Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality, accounting for 27% (including 26% for
females, and 28% for males), and non-small cell lung cancer (NSCLC)
accounts for 80–85% of all lung cancer-associated mortalities
(1,2).
Despite advances in the understanding of the molecular mechanisms
of lung cancer and the development of novel chemotherapeutic
agents, the 5-year survival rates for lung and bronchus cancer
remained <18% from 2004 to 2010 (2). A number of studies have focused on
progressing the understanding of oncogenic kinase signaling
pathways, which has provided targets for developing effective
therapeutic strategies in order to improve clinical outcomes
(3).
One of the potential candidates for therapy is the
Janus kinase/signal transducers and activators of transcription
(JAK/STAT) pathway. JAK/STAT is one of the pleiotropic cascades
that may transduce a multitude of signals for development and
homeostasis in animals, from humans to flies (4). JAK family members have been reported to
be dysregulated in malignant tumors, including in colorectal,
prostate and myeloproliferative cancer (5–8). The
mammalian JAK family includes four members: JAK1, JAK2, JAK3 and
Tyk2. All JAKs exhibit broad patterns of expression with the
exception of JAK3, which is restricted to leukocytes (9).
JAK1 binds to various cytokines non-covalently to
mediate cell proliferation and differentiation (10). JAK1 knockout mice die perinatally
(9). JAK1 mutation has been reported
in hepatocellular carcinoma, acute lymphoblastic leukemia, lung and
gastric cancer (10,11). Phosphorylated (p)-JAK1, the active
form of JAK1, mediates the phosphorylation of receptors and the
major substrates for JAK family members, STATs (4). For example, p-JAK1 expression was
detected in primary esophageal squamous cell carcinoma and not in
normal esophageal squamous cells. p-JAK1 expression was associated
with a reduced overall survival time (12). Additionally, a previous study by the
present authors demonstrated that JAK1 expression was significantly
increased in NSCLC clinical samples compared with normal samples
(P>0.001), while p-JAK1 (Tyk 1022) showed trends of positive
expression, though these did not reach statistical significance
(P=0.055), potentially owing to a small sample size (13). This indicated that JAK1 activation was
abnormal in NSCLC.
Lung adenocarcinoma (ADCC) is the most common
histological subtype of NSCLC. Patients with ADCC and a high
epidermal growth factor receptor (EGFR) copy number can be treated
with EGFR-tyrosine kinase inhibitors (14,15). EGFR
gene amplification has also been reported to be associated with
prognosis of lung cancer, though there is some controversy in this
regard (16,17). However, the combination of EGFR gene
amplification and JAK1 activation for predicting cancer prognosis
has not been extensively studied. This has incited the present
study, which will investigate associations between JAK1 activation,
EGFR gene amplification and survival status in patients with
NSCLC.
Materials and methods
Tissue collection
The study cohort consisted of 142 patients (40
female and 102 male) with a median age of 63 years (range, 20–84
years). A total of 142 paraffin-embedded resected primary NSCLC
samples were analyzed from the archives of the Pathology Department
at Sichuan Provincial People's Hospital (Chengdu, China) from
December 2004 to February 2007, including 74 cases of ADCC and 68
cases of squamous cell carcinoma (SqCC) A total of 142 adjacent
normal pulmonary tissue specimens were also resected in the same
tissue blocks. Staging was performed according to the International
Union Against Cancer's tumor-node-metastasis system (18). Differentiation and histological type
were scored according to the World Health Organization
classification for NSCLC (19). None
of the patients had received neoadjuvant therapy prior to surgical
resection. Following the surgery, the patients underwent standard
therapy procedure, according to the National Comprehensive Cancer
Network Clinical Practice Guideline for Oncology, NSCLC, 2004
(20). Informed consent was obtained
from all individuals included in the present study. Institutional
review board approval for the study was obtained from Sichuan
Provincial People's Hospital.
Immunohistochemical staining
(IHC)
IHC staining was performed as previously described
(3). Briefly, the 4-µm sections
underwent deparaffinization, hydration and endogenous peroxide
blocking. Antigen retrieval was performed by heating at 95°C for 30
min in Tris/ethylenediaminetetraacetic acid retrieval solution. The
sections were blocked with 3% bovine serum albumin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and incubated with primary p-JAK1
antibody (cat. no. 11149, 1:100 dilution; Signalway Antibody LLC.,
College Park, MY, ML, USA) overnight at 4°C. The membranes were
subsequently incubated with enough secondary peroxidase-labeled
polymer-conjugated goat anti-rabbit antibody to cover the specimen
(cat. no. K4007; undiluted; EnVsion; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA), according to the manufacturer's
protocol, for 30 min at room temperature. For staining,
3,3-daiminobenzidine chromogen was applied. Finally, the sections
were counterstained with hematoxylin for 3 min at room temperature,
fixed and mounted.
Scoring for immunohistochemical
staining
Scoring for immunochemical staining was evaluated as
previously described (3) by two
independent pathologists. Briefly, the expression of p-JAK1 was
assessed semi-quantitatively on the basis of criteria that
accounted for the fraction and intensity of immunostaining of the
tumor cells. The fraction score was defined as: 0 (0%), 1
(<20%), 2 (20–50%) and 3 (>50%). The intensity of p-JAK1
staining was scored as 0 (no appreciable staining), 1 (barely
detectable staining), 2 (readily identifiable brown staining) and 3
(dark brown staining). A total score was calculated by multiplying
the fraction and the intensity score. A tumor sample was considered
positive if the score was ≥4, and negative otherwise.
Determination of EGFR gene
amplification by fluorescence in-situ hybridization (FISH)
EGFR FISH analysis was performed with the dual-color
EGFR SpectrumOrange/CEP7 SpectrumGreen probe and paraffin
pretreatment reagent kit (both from Vysis; cat. nos. 05J48-001 and
32–801210, respectively; Abbott Laboratories, Chicago, IL, USA) as
previously described (21). Briefly,
the paraffin sections were deparaffinized, dehydrated and digested
with protease K. The slides were denatured by heating to 75°C for 5
min and dehydrated in ethanol. The probes were denatured for 5 min
at 75°C prior to hybridization. Each slide was hybridized at 37°C
overnight and washed in 2X saline-sodium citrate buffer/0.3% NP40
at 72°C for 2 min. The nuclei were counterstained with
DAPI/antifade 1 (Vysis; Abbott Laboratories, Chicago, IL, USA). A
minimum of 100 non-overlapping tumor cell nuclei were scored in
each case, according to the University of Colorado Cancer Center
criteria (22). The BX-51/Genus FISH
Imaging system (DP71/70/DP30BW software, DP-BSW Ver.03.02) was used
for imaging (Olympus, Tokyo, Japan).
Statistical analysis
The association between clinical characteristics and
p-JAK1 expression was determined using Pearson's χ2
test. When one of the expected values in a 2×2 table was <5,
Fisher's exact test would be used. The Kaplan-Meier method was used
for the univariate analysis of survival time between groups based
on clinical characteristics and p-JAK1 expression. Multivariate
analysis was performed using Cox regression model analysis. Only
markers that were significant predictors in univariate analysis
were included in the multivariate analysis. All tests were
two-sided and P<0.05 was considered to indicate a statistically
significant difference. SPSS 17.0 for Windows (SPSS Inc., Chicago,
IL, USA) was used for these analyses.
Results
JAK1 expression in non-small cell lung
cancer tissues
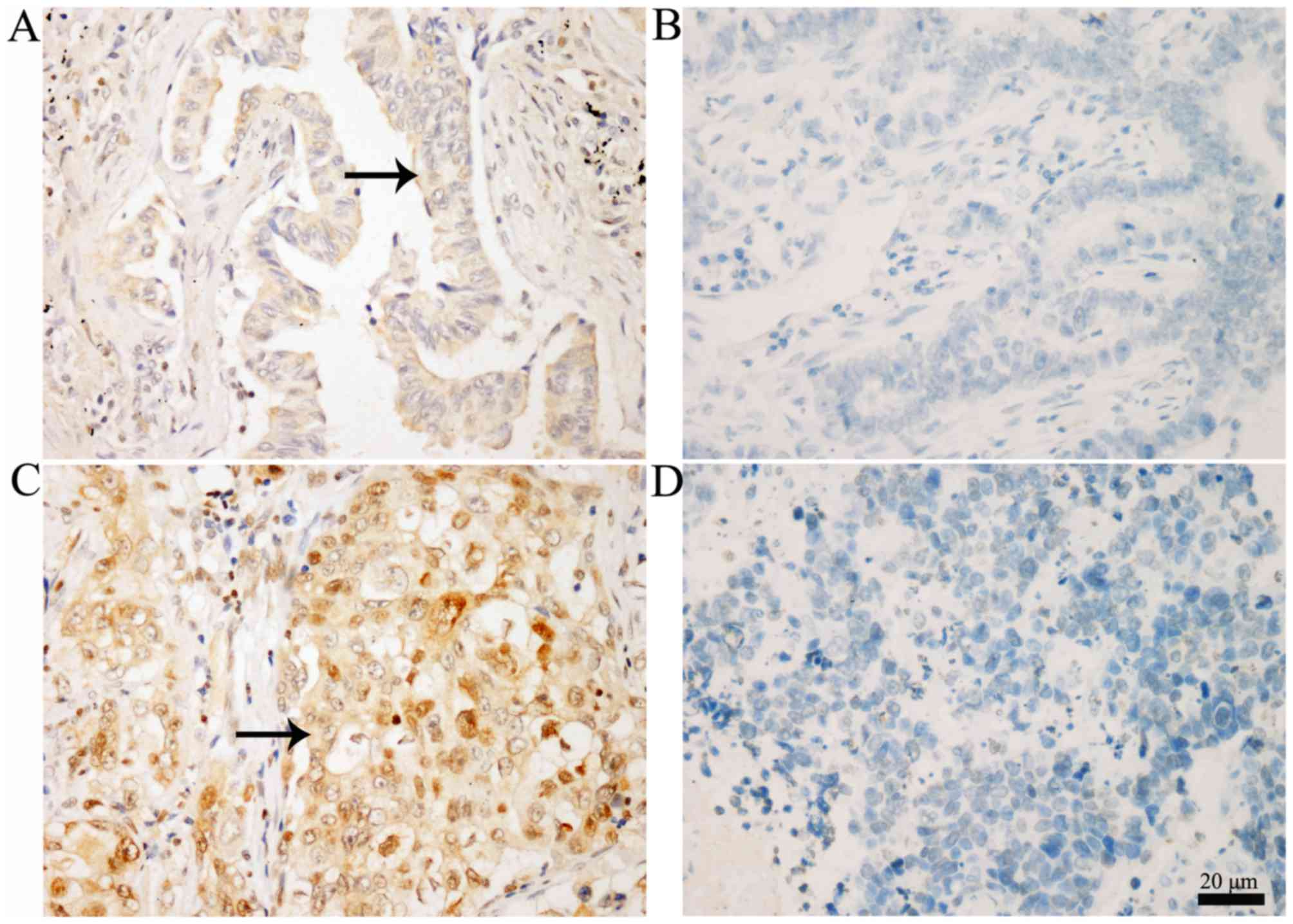
Representative IHC staining images of p-JAK1
expression in NSCLC and adjacent normal lung tissues are displayed
in Fig. 1. Positive p-JAK1 expression
was observed in the cytoplasm and nucleus, as indicated by arrows.
In the NSCLC tissues, 56/142 cases (39.4%) exhibited positive
p-JAK1 expression, whereas 11/142 cases (7.7%) were positive in the
adjacent normal tissues (Table I).
The difference was highly significant (P<0.001).
 | Table I.p-JAK1 expression in NSCLC and
adjacent normal tissues. |
Table I.
p-JAK1 expression in NSCLC and
adjacent normal tissues.
| Parameter | p-JAK1+, n
(%) | p-JAK1−, n
(%) | P-valuea |
|---|
| NSCLC tissue | 56 (39.4) | 86 (60.6) | <0.001 |
| Normal control | 11 (7.7) | 131 (92.3) |
|
Association between clinical
characteristics and prognosis in patients with NSCLC
As listed in Table
II, follow-up information was available for all patients.
Survival time differed significantly by age (>60 vs. ≤60), lymph
node invasion (N0/1 vs. N2/3) and stage (I/II vs. III/IV; P=0.005,
P<0.001 and P=0.001, respectively). Gender, tumor size and
histological type were not significantly associated with survival
time.
 | Table II.Clinical characteristics and prognosis
of patients with non-small cell lung carcinoma. |
Table II.
Clinical characteristics and prognosis
of patients with non-small cell lung carcinoma.
| Parameter | Patients, n | Median survival time,
range (months) | P-valuea |
|---|
| Age |
|
| 0.005 |
| <60
years | 56 | 63 (3–94) |
|
| ≥60
years | 86 | 60 (1–96) |
|
| Gender |
|
| 0.561 |
|
Female | 40 | 63 (3–96) |
|
| Male | 102 | 61 (1–94) |
|
| Histological
type |
|
| 0.959 |
|
Adenocarcinoma | 74 | 61 (3–96) |
|
| Squamous
cell carcinoma | 68 | 62.5 (1–94) |
|
| Tumor size |
|
| 0.446 |
| <3
cm | 59 | 61.5 (6–96) |
|
| ≥3
cm | 83 | 60 (1–94) |
|
| Lymph node
invasion |
|
| <0.001 |
|
N0/1 | 122 | 63 (1–96) |
|
|
N2/3 | 20 | 39 (3–67) |
|
| Distant
metastasis |
|
| 0.973 |
| M0 | 138 | 61 (1–96) |
|
| M1 | 4 | 57.5 (41–85) |
|
| TNM stage |
|
| 0.001 |
|
I/II | 92 | 62.5 (1–96) |
|
|
III/IV | 50 | 51.5 (3–89) |
|
Association between clinical
characteristics and p-JAK1 expression
Pearson's χ2 or Fisher's exact test were
performed to assess the significance of associations between
clinical characteristics and p-JAK1 expression. As shown in
Table III, p-JAK1 expression was
associated with histological type, as 29.7% of ADCC samples and
50.0% of SqCC samples were p-JAK1+ (P=0.014). p-JAK1
expression status was also associated with tumor size and stage
(P=0.021 and P=0.009, respectively). Other clinical
characteristics, including age, gender, lymph node invasion and
distant metastasis, were not associated with p-JAK1 expression
status (P>0.05).
 | Table III.p-JAK1 expression and clinical
characteristics in patients with non-small cell lung cancer. |
Table III.
p-JAK1 expression and clinical
characteristics in patients with non-small cell lung cancer.
| Characteristic |
p-JAK1+ |
p-JAK1− | P-value |
|---|
| Age |
|
| 0.867a |
|
<60 | 22 | 35 |
|
|
≥60 | 34 | 51 |
|
| Gender |
|
| 0.290a |
|
Female | 13 | 27 |
|
|
Male | 43 | 59 |
|
| Histological
type |
|
| 0.014a |
|
Adenocarcinoma | 22 | 52 |
|
|
Squamous cell carcinoma | 34 | 34 |
|
| Tumor size |
|
| 0.029a |
| <3
cm | 17 | 42 |
|
| ≥3
cm | 39 | 44 |
|
| Lymph node
invasion |
|
| 0.297a |
|
N0/1 | 46 | 76 |
|
|
N2/3 | 10 | 10 |
|
| Distant
metastasis |
|
| 0.300b |
| M0 | 53 | 85 |
|
| M1 | 3 | 1 |
|
| TNM stage |
|
| 0.009a |
|
I/II | 29 | 63 |
|
|
III/IV | 27 | 23 |
|
Survival status and p-JAK1
expression
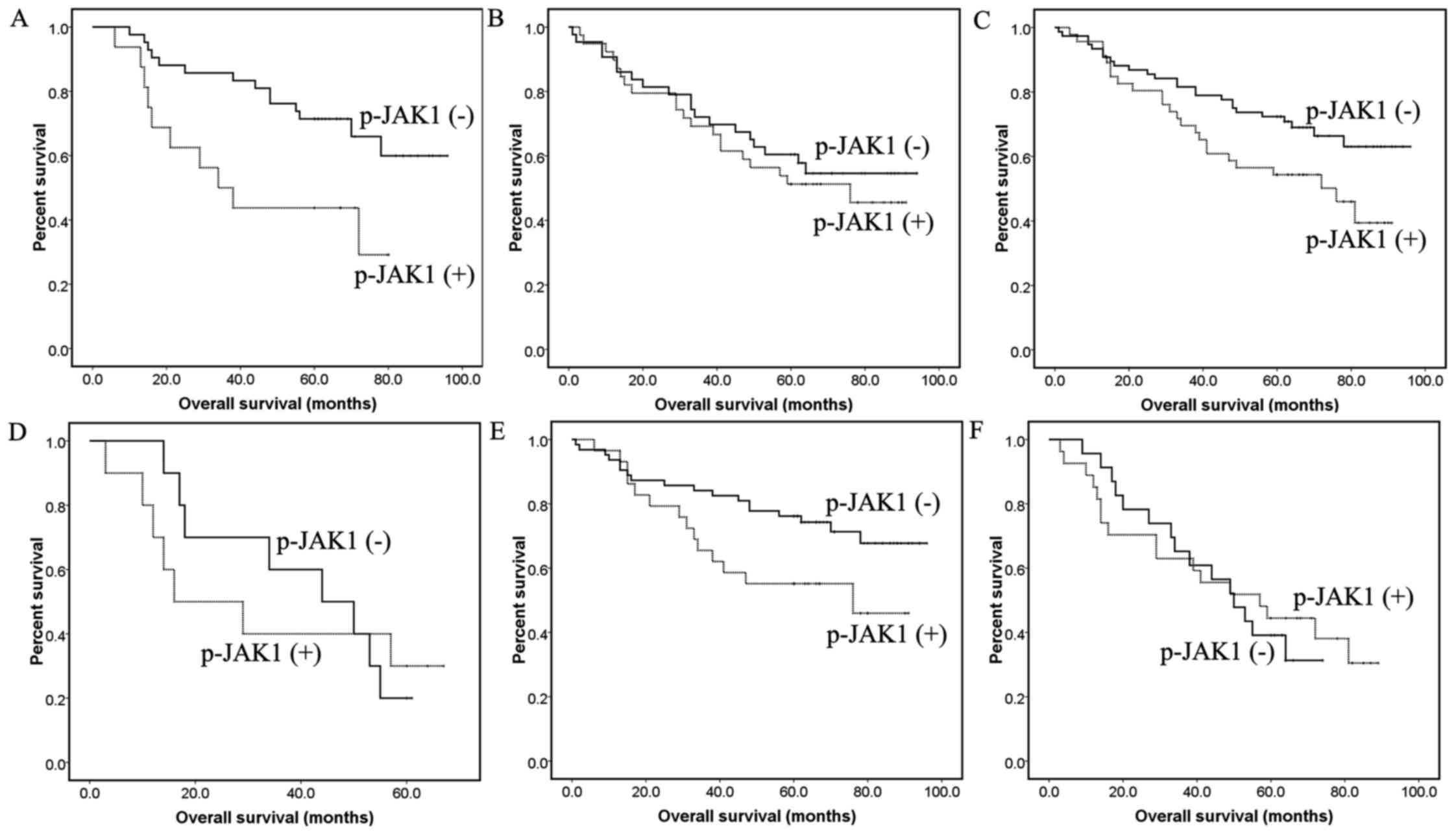
Positive p-JAK1 expression was associated with a
poor prognosis in NSCLC (P=0.039; Fig.
2A-C). However when divided by histological type, the
difference was only statistically significant in ADCC samples
(P=0.007) and not in SqCC (P=0.612). Subjects with p-JAK1
expression demonstrated reduced survival time in early stage NSCLC,
including patients with tumor size <3 cm, N0/1 and stage I/II
(P=0.016, P=0.034 and P=0.048, respectively; Fig. 3A-F). The difference in survival time
in patients with late stage disease, including tumor size ≥3 cm,
N2/3 or stage III/IV was not significant.
Survival status and p-JAK1 and EGFR
expression in ADCC
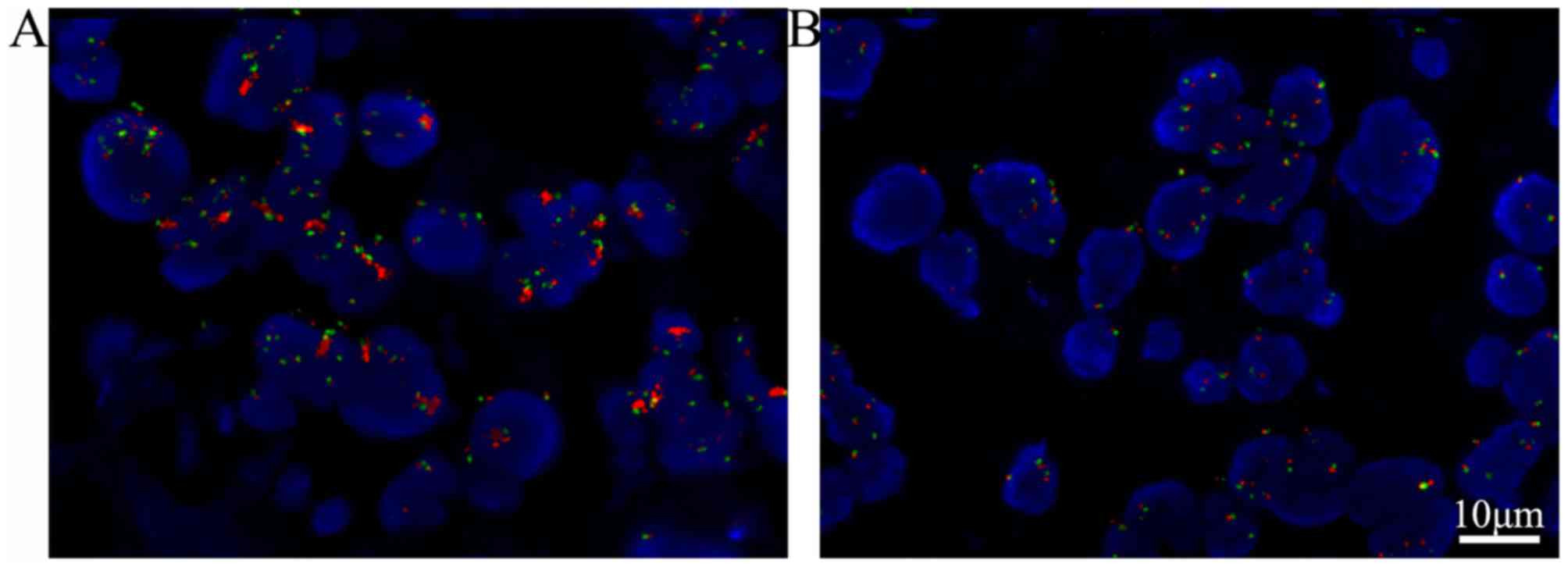
To investigate whether associations between JAK1
activation, EGFR gene amplification and prognosis in patients with
ADCC were significant, EGFR status was examined with FISH analysis,
and univariate survival analyses were performed for the 74 ADCC
subjects (Table V). EGFR gene
amplification was determined by FISH (Fig. 4), 29/74 ADCC cases exhibited EGFR gene
amplification (designated as EGFR+). As shown in
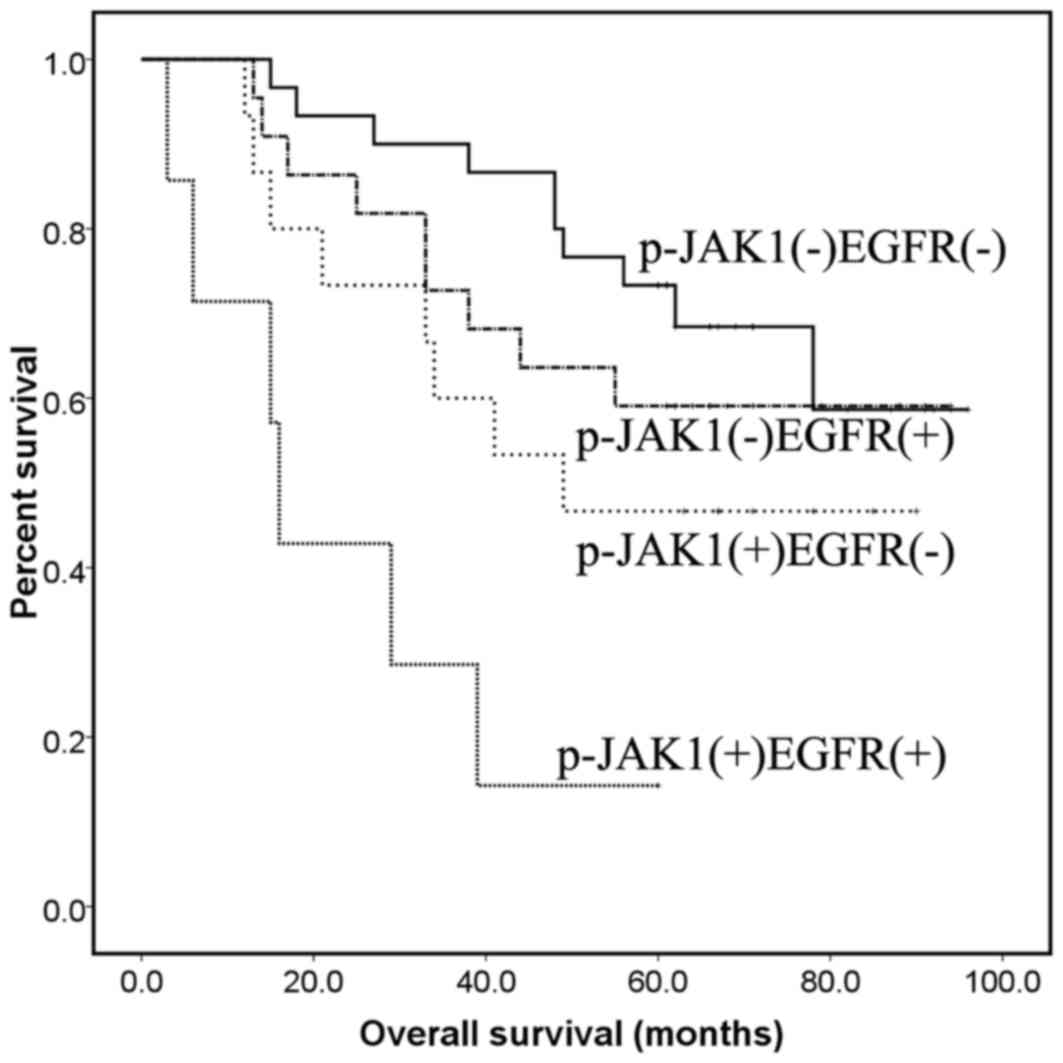
Table V, survival time for patients
with p-JAK1+/EGFR+ was significantly reduced
compared with those with p-JAK1−/EGFR− or
p-JAK1−/EGFR+ combinations (P<0.001 and
P=0.004, respectively), but not the
p-JAK1+/EGFR− combination (P=0.052; Fig. 5). The results indicated that the EGFR
amplification and p-JAK1+ combination may be a novel
target to inform the selection of individual therapy strategies and
for predicting the effect of therapy in ADCC.
 | Table V.Effects of p-JAK1 expression and EGFR
gene duplication status on the prognosis of patients with lung
adenocarcinoma, as determined by Kaplan-Meier analysis. |
Table V.
Effects of p-JAK1 expression and EGFR
gene duplication status on the prognosis of patients with lung
adenocarcinoma, as determined by Kaplan-Meier analysis.
| Status | Patients, n | Median survival
time (months) |
P-valuea |
|---|
|
p-JAK1+/EGFR+ | 7 | 16 (3–60) |
|
|
p-JAK1−/EGFR− | 30 | 61.5 (15–96) | <0.001 |
|
p-JAK1−/EGFR+ | 22 | 62 (13–94) | 0.004 |
|
p-JAK1+/EGFR− | 15 | 49 (12–90) | 0.052 |
Discussion
Phosphorylation of JAK1 is important for the
activation of JAK/STAT pathways and their downstream cascades
(10). In the present study, it was
identified that the activation of JAK1 was associated with a poorer
prognosis in NSCLC (P=0.039), particularly in ADCC (P=0.007).
p-JAK1 was identified as an independent predictor for poor
prognosis (P=0.022). Overall survival time for patients with
p-JAK1+ and EGFR gene duplication was significantly
reduced compared with patients with one or neither trait
(P=0.001).
A previous study by the present authors and studies
by others have indicated that inhibition of JAK signaling exhibits
anticancer and anti-angiogenetic effects in human cancer lines and
xenograft tumors, including lung cancer (13,23–25). These
results support the hypothesis that JAK1 activation is important
for tumorigenesis in NSCLC. In the present study, when subdivided
by histotype, differences in prognosis were identified as
statistically significant. Positive p-JAK1 was an independent
predictor for decreased survival times only for patients with ADCC,
not SqCC. This finding suggests a specific role for JAK1 in lung
ADCC. In several lung ADCC cell lines, JAK family inhibitor AZD1480
has demonstrated to be able to potently block STAT3 signaling and
oncogenesis (24), which supports the
results of the present study to some extent. However, the research
on the effect of p-JAK1 expression on prognosis in lung SqCC has
been limited. You et al (12)
reported that JAK/STAT pathway activations were associated with
shorter survival time in esophageal SqCC cases. As only 68 cases of
SqCC patients were recruited to the present study, further
evaluation should be performed using a larger amount of cases.
In the present study, it was further identified that
tumor size <3 cm, N0/1 and stage I/II patients with positive
p-JAK1 expression had shorter survival time (all P<0.05),
whereas the difference was not significant for patients in advanced
stages (Fig. 3). This finding
indicated that the activation of JAK1 may be an early event in the
tumorigenesis of NSCLC. Therefore, p-JAK1 could be a target for
early diagnosis and prognosis prediction.
In the present study, patients with
p-JAK1+ and EGFR duplication exhibited the poorest
prognosis while patients with p-JAK1− and no EGFR
duplication exhibited the most favorable prognosis (median survival
time, 16 vs. 61.5 months). Although contradictory results were
previously reported (14,15), EGFR FISH status alone was not
associated with the patient survival times (data not shown). To
date, few studies have focused on the interaction between EGFR and
JAKs at the cellular and molecular level. However, it has been
identified that crosstalk between the JAK/STAT and EGFR pathways
mediates adenomatous polyposis coil 1-driven intestinal stem cell
hyperplasia in drosophila (26).
Furthermore, prolactin, a JAK2-coupled cytokine receptor, has also
been demonstrated to synergistically augment EGF signaling in T47D
breast cancer cells (27). In NSCLC,
how EGFR and JAK1 interact to exert biological effects should be
further elucidated.
The present study indicated that JAK1 activation is
associated with a poor prognosis in NSCLC, particularly in lung
ADCC. p-JAK1 is an independent poor prognostic factor and a
potential target for early diagnosis. The combination of EGFR gene
amplification and JAK1 activation may be a novel tool for prognosis
prediction in patients with lung ADCC.
Acknowledgements
The authors of the present study are grateful to the
pathologist from the Department of Pathology, Sichuan Provincial
People's Hospital for assisting in the collection of the tissue
samples. The present study was supported by grants from the
National Natural Foundation of China (grant no. 81201851), Support
Program of Science & Technology Department of Sichuan Province
(grant no. 2014SZ0231) and Doctoral Foundation of Sichuan
Provincial People's Hospital (grant no. 30305030563).
References
|
1
|
Peters S, Adjei AA, Gridelli C, Reck M,
Kerr K and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23:(Suppl 7). vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu D, Huang Y, Chen B, Zeng J, Guo N,
Zhang S, Liu L, Xu H, Mo X and Li W: Activation of mammalian target
of rapamycin pathway confers adverse outcome in nonsmall cell lung
carcinoma. Cancer. 117:3763–3773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rawlings JS, Rosler KM and Harrison DA:
The JAK/STAT signaling pathway. J Cell Sci. 117:1281–1283. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Constantinescu SN, Leroy E, Gryshkova V,
Pecquet C and Dusa A: Activating Janus kinase pseudokinase domain
mutations in myeloproliferative and other blood cancers. Biochem
Soc Trans. 41:1048–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergmann AK, Schneppenheim S, Seifert M,
Betts MJ, Haake A, Lopez C, Maria Murga, Penas E, Vater I, Jayne S,
Dyer MJ, et al: Recurrent mutation of JAK3 in T-cell prolymphocytic
leukemia. Genes Chromosomes Cancer. 53:309–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gurbuz V, Konac E, Varol N, Yilmaz A,
Gurocak S, Menevse S and Sozen S: Effects of AG490 and S3I-201 on
regulation of the JAK/STAT3 signaling pathway in relation to
angiogenesis in TRAIL-resistant prostate cancer cells. Oncol Lett.
7:755–763. 2014.PubMed/NCBI
|
|
9
|
Schindler C, Levy DE and Decker T:
JAK-STAT signaling: From interferons to cytokines. J Biol Chem.
282:20059–20063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie H, Bae H, Noh J, Eun JW, Kim JK, Jung
KH, Ryu JC, Ahn YM, Kim SY, Lee SH, et al: Mutational analysis of
JAK1 gene in human hepatocellular carcinoma. Neoplasma. 56:136–140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong EG, Kim MS, Nam HK, Min CK, Lee S,
Chung YJ, Yoo NJ and Lee SH: Somatic mutations of JAK1 and JAK3 in
acute leukemias and solid cancers. Clin Cancer Res. 14:3716–3721.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
You Z, Xu D, Ji J, Guo W, Zhu W and He J:
JAK/STAT signal pathway activation promotes progression and
survival of human oesophageal squamous cell carcinoma. Clin Transl
Oncol. 14:143–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, Huang Y, Zeng J, Chen B, Huang N,
Guo N, Liu L, Xu H, Mo X and Li W: Down-regulation of JAK1 by RNA
interference inhibits growth of the lung cancer cell line A549 and
interferes with the PI3K/mTOR pathway. J Cancer Res Clin Oncol.
137:1629–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch FR, Varella-Garcia M, McCoy J, West
H, Xavier AC, Gumerlock P, Bunn PA Jr, Franklin WA, Crowley J and
Gandara DR: Southwest Oncology Group: Increased epidermal growth
factor receptor gene copy number detected by fluorescence in situ
hybridization associates with increased sensitivity to gefitinib in
patients with bronchioloalveolar carcinoma subtypes: A southwest
oncology group study. J Clin Oncol. 23:6838–6845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee Y, Shim HS, Park MS, Kim JH, Ha SJ,
Kim SH and Cho BC: High EGFR gene copy number and skin rash as
predictive markers for EGFR tyrosine kinase inhibitors in patients
with advanced squamous cell lung carcinoma. Clin Cancer Res.
18:1760–1768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling
Y, Li W, Li L, Liu X, Zheng B, et al: Concurrence of EGFR
amplification and sensitizing mutations indicate a better survival
benefit from EGFR-TKI therapy in lung adenocarcinoma patients. Lung
Cancer. 89:337–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koh Y, Jang B, Jeon YK, Kim TM, Lee SH,
Kim DW, Chung DH, Kim YT, Kim YW and Heo DS: EGFR gene copy number
gain is related to high tumor SUV and frequent relapse after
adjuvant chemotherapy in resected lung adenocarcinoma. Jpn J Clin
Oncol. 41:548–554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
UICC and IUAC: TNM classification of
malignant tumours. Wiley; New York, NY: 2002
|
|
19
|
Travis W, Brambilla E, Muller-Hermlink H
and Harris C: World Health Organization classification of
tumoursPathology and genetics of tumours of the lung, pleura,
thymus and heart. IARC Press; Lyon: 2004
|
|
20
|
NCCN: NCCN Clinical practice guideline in
oncology-non-small cell lung cancer guideline 2004. http://www.nccn.org2004.
|
|
21
|
Dacic S, Flanagan M, Cieply K, Ramalingam
S, Luketich J, Belani C and Yousem SA: Significance of EGFR protein
expression and gene amplification in non-small cell lung carcinoma.
Am J Clin Pathol. 125:860–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varella-Garcia M: Stratification of
non-small cell lung cancer patients for therapy with epidermal
growth factor receptor inhibitors: The EGFR fluorescence in situ
hybridization assay. Diagn Pathol. 1:192006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murakami T, Takigawa N, Ninomiya T, Ochi
N, Yasugi M, Honda Y, Kubo T, Ichihara E, Hotta K, Tanimoto M and
Kiura K: Effect of AZD1480 in an epidermal growth factor
receptor-driven lung cancer model. Lung Cancer. 83:30–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hedvat M, Huszar D, Herrmann A, Gozgit JM,
Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et
al: The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torres AF, Nogueira C, Magalhaes J, Costa
IS, Aragao A, Gomes Neto A, Martins F and Tavora F: Expression of
EGFR and molecules downstream to PI3K/Akt, Raf-1-MEK-1-MAP
(Erk1/2), and JAK (STAT3) pathways in invasive lung adenocarcinomas
resected at a single institution. Anal Cell Pathol (Amst).
2014:3529252014.PubMed/NCBI
|
|
26
|
Cordero JB, Stefanatos RK, Myant K, Vidal
M and Sansom OJ: Non-autonomous crosstalk between the Jak/Stat and
Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia
in the Drosophila adult midgut. Development. 139:4524–4535. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Y, Li X, Jiang J and Frank SJ:
Prolactin modulates phosphorylation, signaling and trafficking of
epidermal growth factor receptor in human T47D breast cancer cells.
Oncogene. 25:7565–7576. 2006. View Article : Google Scholar : PubMed/NCBI
|