), alcohol abuse (n=3),
hepatitis C (n=1), and unknown cause (n=5).
The controls consisted of 22 patients (16 men, 6
women; mean age, 56.91 years; range, 34–67 years) with HCC and
cirrhosis. They were matched to the IMCC cases for sex (P=1.000)
and age (P=0.600). The controls were recruited during the same
study period as the cases. The inclusion criteria were (1) pathology-confirmed diagnosis of HCC
excluding patients with mixed hepatocellular-cholangiocarcinoma and
multiple lesions; and (2), (3) the same as above. CT (n=18), MRI (n=21)
and serum tests (n=22) features were retrospectively reviewed. The
underlying causes of liver cirrhosis included hepatitis B (n=19),
hepatitis B with alcohol abuse (n=2), and unknown cause (n=1).
Image acquisition
CT imaging was performed with a multidetector-row
helical CT scanners (Somatom Definition AS 40-row, Siemens Medical
Systems, Erlangen, Germany) with 5 mm axial sections from the dome
of the diaphragm to the last plane of the liver in 38 patients. All
patients were examined in a fasting state with plain scanning at
first, and then non-ionic contrast medium (Omnipaque 300 g/l; GE
Healthcare, Milwaukee, WI, USA) 80 ml per bolus injection was given
via antecubital vein for enhanced scanning. Images were obtained
separately at the arterial phase (25 sec after injection), portal
venous phase (60 sec after injection) and equilibrium phase (100
sec after injection).
MR scanning was performed using a 3.0-T magnet
(Signa or Discovery 750; GE Healthcare) with an eight-channel
torso-array coil. Axial T1-weighted images (T1WI) and T2-weighted
images (T2WI) were obtained from 36 patients, and additional
contrast-enhanced T1WI (Omniscan, 0.1 mmol/kg body weight; GE
Healthcare) images were obtained from all patients. Dynamic
breath-hold T1WI acquisitions were obtained at arterial phase,
portal venous phase, equilibrium phase and delayed phase (20–27,
45–52, 75–82 and 135–142 sec after contrast enhancement).
The imaging parameters for T1WI and T2WI were as
follows: Repetition time/echo time (TR/TE) of 205/3.2 msec (or
3.9/1.8 msec) and 6,000/102.5 msec (or 12,000/86.3). The matrix was
256×256, the standard field-of-view was 400 mm and slice thickness
was 4.0 mm with no interslice gap. Additional diffusion weighted
imaging (DWI) was performed in all patients using the following
parameters: TR/TE=1,300/60.6 or 6,000/52.5 msec, 5 mm thickness,
water selective excitation for fat suppression, matrix
size=128×128, field of view=36×36 cm, number of excitations=6.0,
slice thickness/gap=5 mm/1.0 mm, 20 axial slices, scan time=2 min
24 sec, b value=0 and 600 s/mm2, under breath-hold.
Pathological examination
Histologic specimens of IMCC and HCC were obtained
by percutaneous needle biopsy in 12 patients and by exploratory
laparotomy and nodule biopsy in 32 patients. IMCC and HCC were
diagnosed on the basis of light microscopic examinations of
histologic specimens. Hematoxylin and eosin staining and
immunohistochemical staining were performed on all tumors. All IMCC
and HCC specimen analyses were confirmed by an experienced
pathologist for diagnostic accuracy.
Image analysis
All CEMP CT and MR images were retrospectively
analyzed separately by two abdominal radiologists (Y.C. and R.S.Y.)
with 5 and 25 years of experience in abdominal radiology,
respectively, in a blind manner. Discordance between the two was
resolved by consensus. The reviewers knew that the patients had
liver tumors but were unaware of pathological outcome. Each lesion
was evaluated as following: The maximum diameter, morphology,
location, signal and/or density of the tumors on each phase and
enhancement patterns of the tumors. Accompanying findings including
hepatic capsule retraction, cholangiolithiasis, bile duct dilation,
portal vein invasion and lymphadenectasis were also noted. The
dynamic enhancement patterns were defined as follows: i)
Progressive: The nodule enhances progressively over time, reaching
maximal intensity on equilibrium phase or delayed phase, including
centripetal enhancement; ii) rim-like: The enhancement limited to
the periphery of the lesion and remains invariable from the
arterial to the portal venous and delayed phases; iii) stable: The
enhancement is unmodified through the whole process but not
restricted to the periphery; iv) wash-out: Intense contrast
enhancement during the arterial and/or portal venous phase followed
by contrast washout on equilibrium phase or delayed phase.
Statistical analysis
Statistical package for the social sciences for
windows, v20.0, (SPSS, Chicago, IL, USA) was used in this
statistical analysis. Categorical variables were tested using
χ2 test or Fishers exact test. Continous variables were
compared with the independent-samples t-test if they were
homogeneity of variances; otherwise, if continuous variables were
heterogeneity of variances, non-parametric test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Morphologic features of tumors
A total of 22 patients with diagnosis of IMCC with
cirrhosis ranging from 2.2 to 18.1 cm in maximum diameter (mean,
8.18 cm), and 22 control patients of HCC in cirrhotic liver ranging
from 1.5 to 13.1 cm in maximum diameter (mean, 5.31 cm) were
assessed (P=0.010), and the main features were summarized in
Table I. The frequency of capsule
retraction (P=0.001), portal vein invasion (P=0.002), bile duct
dilation (P=0.007) and abdominal lymphadenectasis (P<0.001) as
well as maximum diameter (P=0.010) were statistically significant
differences.
 | Table I.Main patient and tumor features. |
Table I.
Main patient and tumor features.
| Characteristic | IMCC (n=22) | HCC (n=22) | P-value |
|---|
| Age | 58.32 | 56.91 | NS (0.584) |
| Sex |
|
| NS (1.000) |
| Man | 16 (72.7%) | 16 (72.7%) |
|
|
Female | 6 (27.3%) | 6 (27.3%) |
|
| Nodule
size(cm) | 8.177 | 5.305 | 0.010 |
| Location of
nodules |
|
| NS (1.000) |
| Left
lobe | 7 (31.8%) | 7 (31.8%) |
|
| Right
lobe | 14 (63.6%) | 14 (63.6%) |
|
| Caudal
lobe | 1 (4.6%) | 1 (4.6%) |
|
|
Morphology |
|
| NS (0.176) |
|
Regular | 14 (63.6%) | 18 (81.8%) |
|
|
Irregular | 8 (36.4%) | 4 (18.2%) |
|
| Bile duct
dilation | 10 (45.5%) | 2 (9.1%) | 0.008 |
| Portal
vein invasion | 14 (63.6%) | 4 (18.2%) | 0.002 |
|
Lymphadenectasis | 20 (90.9%) | 8 (36.4%) | <0.001 |
Enhancement patterns
The enhancement patterns on CEMP CT of IMCC and HCC
with cirrhosis were summarized in Table
II. In the IMCC group, all the tumors appeared as hypodensity
compared to the surrounding parenchyma on unenhanced scans. The
analysis of the dynamic enhancement pattern throughout the
different phases showed that 6 (30.0%) and 11 (55.0%) tumors
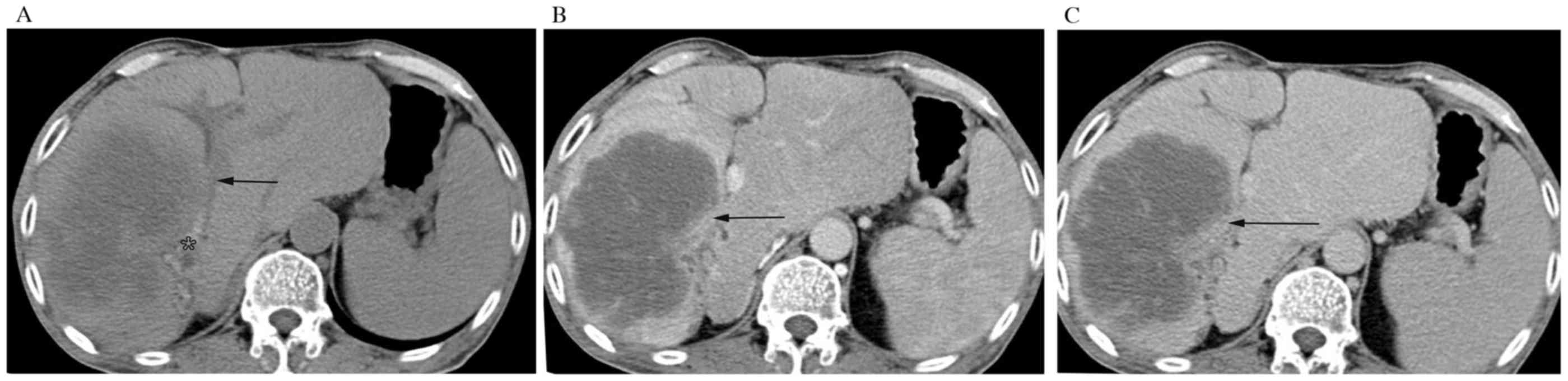
displayed as rim-like (Fig. 1A-C) and
progressive contrast enhancement. One (5.0%) tumor had a stable
enhancement pattern. The remaining 2 (10.0%) tumors represented a
washout pattern. In the HCC group, most (15, 83.3%) tumors
visualized on plain scans were hypodensity and the rest (3, 16.7%)
tumors were isodensity compared to the surrounding parenchyma.
After injection of contrast agent, 15 (83.3%) tumors showed a
washout enhancement pattern (P<0.001). One (5.6%) and two
(11.1%) tumors have a progressive (P=0.001) and stable enhancement
pattern, respectively. But no one having a rim-like enhancement
pattern (P=0.021).
 | Table II.The enhancement patterns on CEMP CT of
IMCC and HCC with cirrhosis. |
Table II.
The enhancement patterns on CEMP CT of
IMCC and HCC with cirrhosis.
| Enhancement
pattern | IMCC (n=20) (%) | HCC (n=18) (%) | P-value |
|---|
| Rim-like | 6 (30.0) | 0 (0.0) | 0.021 |
| Progressive | 11 (55.0) | 1 (5.6) | 0.001 |
| Stable | 1 (5.0) | 2 (11.1) | NS (0.595) |
| Wash-out | 2 (10.0) | 15 (83.3) | <0.001 |
The enhancement patterns and hypointense signal at
delayed phase on CEMP MRI of IMCC and HCC with cirrhosis were
summarized in Table III. All the
IMCC with cirrhosis were either hypointense (14, 93.3%) or
isointense (1, 6.7%) on T1WI with a presence of homogeneous or
heterogeneous hyperintense on T2WI. On DWI, all the tumors were
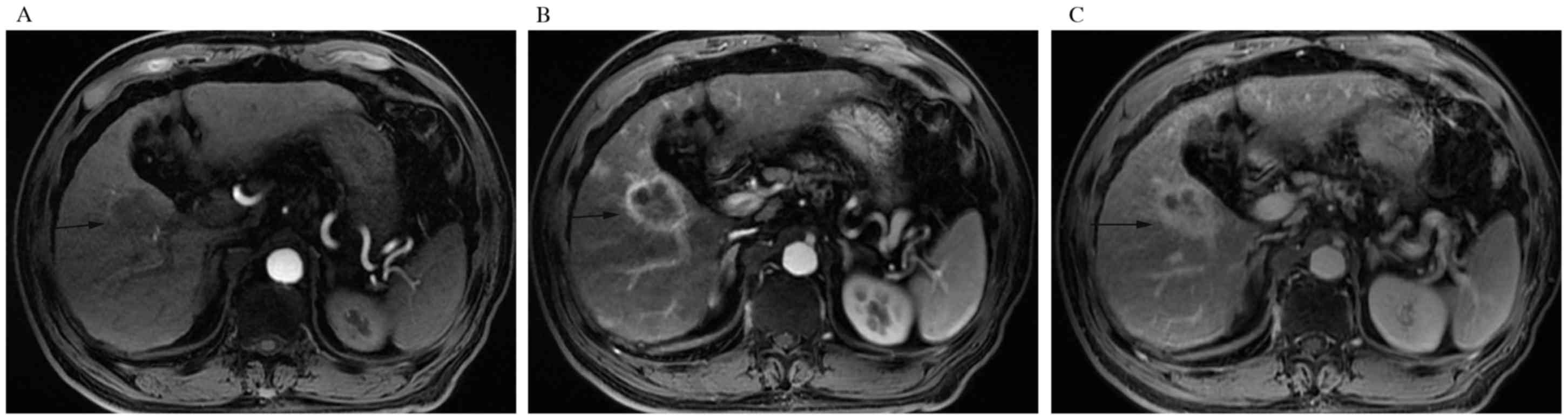
hyperintense except one. The prevalent tumor enhancement pattern
was progressive (10, 66.6%) (Fig.
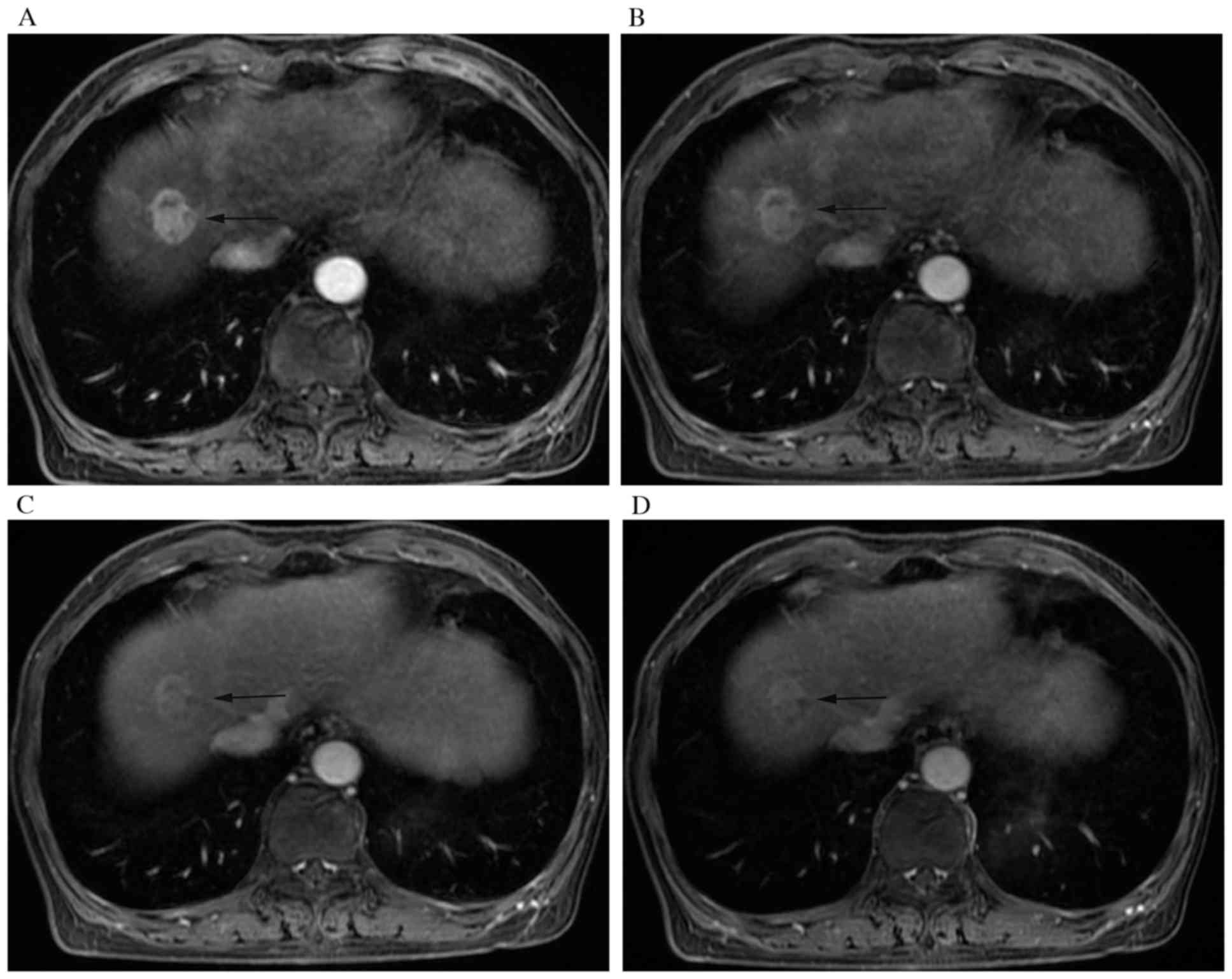
2A-C). The rim-like, stable and washout (Fig. 3A-D) enhancement patterns accounted for
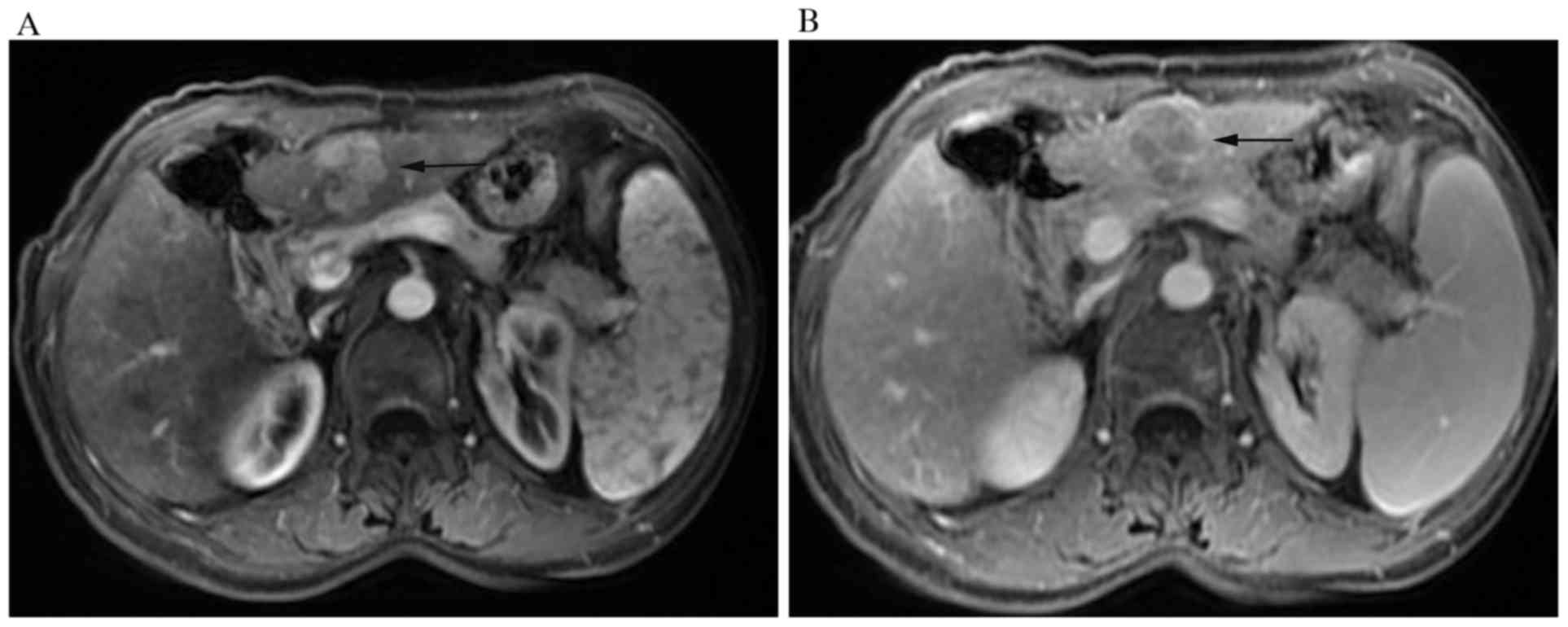
6.7, 6.7 and 20%. As for HCC with cirrhosis, three (14.3%) tumors
appeared high-low mixed signals owing to hemorrhage and the
remaining (18, 85.7%) were hypointense on T1WI. All the lesions
were homogeneous or heterogeneous hyperintense on T2WI and
hyperintense on DWI. Among these tumors, washout (Fig. 4A and B) was the most common type
throughout the different phases of the study (19, 90.4%)
(P<0.001). And there were respectively 1 (4.8%) and 1 (4.8%)
displaying progressive (P<0.001) and stable enhancement patterns
with no one having a rim-like enhancement pattern.
 | Table III.The enhancement patterns on CEMP MRI
of IMCC and HCC with cirrhosis. |
Table III.
The enhancement patterns on CEMP MRI
of IMCC and HCC with cirrhosis.
| Enhancement
pattern | IMCC (n=15) (%) | HCC (n=21) (%) | P-value |
|---|
| Rim-like | 1 (6.7) | 0 (0.0) | NS (0.417) |
| Progressive | 10 (66.6) | 1 (4.8) |
<0.001 |
| Stable | 1 (6.7) | 1 (4.8) | NS (1.000) |
| Wash-out | 3 (20.0) | 19 (90.4) |
<0.001 |
| Hyperintense signal
at delayed phase | 12 (80.0) | 2 (9.5) |
<0.001 |
In addition, we found 12 (80.0%) of the 15 IMCC
lesions while only 2 (9.5%) of 21 HCC lesions with cirrhosis
appearing as hyperintense at delayed phases on CEMP MRI
(P<0.001).
Tumor markers detection
There were 21 patients with IMCC and 22 patients
with HCC having a record of tumor markers detection (Table IV). The average ± SD numbers of
CA199, AFP and CEA were 1497.53±3626.50 U/ml, 517.92±2047.69 and
58.04±225.09 respectively in IMCC group. While, the average ± SD
numbers of CA199, AFP and CEA were 25.26±30.29 U/ml,
5460.10±17028.11 and 2.94±1.65 ng/ml, respectively in HCC group.
These continuous variables were heterogeneity of variances, so
non-parametric test was used. There were statistically differences
in CA199 (P=0.022) and AFP (P=0.013) between IMCC group and HCC
group, but no statistically difference in CEA between the two
groups.
 | Table IV.Tumor markers detection of IMCC and
HCC with cirrhosis. |
Table IV.
Tumor markers detection of IMCC and
HCC with cirrhosis.
| Tumor marker | IMCC (n=21) | HCC (n=22) | P-value |
|---|
| CA199 (U/ml) |
1,497.53±3626.50 | 25.26±30.29 | 0.022 |
| AFP (ng/ml) | 517.92±2047.69 |
5,460.10±17,028.11 | 0.013 |
| CEA (ng/ml) | 58.04±225.09 | 2.94±1.65 | 0.076 |
Discussion
Cholangiocarcinoma arises from the ductular
epithelium of the biliary tree first reported by Durand Fardel in
1840. It is classified into intrahepatic (including hilar) and
extrahepatic cholangiocarcinoma (12). ICC is the second most common primary
hepatic tumor after HCC, which accounts for 15–20% of all primary
hepatic tumors (13). And IMCC is the
most common type of ICC and clinically is comparable to HCC
(14). With respect to HCC, IMCC has
dismal prognosis and limited choices of treatment. Therefore, its
critical to accurately distinguish the two entities (6). However, the discrimination between IMCC
and HCC in cirrhotic patients remains challenging because of
atypical enhancement patterns of IMCC and HCC (15). Our research may provide some valuable
informations for distinguishing IMCC from HCC in patients with
cirrhosis.
In the present study, the mean diameter of IMCCs was
larger than that of HCCs, and there was statistically significant
difference (P=0.010), which was not comparable with other reports
in the literature (9). We considered
that the reason might be that IMCC patients were usually silent in
clinical and patients were discovered with symptoms frequently at
an advanced stage (8). While HCC
patients were usually discovered by physical examination. As we
could see in our cases, there were 13 patients with IMCC having
symptoms of abdominal pain, abdominal discomfort or feeble.
However, only 4 HCC patients were symptomatic and the remaining
were discovered by physical examination.
The CEMP CT and MRI results of our research showed
that the most prevalent enhancement pattern of IMCC in the setting
of cirrhosis was progressive enhancement (CT 55.0% and MRI 66.6%),
while only 5.6% (CT) and 4.8% (MRI) in HCC group, and there were
statistically significant differences (P=0.001 and P<0.001),
which were not only consistent with other observations in the
setting of cirrhosis (4,6,9,11) but also resembled as previously
reported in normal livers (3,13,16).
According to this enhancement pattern, radiologists could
differentiate IMCC from HCC in patients with cirrhosis, which
appeared peripheral hyperdensity/hyperintensity at hepatic arterial
phase (HAP) or partial hyperdensity/hyperintensity with gradual and
centripetal enhancement during later phases. According to our
observation and a previous report (17) that the HAP peripheral or partial
hyperintensity was owing to the greater density of viable tumor
cells, and the centripetal progressive enhancement was attributed
to the variable degree of fibrosis in the center of IMCC.
The second prevalent enhancement pattern of IMCC in
the setting of cirrhosis on CEMP CT was rim-like enhancement
(30.0%), while no one in HCC group. Noticeably, there was
statistically significant difference (P=0.021), which was also
demonstrated in the research of Kim et al (9) and Iavarone et al (11), indicating that this pattern was
related to tumor size, mostly seen in large lesions ≥3 cm, which
supported our results, and this provided significant information
for diagnosis IMCC in patients with cirrhosis. However,
interestingly, we noticed that two IMCC lesions showing rim-like
enhancement on CEMP CT were progressive enhancement on CEMP MRI.
Previous studies (11,18) also demonstrated the discrepancies in
the diagnostic accuracy between CT-scan and MRI, considering it was
owing to the stronger arterial uptake and greater sensitivity for
intravascular contrast agent on MRI than CT. So we thought that
equivocal imaging patterns need to be confirmed by MRI for further
examination.
Although some previous researches (6,11) showing
that there was no IMCC having a hallmark of HCC which represented
by hyperenhancement on the arterial phase followed by contrast
wash-out on the portal venous and/or delayed phase, we observed
that 10.0 and 20.0% IMCCs displayed wash-out enhancement pattern on
CEMP CT and MRI in our study (P<0.001), which may lead to
misdiagnosis with HCCs, which was also found in some recent reports
(4,9).
Whats more, we also noticed that the signals at delayed phase of
the IMCCs with wash-out enhancement pattern were hyperintense,
while, the overwhelming majority HCCs were observed hypointense at
delayed phase. And there was statistically significant difference
in hyperintense at delayed phases on CEMP MRI (P<0.001), which
was of great help in making correct diagnoses of the two entities
in patients with cirrhosis. To the best of our knowledge, there
have not been described previously in the English language
literature.
In addition, there were 5.0 and 6.7% IMCCs in our
research manifested stable enhancement pattern on CEMP CT and MRI,
which were also found in other observations (4) on CEMP MRI and indicated that this stable
enhancement pattern was related to tumor size and was mostly seen
in small lesions <3 cm. And there was no significant difference
between IMCC and HCC which might result in increasing risk of
misdiagnosis, if radiologists didnt take notice to this potential
similarity between IMCC and HCC.
Accompanying characteristics also played an
important role in diagnosis of IMCC in cirrhotic liver. IMCC
usually have an appearance of capsule retraction. Some researchers
thought scirrhous stroma of ICC was related to the imaging of
capsular retraction (19). Resembling
the classic findings of IMCC in noncirrhotic liver, bile duct
dilatation is also a characteristic of IMCC in cirrhotic liver,
because IMCC arises from the ductular epithelium of the biliary
tree and regularly accompanies with bile duct wall infiltration
leading to bile ducts stricture or blocking while the adjacent bile
ducts dilatate. Another reason is IMCC occasionally coexisted with
hepatolithiasis which also results in bile duct dilatation.
Narrowing or obstruction of portal vein is frequently seen in IMCC
due to invasion or external compression. In the present study, we
found that 63.6% IMCCs had a sign of portal vein invasion and only
9.1% HCCs did (P=0.008). The accompanying characteristics above
were comparable with other reports in the literature (4,6,9,11) and
could help to suggest the correct diagnoses of the two tumors.
Another difference between IMCCs and HCCs in cirrhotic liver was
lymphadenectasis. Images showed 90.9% IMCCs in our research
accompanied with lymphadenectasis in porta hepatis, retroperitoneum
or cardiodiaphragmatic angle, while only 36.4% HCCs had
lymphadenectasis and no one showed lymphadenectasis in
cardiodiaphragmatic angle, which were not been described previously
in the English language literature, to the best of our
knowledge.
As is known, AFP is a specific tumor marker of HCC.
However, there are no tumour markers specific for
cholangiocarcinoma. But some can be used as a diagnostic guide such
as CA199 and CEA (12). Previous
papers reported that the sensitivity and specificity of CA199
concentrations of more than 100 U/ml in diagnosing
cholangiocarcinoma were relatively high (12,20). In
our cases, compared with HCCs group, expression level of CA199
significantly increased in IMCCs group (P=0.022), which might be
useful in correct diagnosis of IMCC, but was not in conformity with
the research of Sheng et al (4), and further studies were needed.
We acknowledge that our study had several
limitations. Firstly, the sample size of IMCCs was relatively small
but previous study also included small samples, which reflected the
low incidence (less that 5%) of ICC in patients with cirrhosis.
Secondly, it was a retrospective study and selection bias might
exist. Thirdly, there was no data about diagnostic sensitivity and
specificity of CEMP CT and MRI in differential diagnosis owing to
the retrospective nature and further prospective studies would be
needed.
In conclusion, CEMP MRI is more advantageous over CT
in displaying the imaging features of progressive enhancement. The
CEMP CT and MRI features may be able to aid in differentiating IMCC
and HCC in cirrhosis and choice of appropriate treatment strategy.
It should be seriously considered the diagnosis of IMCC in
cirrhotic patients that progressive and/or peripheral rim-like
enhancement on CEMP CT and MRI, hyperintense at delayed phase on
CEMP MRI with capsule retraction, portal vein invasion, bile duct
dilation, abdominal lymphadenectasis and CA199 elevated.
Acknowledgements
This study was supported by funding from the Medical
Science and Technology Project of the Health Department of Zhejiang
Province of China (no. 2016147345).
References
|
1
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SA, Lee JM, Lee KB, Kim SH, Yoon SH,
Han JK and Choi BI: Intrahepatic mass-forming cholangiocarcinomas:
Enhancement patterns at multiphasic CT, with special emphasis on
arterial enhancement pattern-Correlation with clinicopathologic
findings. Radiology. 260:148–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim JH: Cholangiocarcinoma: Morphologic
classification according to growth pattern and imaging findings.
AJR Am J Roentgenol. 181:819–827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheng RF, Zeng MS, Rao SX, Ji Y and Chen
LL: MRI of small intrahepatic mass-forming cholangiocarcinoma and
atypical small hepatocellular carcinoma (≤3 cm) with cirrhosis and
chronic viral hepatitis: A comparative study. Clin Imaging.
38:265–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rimola J, Forner A, Reig M, Vilana R, de
Lope CR, Ayuso C and Bruix J: Cholangiocarcinoma in cirrhosis:
Absence of contrast washout in delayed phases by magnetic resonance
imaging avoids misdiagnosis of hepatocellular carcinoma.
Hepatology. 50:791–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmer WC and Patel T: Are common factors
involved in the pathogenesis of primary liver cancers? A
meta-analysis of risk factors for intrahepatic cholangiocarcinoma.
J Hepatol. 57:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and
treatment. Hepatology. 48:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Lee JM, Han JK, Kim KH, Lee JY and
Choi BI: Peripheral mass-forming cholangiocarcinoma in cirrhotic
liver. AJR Am J Roentgenol. 189:1428–1434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quaia E, Angileri R, Arban F, Gennari AG
and Cova MA: Predictors of intrahepatic cholangiocarcinoma in
cirrhotic patients scanned by gadobenate dimeglumine-enhanced
magnetic resonance imaging: Diagnostic accuracy and confidence.
Clin Imaging. 39:1032–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iavarone M, Piscaglia F, Vavassori S,
Galassi M, Sangiovanni A, Venerandi L, Forzenigo LV, Golfieri R,
Bolondi L and Colombo M: Contrast enhanced CT-scan to diagnose
intrahepatic cholangiocarcinoma in patients with cirrhosis. J
Hepatol. 58:1188–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciresa M, De Gaetano AM, Pompili M,
Saviano A, Infante A, Montagna M, Guerra A, Giuga M, Vellone M,
Ardito F, et al: Enhancement patterns of intrahepatic mass-forming
cholangiocarcinoma at multiphasic computed tomography and magnetic
resonance imaging and correlation with clinicopathologic features.
Eur Rev Med Pharmacol Sci. 19:2786–2797. 2015.PubMed/NCBI
|
|
14
|
Kang Y, Lee JM, Kim SH, Han JK and Choi
BI: Intrahepatic mass-forming cholangiocarcinoma: Enhancement
patterns on gadoxetic acid-enhanced MR Images. Radiology.
264:751–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim R, Lee JM, Shin CI, Lee ES, Yoon JH,
Joo I, Kim SH, Hwang I, Han JK and Choi BI: Differentiation of
intrahepatic mass-forming cholangiocarcinoma from hepatocellular
carcinoma on gadoxetic acid-enhanced liver MR imaging. Eur Radiol.
26:1808–1817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maetani Y, Itoh K, Watanabe C, Shibata T,
Ametani F, Yamabe H and Konishi J: MR imaging of intrahepatic
cholangiocarcinoma with pathologic correlation. AJR Am J
Roentgenol. 176:1499–1507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung YE, Kim MJ, Park YN, Choi JY, Pyo
JY, Kim YC, Cho HJ, Kim KA and Choi SY: Varying appearances of
cholangiocarcinoma: Radiologic-pathologic correlation.
Radiographics. 29:683–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semelka RC, Martin DR, Balci C and Lance
T: Focal liver lesions: Comparison of dual-phase CT and
multisequence multiplanar MR imagingincluding dynamic gadolinium
enhancement. J Magn Reson Imaging. 13:397–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsunematsu S, Chuma M, Kamiyama T,
Miyamoto N, Yabusaki S, Hatanaka K, Mitsuhashi T, Kamachi H, Yokoo
H, Kakisaka T, et al: Intratumoral artery on contrast-enhanced
computed tomography imaging: Differentiating intrahepatic
cholangiocarcinoma from poorly differentiated hepatocellular
carcinoma. Abdom Imaging. 40:1492–1499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosconi S, Beretta GD, Labianca R, Zampino
MG, Gatta G and Heinemann V: Cholangiocarcinoma. Crit Rev Oncol
Hematol. 69:259–270. 2009. View Article : Google Scholar : PubMed/NCBI
|