Introduction
Epidermal growth factor receptor (EGFR) and human
EGFR2 (HER2) are frequently upregulated or mutated in various types
of cancer, and serve essential roles in cancer cell proliferation,
survival, migration and differentiation (1,2). EGFR is
frequently mutated or overexpressed in lung, brain, colon,
pancreatic and breast cancer (3–6).
Furthermore, HER2 is often overexpressed in breast, gastric,
esophageal, pancreatic and ovarian cancer (7,8).
EGFR-tyrosine kinase inhibitors (TKIs), including gefitinib and
erlotinib, are used either alone or in combination with radiation
or chemotherapy in cancer therapy (9). The EGFR-TKI lapatinib, and the
monoclonal antibody trastuzumab have been approved for the
treatment of HER2-overexpressing breast cancer (10). Lapatinib is a potent adenosine
trisphosphate (ATP)-competitive dual kinase inhibitor that inhibits
EGFR and HER2, and has demonstrated antiproliferative activity
against human HER2-amplified breast cancer cell lines (11). Wainberg et al (12) reported that lapatinib selectively
inhibits the proliferation of HER2-amplified human gastric cancer
cells. However, lapatinib is currently unsatisfactory for the
treatment of patients with gastric cancer (13). A previous clinical trial suggested
that HER2-targeted therapy is associated with drug resistance in
cancer cells (14). However, it is
unknown whether such resistance mechanisms are associated with
gastric cancer stem cells (GCSCs).
The CSC hypothesis was introduced to explain the
pathogenesis of numerous cancer types. CSCs have the ability to
self-renew and proliferate indefinitely, which can initiate tumor
formation and cause tumor recurrence. CSCs are distinguished from
the bulk of the tumor cell population by their ability to
successfully seed new tumors when implanted in low numbers into
experimental animals (15).
Furthermore, CSCs are more resistant to chemotherapy and
radiotherapy than their corresponding differentiated cancer cells
(16–18). The neurogenic locus notch homolog
protein (Notch) signaling pathway serves an important role in the
determination of cell fate in various organ systems (19). The Notch pathway encompasses four
types of receptors (Notch1, −2, −3 and −4) and five membrane
proteins ligands that include Delta-like ligands (DLL1, −2 and −3)
and Serrate/Jagged (JAG1 and −2). The Notch1 signaling pathway is
essential in maintaining the characteristics of CSCs and is
associated with the self-renewal of various types of CSCs,
including breast and pancreatic CSCs (20). Hes1 is the downstream target gene of
Notch1 pathway (21). Hes1 may have
an important function in the maintenance of cancer stem cells
self-renewal and epithelial-mesenchymal transition (EMT) process
(22).
In the present study, the role of the Notch1
signaling pathway in GCSCs and lapatinib sensitivity was examined.
Furthermore, the current study aimed to elucidate the molecular
mechanism underlying resistance to lapatinib in GCSCs.
Materials and methods
Culture of sphere-forming cells
MKN45 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China),
which were seeded at a density of 2×104 cells/ml in 100
mm ultralow attachment plates (Costar; Corning Incorporated,
Corning, NY, USA). Cells were grown in serum-free RPMI-1640 and
Nutrient Mixture F-12 medium (both Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with B27 (1:50; Hyclone; GE
Healthcare Life Sciences), 20 ng/ml EGF, 20 ng/ml basic fibroblast
growth factor (bFGF) (both R&D Systems, Minneapolis, MN, USA),
5 µg/ml bovine insulin (Cell Applications, Inc., San Diego, CA,
USA), 0.5 µg/ml hydrocortisone (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and penicillin/streptomycin (Hyclone; GE
Healthcare Life Sciences). MKN45 cells were grown for 3 days in the
above sphere culture maintained at 37°C in a 5% CO2
atmosphere and produced spheres, which were dissociated through
incubation with 0.05% trypsin/EDTA (Sigma-Aldrich; Merck KGaA) on
day 4. Dissociated MKN45 cells were cultured in the aforementioned
sphere conditions maintained at 37°C in a 5% CO2
atmosphere for another 3 days and then harvested.
Tumorigenicity assay
A total of 40 4-week-old female nude mice (weight
18–20 g) were obtained from the Shanghai Experimental Animal Center
of the Chinese Academy of Science (Shanghai, China). The mice, were
given SPF grade feed and purified water and were kept in cages (5
mice/cage) in a room with a constant temperature (22±1°C) and a 12
h dark/light cycle. For in vivo experiments, sphere-forming
and parental cells were resuspended in PBS (Hyclone; GE Healthcare
Life Sciences), and injected subcutaneously into the limbs of mice.
The protocol used in the present study was approved by the Ethics
Committee of Chongqing Cancer Institute (Chongqing, China). Groups
of mice were inoculated with sphere-forming or parental cells at
5×102, 5×103, 5×104 or
5×105 (five mice/group). Tumor growth was monitored
every 2 days following the second week of inoculation.
Lentivirus transfection
Inhibition of transcription factor Hes1 (Hes1) was
achieved by infecting cells with the Hes1-small interfering (si)RNA
lentivirus (Shanghai GenePharma Co., Ltd., Shanghai, China).
Transduction was performed using a GenePharma Lentivirus
Transduction kit (Shanghai GenePharma Co., Ltd.). The target
sequence for the Hes1 siRNA was 5′-AGATCAATGCCATGACCTA-3′. Cells
were cultured in six-well plates at 20–30% confluence and incubated
for 12 h at 37°C with 5% CO2. Cells were then infected
with Hes1-siRNA- or scrambled control-siRNA-expressing lentiviruses
(5×108 TU/ml, 40 µl, Shanghai GenePharma Co., Ltd.).
Following the infections, the culture medium was replaced with
supernatant fluid that contained an appropriate viral titer (1
ml/well). After incubating at 37°C for 12 h, the viral supernatant
was replaced with fresh medium. The infected cells were selected
using 2 mg/ml puromycin (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) following incubation for 48 h. Successful infection was
confirmed by expression of green fluorescent protein using an
inverted fluorescence microscope (Leica DMI4000 B; Leica
Microsystems GmbH, Wetzlar, Germany). The knockdown efficiency was
determined using western blotting as described below.
Spheroid colony formation assay
Cells were inoculated into each well (20 cells/well)
of ultralow attachment 48-well plates (Costar; Corning
Incorporated) and supplemented with 300 µl of RPMI-1640 containing
40 ng/ml bFGF and 20 ng/ml EGF. After incubation for 4 weeks at
37°C with 5% CO2, the total number of spheroid
colonies/well was counted.
Cell chemosensitivity examination
Cells cultured in medium were incubated at 37°C with
5% CO2 and treated with 6 mM 5-fluorouracil (5-Fu) or 5
µmol/l lapatinib (both Sigma-Aldrich; Merck KGaA). After 48 h of
exposure, 20 ml of 0.5 mg/ml MTT solution (Sigma-Aldrich; Merck
KGaA) was added for an additional 4 h and then 100 ml dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added for 15 min. The
plates were agitated at a low speed for 5 min and the absorbance
was measured at a wavelength of 570 nm using a spectrophotometer.
Five wells were assayed for each condition.
Western blotting
According to the manufacturer's protocol, total
protein samples for immunoblots were extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Following quantification of the
protein extracts using a BCA protein assay, equivalent amounts of
protein (40 µg/lane) were resolved using 10% SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane (both Beyotime
Institute of Biotechnology). Membranes were subsequently blocked
with 5% non-fat milk in TBS-Tween-20 (Beyotime Institute of
Biotechnology) for 1 h at 4°C. Next, the blots were incubated with
the appropriate primary antibody for 12 h at 4°C and then with the
corresponding horseradish peroxidase (HRP)-conjugated secondary
antibody for 2 h at 37°C. Signals were detected using an enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA). The
results were analyzed by Quantity One software (version 4.6.2,
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Antibodies directed against GAPDH (cat. no. 560005)
and zinc finger protein SNAI1 (Snail, cat. no. 550589) were
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Antibodies
directed against epithelial cell adhesion molecule (EpCAM, cat. no.
ab71916), the homeobox protein Nanog (Nanog, cat. no. ab109250),
the transcription factor sex determining region Y-box 2 (Sox2, cat.
no. ab92494), multidrug resistance protein 1 (MDR1, catalog no.
ab170904), ATP-binding cassette sub-family G member ABCG1 (cat. no.
ab52617), ABCG2 (cat. no. ab24115), DNA repair protein RAD51
homolog 1 (RAD51, cat. no. ab88572), tight junction protein ZO-1
(ZO1, cat. no. ab59720), N-cadherin (cat. no. ab76057), E-cadherin
(cat. no. ab1416) and vimentin (cat. no. ab92547) were purchased
from Abcam (Cambridge, UK). The monoclonal goat anti-rabbit IgG and
goat anti-mouse HRP-conjugated antibodies (cat. no. sc-2354) were
purchased from Santa Cruz Biotechnology Inc.
The following antibody dilutions were used:
Anti-Notch1, 1:1,200; anti-Hes1, 1:10,000; anti-E-cadherin,
1:1,200; anti-N-cadherin, 1:1,200; anti-vimentin, 1:1,200;
anti-Snail, 1:500; anti-ZO1, 1:500; anti-EpCAM, 1:1,500;
anti-Nanog, 1:1,500; anti-Sox2, 1:1,500; anti-MDR1, 1:1,000;
anti-ABCG1, 1:1,000; anti-ABCG2, 1:1,000; anti-RAD51, 1:500;
anti-GAPDH, 1:500; and HRP-conjugated IgG antibodies, 1:7,000.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from the cells using RNAiso
(Takara Bio, Inc., Otsu, Japan) and complementary DNA (cDNA) was
then synthesized using the PrimeScript II First Strand cDNA
Synthesis kit (Takara Bio, Inc.) according to the manufacturers'
protocols. PCR (100 ng cDNA used per qPCR) was performed using a
CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.)
with SYBR® Premix Ex Taq™ II (Takara Bio, Inc.). The PCR
conditions were as follows: 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec and 60°C for 30 sec. Data were normalized against
β-actin messenger RNA (mRNA). The sequences of the PCR primers were
as follows: Notch1 forward, 5′-TGCCGAACCAATACAACCCTC-3′ and
reverse, 5′-TGGTAGCTCATCATCTGGGACA-3′; Hes1 forward,
5′-GTGCATGAACGAGGTGACCC-3′ and reverse, 5′-GTATTAACGCCCTCGCACGT-3′;
and β-actin forward, 5′-CCACGAAACTACCTTCAACTCC-3′ and reverse,
5′-GTGATCTCCTTCTGCATCCTGT-3′. qPCR was performed according to the
2−ΔΔCq method (23).
Histological examination
Tumor tissues were fixed in 10% neutral-buffered
formalin for 24 h at room temperature, embedded in paraffin, and
then the sections (5 um) were stained with hematoxylin for 10 min
and eosin (both Beyotime Institute of Biotechnology) for 5 sec at
room temperature. Histological differences were examined using an
inverted microscope.
Statistical analysis
All experiments were repeated three times and the
results were analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). Data are presented as the mean ± standard
deviation. The statistical significance of the differences among
the groups was evaluated using the Student's t-test (two-tailed).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sphere-forming cells isolated from
gastric cancer MKN45 cells exhibit the characteristics of CSCs
Tumors contain a small number of CSCs that possess
self-renewal and tumor-initiating abilities (24). To isolate sphere-forming cells, MKN45
cells were grown in ultralow attachment plates with sphere culture
medium for 7 days. Subsequently, the expression levels of CSC
markers, including EpCAM, Nanog and Sox2, were determined using
western blotting. Concordant with previous studies the protein
expression levels of these CSC markers were significantly higher in
sphere-forming cells than in parental cells (Fig. 1A). Furthermore, in the spheroid colony
formation assay, sphere-forming cells formed significantly more
spheroids compared with those in parental cells (Fig. 1B).
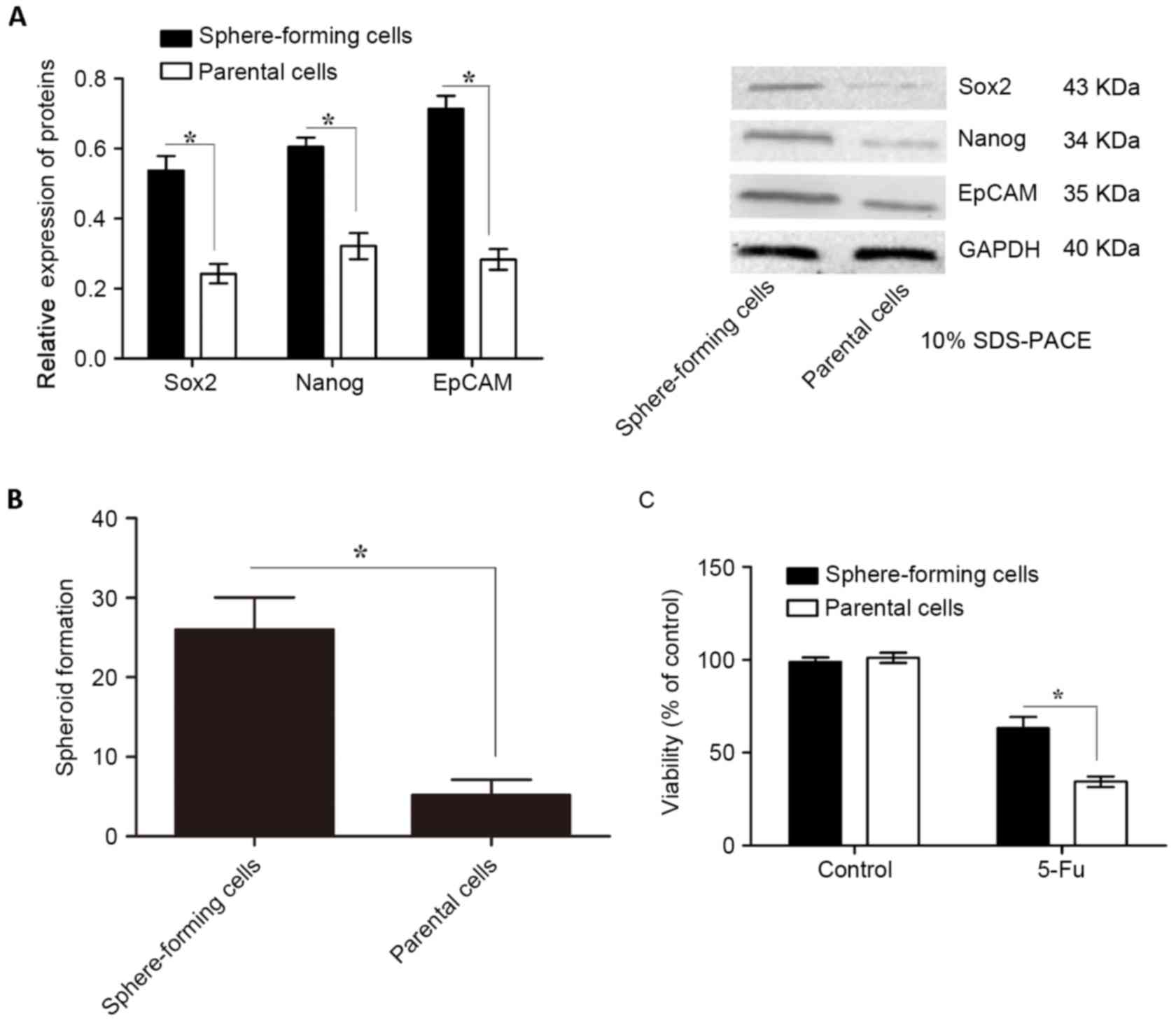 | Figure 1.Expression of CSC markers,
spheroid-forming ability and chemotherapy susceptibility of
sphere-forming and parental cells isolated from MKN45 cells. (A)
CSC markers, including EpCAM, Nanog and Sox2, were evaluated using
western blotting relative to GAPDH. The protein expression levels
of these CSC markers were significantly higher in sphere-forming
cells compared with those in parental cells. (B) Sphere-forming
cells formed significantly more spheroids than parental cells. (C)
Sphere-forming cells were significantly more chemoresistant to 5-Fu
than parental cells, as indicated by their cell viability levels
determined using an MTT assay. Data are presented as the mean ±
standard deviation (n=5). *P<0.05. CSC, cancer stem cell; EpCAM,
epithelial cell adhesion molecule; Nanog, homeobox protein Nanog;
Sox2, sex determining region Y-box 2; 5-Fu, 5-fluorouracil. |
In the tumorigenicity assay, nude mice were injected
with 5×102-5×105 sphere-forming or parental
cells. Transplantation of 5×102, 5×103 or
5×104 parental cells consistently failed to form tumors
in all mice, while 5×105 parental cells led to tumor
formation in 1/5 mice (Table I). By
contrast, transplantation of 5×102 sphere-forming cells
resulted in tumor formation in 2/5 mice, while transplantation of
5×103, 5×104 or 5×105
sphere-forming cells resulted in tumor formation in all mice
(Table I). Subsequently, the
chemotherapy susceptibility of sphere-forming and parental cells to
5-Fu, which is generally used for the treatment of gastric cancer,
was examined. Sphere-forming cells were significantly more
chemoresistant to 5-Fu than parental cells, as determined using an
MTT cell viability assay (Fig. 1C;
P<0.05). These data suggest that sphere-forming cells are
tumorigenic and possess CSC characteristics.
 | Table I.Tumorigenicity of sphere-forming and
parental cells in nude micea. |
Table I.
Tumorigenicity of sphere-forming and
parental cells in nude micea.
|
| No. of cells
injected |
|---|
|
|
|
|---|
| Tumor marker
presence |
5×102 |
5×103 |
5×104 |
5×105 |
|---|
|
CD44+ | 2/5 | 4/5 | 5/5 | 5/5 |
|
CD44− | 0/5 | 0/5 | 0/5 | 1/5 |
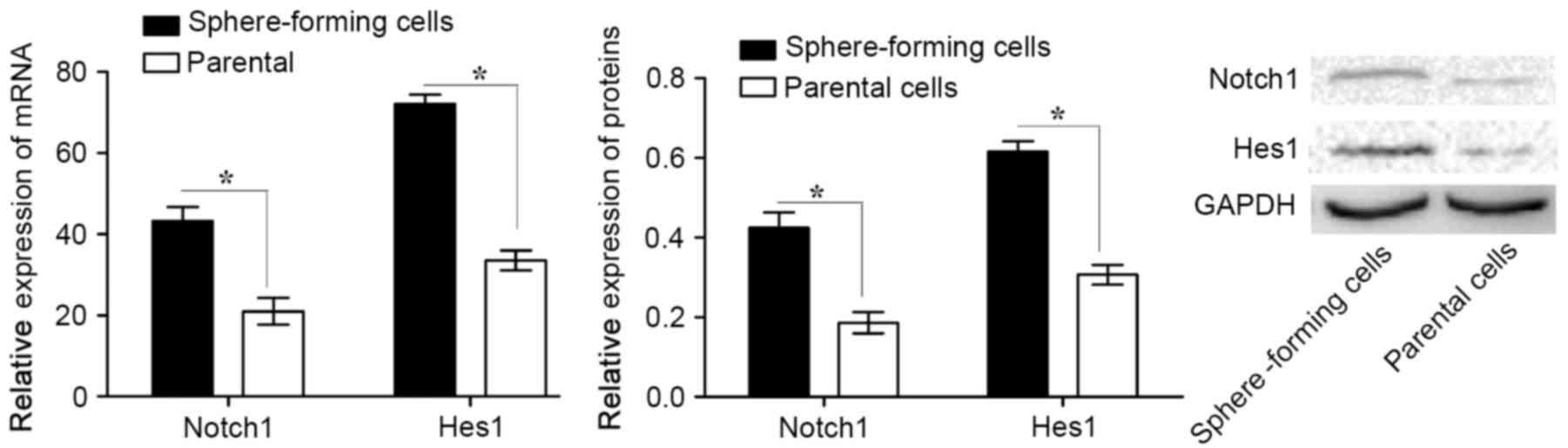
Notch1 signaling pathway is activated
in sphere-forming MKN45 cells
To investigate the role of the Notch1 signaling
pathway in CSCs, the expression of Notch1 and Hes1 in
sphere-forming and parental cells was analyzed. It was revealed
that Notch1 and Hes1 mRNA and protein expression levels were
significantly higher in sphere-forming cells compared with those in
parental cells (Fig. 2). These data
suggest that the Notch1 signaling pathway is activated in
sphere-forming cells.
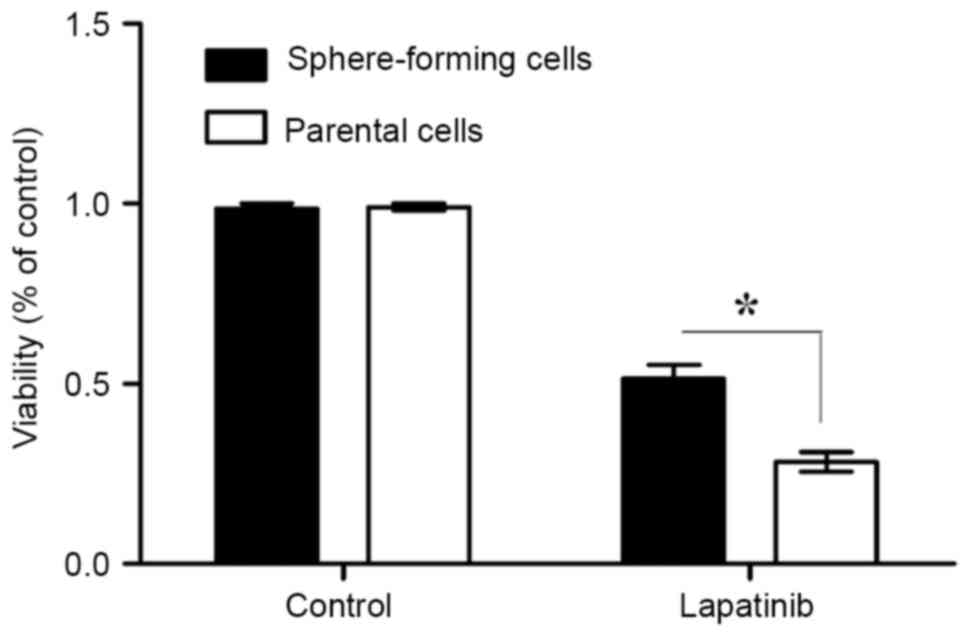
Sphere-forming MKN45 cells are
resistant to the EGFR-TKI lapatinib
The sensitivities of sphere-forming and parental
cells to lapatinib were next assessed. The results of the MTT
assays demonstrated that the viability of sphere-forming cells was
significantly increased compared with that of parental cells
(P<0.05; Fig. 3). Therefore, this
suggests that sphere-forming cells are more resistant to lapatinib
than parental cells.
Inhibition of Hes1 expression
attenuates the characteristics of CSCs and resistance to lapatinib
in sphere-forming cells
To examine the effects of the Notch1 signaling
pathway in GCSCs, sphere-forming cells were infected with
lentivirus containing Hes1-siRNA or scrambled control-siRNA. The
protein expression levels of Hes1 were determined using western
blot analysis. Infection of sphere-forming cells with lentivirus
containing Hes1-siRNA significantly reduced Hes1 protein expression
levels compared with those in control-siRNA-treated and untreated
cells, while the control siRNA group exhibited no significant
effect compared with the untreated control group (Fig. 4A). Simultaneously, the protein
expression levels of the CSC markers EpCAM, Nanog and Sox2 were
significantly downregulated in Hes1-siRNA lentivirus-infected
sphere-forming cells compared with those in the control-siRNA group
(Fig. 4B).
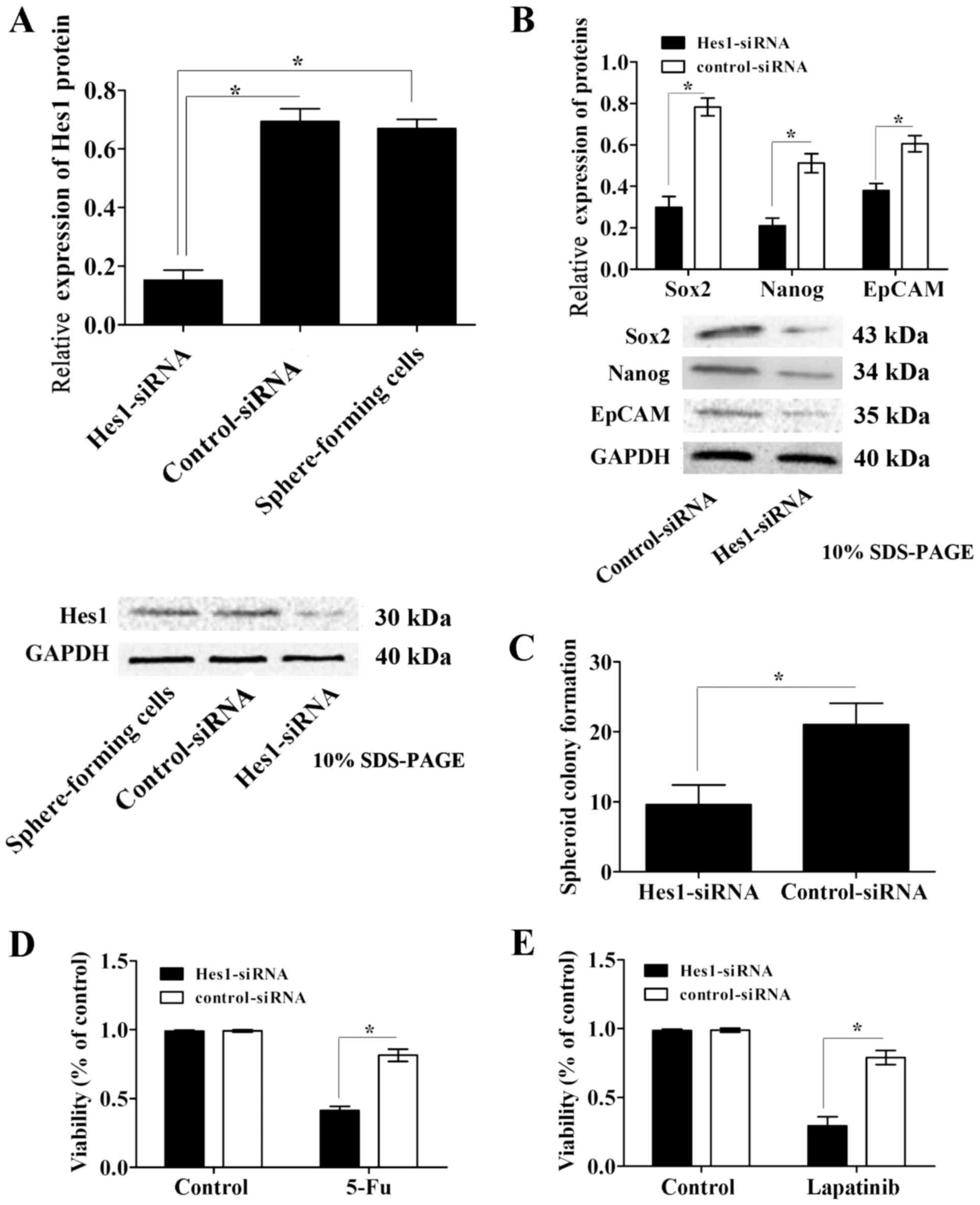 | Figure 4.Inhibition of Hes1 expression
attenuates the characteristics of CSCs and resistance to lapatinib
in sphere-forming cells. (A) Hes1 protein expression levels were
significantly downregulated in sphere-forming cells infected with
lentivirus containing Hes1-siRNA compared with those in the
control-siRNA infected and untreated groups. (B) The protein
expression levels of the CSC markers EpCAM, Nanog and Sox2, were
significantly decreased in Hes1-siRNA lentivirus-infected
sphere-forming cells compared with those in the control-siRNA
group. (C) Sphere-forming cells infected with Hes1-siRNA lentivirus
formed significantly fewer spheroids than control-siRNA
lentivirus-infected cells. The chemotherapy susceptibly of
Hes1-siRNA lentivirus-infected sphere-forming cells to (D) 5-Fu and
(E) lapatinib was significantly increased compared with that of
control-siRNA lentivirus-infected cells. Protein expression levels
were normalized to GAPDH. Data are presented as the mean ± standard
deviation (n=5). *P<0.05. Notch1, neurogenic locus notch homolog
protein 1; Hes1, transcription factor Hes1; EpCAM, epithelial cell
adhesion molecule; Nanog, homeobox protein Nanog; Sox2, sex
determining region Y-box 2; siRNA, small interfering RNA; CSC,
cancer stem cell; 5-Fu, 5-fluorouracil. |
In the spheroid colony formation assay,
sphere-forming cells infected with Hes1-siRNA lentivirus formed
significantly less spheroids compared with those in the
control-siRNA lentivirus-infected cells (Fig. 4C). In addition, the results of the MTT
assay revealed that the susceptibility of Hes1-siRNA
lentivirus-infected cells to the chemotherapeutic agent 5-Fu was
significantly increased compared with that of the control-siRNA
group (Fig. 4D).
To investigate the influence of the Notch1 signaling
pathway on the susceptibility of sphere-forming cells to EGFR-TKIs,
the susceptibility of Hes1-siRNA lentivirus-infected sphere-forming
cells to lapatinib was assessed. Data from the MTT assays
demonstrated that the survival rate of Hes1-siRNA
lentivirus-infected sphere-forming cells was significantly
decreased compared with that of control-siRNA lentivirus-infected
cells (P<0.05; Fig. 4E). These
data indicate that inhibition of Hes1 expression attenuates the
characteristics of CSCs and increases susceptibility to lapatinib
in sphere-forming cells.
Inhibition of Hes1 prevents EMT and
decreases the expression of chemoresistance-associated proteins in
sphere-forming MKN45 cells
To further investigate the molecular mechanisms of
the Notch1 signaling pathway on the susceptibility of
sphere-forming cells, the expression of EMT markers and
chemoresistance-associated proteins was examined. Western blot
analysis demonstrated that the expression levels of the epithelial
markers E-cadherin and ZO1 were significantly upregulated, while
the expression levels of the mesenchymal markers N-cadherin,
vimentin and Snail were significantly downregulated in Hes1-siRNA
lentivirus-infected sphere-forming cells compared with those in the
control-siRNA group (Fig. 5A).
Furthermore, the expression levels of the
chemoresistance-associated proteins MDR1, ABCG1, ABCG2 and RAD51
were significantly decreased in Hes1-siRNA lentivirus-infected
sphere-forming cells (Fig. 5B).
Together, these results indicate that inhibition of Hes1 can impair
EMT and decrease the expression of chemoresistance-associated
proteins in sphere-forming MKN45 cells.
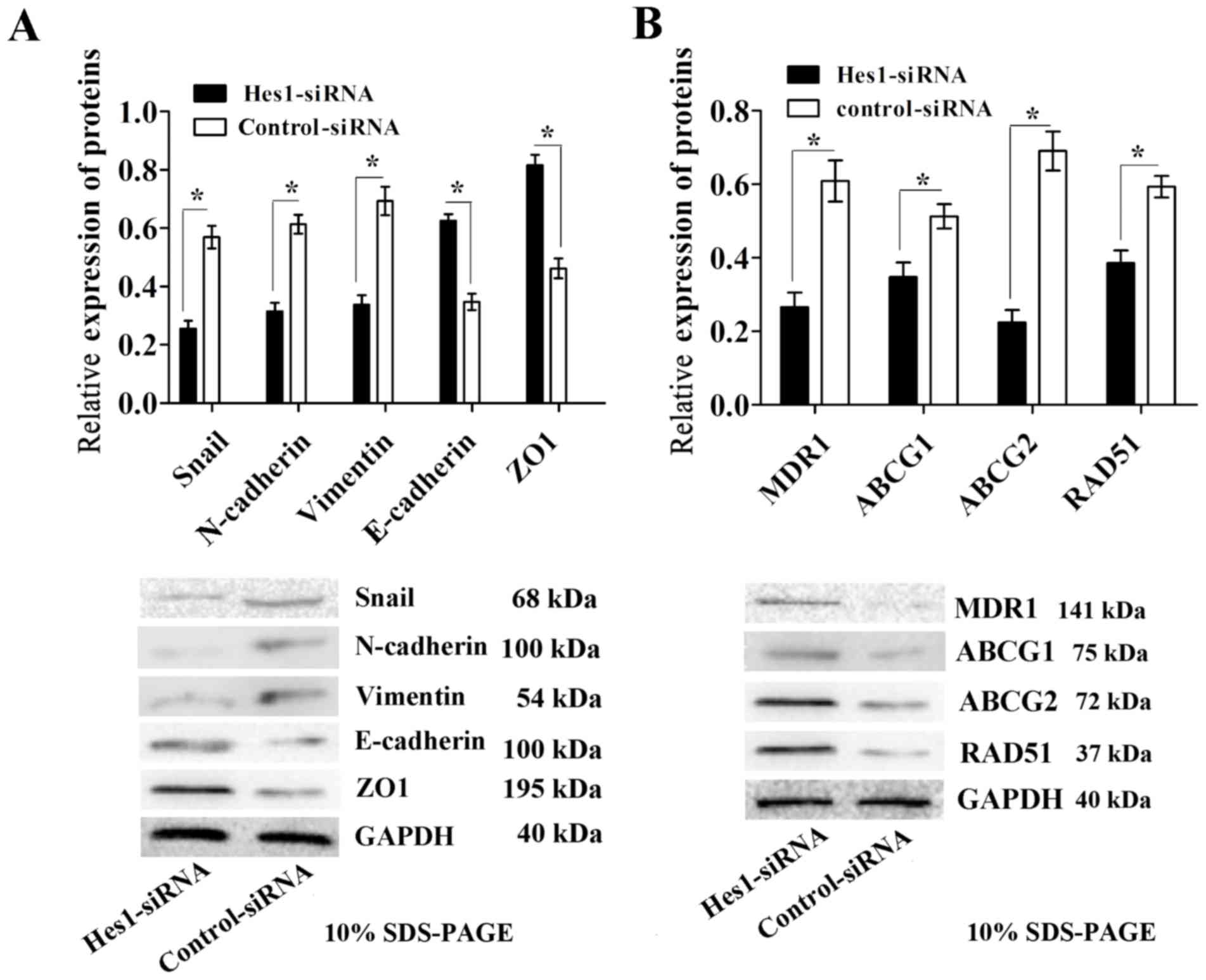 | Figure 5.Infection with Hes1-siRNA lentivirus
results in impaired EMT and decreased expression of
chemoresistance-associated proteins in sphere-forming MKN45 cells.
(A) EMT was impaired in sphere-forming cells infected with
Hes1-siRNA lentivirus, as demonstrated by a significant decrease in
the expression levels of the EMT-associated proteins Snail,
N-cadherin and vimentin, and an increase in expression level of
E-cadherin and ZO1 compared with those in the control-siRNA group.
(B) The expression levels of the chemoresistance-associated
proteins MDR1, ABCG1, ABCG2 and RAD51 were significantly
downregulated in sphere-forming cells infected with Hes1-siRNA
lentivirus compared with those in the control-siRNA group. Protein
expression levels were normalized to GAPDH. Data are presented as
the mean ± standard deviation (n=5). *P<0.05. Notch1, neurogenic
locus notch homolog protein 1; Hes1, transcription factor Hes1;
siRNA, small interfering RNA; Snail, zinc finger protein SNAI1;
MDR1, multidrug resistance protein 1; ABCG, ATP-binding cassette
sub-family G member; RAD51, DNA repair protein RAD51 homolog 1;
ZO1, tight junction protein ZO-1; EMT, epithelial-mesenchymal
transition. |
Discussion
The CSC hypothesis suggests that cancer is
maintained by a subpopulation of stem cells with an indefinite life
span, which raises the possibility that targeting CSCs could
provide an approach for cancer treatment (25). The sphere-forming assay in which cells
are cultured in non-adherent conditions in a serum-free medium
supplemented with bFGF and EGF is a practical approach for
identifying stem cells in individual solid tumor tissue samples or
cancer cell cultures (26,27). In the present study, sphere-forming
cells were developed by cultivating the human gastric cancer cell
line MKN45 in defined serum-free medium. The sphere-forming cells
were able to generate significantly more spheroid bodies than their
parental cells. This phenomenon indicates that sphere-forming cells
are capable of self-renewal and proliferation, which are important
characteristics of CSCs (25).
Chemoresistance is another important characteristic of CSCs
(18). To assess whether the
self-renewing sphere-forming cells possessed a hypothetical CSC
chemoresistant property, the sensitivity of sphere-forming cells to
chemotherapeutic agents was examined. Sphere-forming cells
exhibited significantly greater resistance to 5-Fu compared with
that of their parental cells, as determined using cell viability
assays. Xenotransplantation is generally regarded as the gold
standard for evaluating the tumorigenicity of tumor cells. In the
tumorigenicity assay, nude mice were injected with sphere-forming
or parental cells. As few as 500 sphere-forming cells were able to
generate tumors in mice. Additionally, sphere-forming cells
generated subcutaneous tumors with larger volumes in shorter time
periods compared with those generated from parental cells. To
further investigate the CSC properties of sphere-forming cells, the
expression of the CSC markers Sox2, Nanog and EpCAM was
investigated. Western blot analysis demonstrated that the
expression levels of Sox2, Nanog and EpCAM were significantly
higher in sphere-forming cells than in parental cells. In summary,
sphere-forming cells from the human gastric cancer cell line MKN45
possess GCSC properties, which is in agreement with previous
studies (28).
The Notch signaling pathway serves an important role
in cellular processes during embryonic and postnatal development,
including stem cell renewal, cell fate determination and apoptosis
(15). However, dysregulation of the
Notch signaling pathway also contributes to tumorigenesis (29). The interaction of Notch ligands with
their receptors promotes γ-secretase-dependent cleavage of the
Notch receptor and releases the Notch intracellular domain, which
results in the activation of the signaling pathway and induces
target genes, including Hes1 (30).
The role of the Notch1 signaling pathway in the maintenance of CSCs
has been described in preclinical models and in clinical studies
(31,32). Using gain- and loss-of-function
approaches, the maintenance of CSCs has been attributed to Notch
signaling regulation through Hes1, which dictates cell fate
decisions (33). In the present
study, the expression of Notch1 and its downstream target Hes1 were
significantly higher in sphere-forming cells compared with those in
parental cells.
To the best of our knowledge, the role of the Notch1
signaling pathway in the lapatinib resistance of GCSCs has not been
investigated previously. Therefore, in the present study, the
sensitivities of sphere-forming and parental cells to lapatinib
were assessed, and it was demonstrated that sphere-forming cells
were significantly more resistant to lapatinib than parental cells.
To investigate the potential molecular mechanisms that influence
the Notch1 signaling pathway in lapatinib resistance, the
expression of Hes1 was inhibited via transfection with Hes1-siRNA
lentivirus. Following the downregulation of Hes1 expression, the
CSC properties of sphere-forming cells were significantly impaired
and the expression levels of CSC markers were significantly
downregulated compared with those of the corresponding control
siRNA groups.
The activation of EGFR family proteins is regulated
by ligand binding, with the exception of HER2, which dimerizes
independently of ligand binding (34,35). Once
dimerization occurs, intracellular tyrosine kinases are fully
activated and induce autophosphorylation of their tyrosine
residues. These phosphorylated tyrosines function as docking sites
for several adapter proteins, including growth factor receptor
bound protein 2 and SHC adaptor protein, which further transduce
the signaling pathways through protein-protein interactions and
post-translational modifications (36). Cross-regulation between EGFR/HER2 and
Notch signaling pathways has long been observed in genetic studies
(37). For instance, activation of
the Notch1 signaling pathway is associated with EGFR-TKI resistance
in PC9 cells expressing mutated EGFR (38). In the present study, MKN45 cells were
selected, as HER2 is highly activated in these cells (39). It was revealed that the inhibition of
Hes1 significantly increased the sensitivity of sphere-forming
cells to lapatinib.
To further investigate the role of the Notch1
signaling pathway in lapatinib resistance, EMT and
resistance-associated protein expression levels were examined.
During EMT, epithelial cells lose several of their epithelial
characteristics, including E-cadherin and ZO1 expression, and
acquire properties that are typical of mesenchymal cells, including
the expression of vimentin (40). In
the present study, the inhibition of Hes1 decreased Snail
expression and impaired EMT in sphere-forming cells. Additionally,
it is known that CSCs can effectively increase drug resistance
through upregulating the expression of drug efflux transporter
genes and various multidrug resistance genes (41). CSCs isolated from the sphere-forming
culture of cancer cells were previously demonstrated to possess
high expression of the ABCB1 gene and were identified to be
significantly resistant to various chemotherapeutic agents
(42). In the present study, the
expression levels of the resistance-associated proteins MDR1,
ABCG1, ABCG2 and RAD51 were significantly downregulated following
inhibition of Hes1. These results suggest that inhibiting the
Notch1 signaling pathway through inhibition of Hes1 prevents
evasion of CSCs from HER2-targeted therapy and potentially
increases the sensitivity of GCSCs to EGFR-TKIs.
The results of the present study support the idea
that sphere-forming cells isolated from human gastric cancer cells
possess the properties of CSCs. Furthermore, the role of the Notch1
signaling pathway in GCSCs was demonstrated. In addition, the
inhibition of Hes1 through siRNA knockdown was revealed to
significantly impair the stemness of GCSCs and increase the
sensitivity of GCSCs to EGFR-TKIs. In the future, a combination
strategy using EGFR/HER2 and Notch signaling pathway inhibitors may
become a treatment option for patients with gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172387) and the
National Natural Science Foundation of Chongqing (grant no.
C2011BB5119).
References
|
1
|
Dhomen NS, Mariadason J, Tebbutt N and
Scott AM: Therapeutic targeting of the epidermal growth factor
receptor in human cancer. Crit Rev Oncog. 17:31–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rimawi MF, Shetty PB, Weiss HL, Schiff R,
Osborne CK, Chamness GC and Elledge RM: Epidermal growth factor
receptor expression in breast cancer association with biologic
phenotype and clinical outcomes. Cancer. 116:1234–1242. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan HK, Kaye AH and Luwor RB: The EGFRvIII
variant in glioblastoma multiforme. J Clin Neurosci. 16:748–754.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bronte G, Terrasi M, Rizzo S, Sivestris N,
Ficorella C, Cajozzo M, Di Gaudio F, Gulotta G, Siragusa S, Gebbia
N and Russo A: EGFR genomic alterations in cancer: Prognostic and
predictive values. Front Biosci (Elite Ed). 3:879–887.
2011.PubMed/NCBI
|
|
7
|
Sharma MR and Schilsky RL: GI cancers in
2010: New standards and a predictive biomarker for adjuvant
therapy. Nat Rev Clin Oncol. 8:70–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abramson V and Arteaga CL: New strategies
in HER2-overexpressing breast cancer: Many combinations of targeted
drugs available. Clin Cancer Res. 17:952–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pandya K, Meeke K, Clementz AG, Rogowski
A, Roberts J, Miele L, Albain KS and Osipo C: Targeting both Notch
and ErbB-2 signalling pathways is required for prevention of
ErbB-2-positive breast tumour recurrence. Br J Cancer. 105:796–806.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wainberg ZA, Anghel A, Desai AJ, Ayala R,
Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ and Finn RS:
Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively
inhibits HER2-amplified human gastric cancer cells and is
synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res.
16:1509–1519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 30:2039–2049.
2014. View Article : Google Scholar
|
|
14
|
Shimoyama S: Unraveling trastuzumab and
lapatinib inefficiency in gastric cancer: Future steps (Review).
Mol Clin Oncol. 2:175–181. 2014.PubMed/NCBI
|
|
15
|
Cenciarelli C, Marei HE, Zonfrillo M,
Pierimarchi P, Paldino E, Casalbore P, Felsani A, Vescovi AL, Maira
G and Mangiola A: PDGF receptor alpha inhibition induces apoptosis
in glioblastoma cancer stem cells refractory to anti-Notch and
anti-EGFR treatment. Mol Cancer. 13:2472014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen T, Yang K, Yu J, Meng W, Yuan D, Bi
F, Liu F, Liu J, Dai B, Chen X, et al: Identification and expansion
of cancer stem cells in tumor tissues and peripheral blood derived
from gastric adenocarcinoma patients. Cell Res. 22:248–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu G, Shen J, Ou Yang X, Sasahara M and Su
X: Cancer stem cells: The ‘heartbeat’ of gastric cancer. J
Gastroenterol. 48:781–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim TH and Shivdasani RA: Notch signaling
in stomach epithelial stem cell homeostasis. J Exp Med.
208:677–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cenciarelli C, Marei HE, Zonfrillo M,
Casalbore P, Felsani A, Giannetti S, Trevisi G, Althani A and
Mangiola A: The interference of Notch1 target Hes1 affects cell
growth, differentiation and invasiveness of glioblastoma stem cells
through modulation of multiple oncogenic targets. Oncotarge.
8:17873–17886. 2017.
|
|
22
|
Liu ZH, Dai XM and Du B: Hes1: A key role
in stemness, metastasis and multidrug resistance. Cancer Biol Ther.
16:353–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin B, Zeng Y, Liu G, Wang X, Wang P and
Song Y: MAGE-A3 is highly expressed in a cancer stem cell-like side
population of bladder cancer cells. Int J Clin Exp Pathol.
7:2934–2941. 2014.PubMed/NCBI
|
|
25
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Spheroid body-forming cells in the human gastric
cancer cell line MKN-45 possess cancer stem cell properties. Int J
Oncol. 42:453–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radio-resistance by preferential activation of the
DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li LC, Wang DL, Wu YZ, Nian WQ, Wu ZJ, Li
Y, Ma HW and Shao JH: Gastric tumor-initiating CD44+ cells and
epithelial-mesenchymal transition are inhibited by γ-secretase
inhibitor DAPT. Oncol Lett. 10:3293–3299. 2015.PubMed/NCBI
|
|
29
|
Yamaguchi H, Chang SS, Hsu JL and Hung MC:
Signaling cross-talk in the resistance to HER family receptor
targeted therapy. Oncogene. 33:1073–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z,
Chen L, Zhao J, Liu X, Qi S, et al: Basic fibroblast growth factor
reduces scar by inhibiting the differentiation of epidermal stem
cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell
Res Ther. 8:1142017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aster JC and Blacklow SC: Targeting the
Notch pathway: Twists and turns on the road to rational
therapeutics. J Clin Oncol. 30:2418–2420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu ZH, Dai XM and Du B: Hes1: A key role
in stemness, metastasis and multidrug resistance. Cancer Biol Ther.
16:353–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burgess AW: EGFR family: Structure
physiology signalling and therapeutic targets. Growth Factors.
26:263–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arasada RR, Amann JM, Rahman MA, Huppert
SS and Carbone DP: EGFR blockade enriches for lung cancer stem-like
cells through Notch3-dependent signaling. Cancer Res. 74:5572–5584.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie M, Zhang L, He CS, Xu F, Liu JL, Hu
ZH, Zhao LP and Tian Y: Activation of Notch-1 enhances
epithelial-mesenchymal transition in gefitinib-acquired resistant
lung cancer cells. J Cell Biochem. 113:1501–1513. 2012.PubMed/NCBI
|
|
39
|
Morishita A, Gong J and Masaki T:
Targeting receptor tyrosine kinases in gastric cancer. World J
Gastroenterol. 20:4536–4545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B, Lindley LE, Fernandez-Vega V,
Rieger ME, Sims AH and Briegel KJ: The T box transcription factor
TBX2 promotes epithelial-mesenchymal transition and invasion of
normal and malignant breast epithelial cells. PLoS One.
7:e413552012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Houthuijzen JM, Daenen LG, Roodhart JM and
Voest EE: The role of mesenchymal stem cells in anti-cancer drug
resistance and tumour progression. Br J Cancer. 106:1901–1906.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chockalingam S and Ghosh SS: Amelioration
of cancer stem cells in macrophage colony stimulating
factor-expressing U87MG-human glioblastoma upon 5-fluorouracil
therapy. PLoS One. 8:e838772013. View Article : Google Scholar : PubMed/NCBI
|