Introduction
Triple-negative breast cancer (TNBC) is
characterized by the absence of estrogen receptor (ER) or
progesterone receptor (PgR) expression, and the lack of human
epidermal growth factor receptor 2 (HER2) gene amplification. TNBC
represents 15–20% of all breast cancer cases; it exhibits a
distinctly aggressive nature, with higher rates of relapse and
shorter overall survival times compared with the other breast
cancer subtypes (1,2).
TNBC is characterized by a heterogeneous
immunohistochemical phenotype. The majority of TNBCs have a
high-grade ‘invasive ductal carcinoma, not otherwise specified’
(IDC-NOS) histology. However, a significant proportion of other
relatively rare histotypes (medullary, metaplastic, adenoid cystic
and apocrine carcinomas) may also lack expression of ER, PgR and
HER2 (3). From a genetic standpoint,
TNBCs include different molecular subtypes. Lehmann et al
(4) identified six different TNBC
subtypes that express unique gene expression patterns by analyzing
the mRNA gene expression profiles from 21 breast cancer datasets,
including basal-like (BL)1 and 2, immune-modulatory (IM),
mesenchymal (M), mesenchymal stem-like (MSL), luminal androgen
receptor (LAR) and unstable (UNS) subtypes. Recently, these six
subtypes (TNBC type: BL1, BL2, IM, M, MSL and LAR) were refined to
four (TNBC type-4: BL1, BL2, M and LAR), when considering that the
IM and MSL subtypes represent tumors with substantial infiltrating
lymphocytes and tumor-associated mesenchymal cells (5).
Cytotoxic chemotherapy is currently the only
treatment option for TNBC; it has been demonstrated that TNBC is
more chemotherapy-sensitive than ER-positive tumors (6,7). A
pathological complete response (pCR) to neoadjuvant chemotherapy
(NAC) of TNBC is highly associated with prolonged overall and
event-free survival times. In previous studies, 20–30% of patients
with TNBC achieved pCR in the neoadjuvant setting and the response
of TNBC to chemotherapy was heterogeneous (6,8–10). Masuda et al (11) reported that Lehmann's six gene
expression subtypes (TNBCtype) were associated with pCR status,
with the BL1 subtype presenting a high rate of pCR (52%) compared
with the M, IM, MSL and LAR subtypes, which presented relatively
low pCR rates (31, 30, 23 and 10%, respectively).
However, subtype classification by mRNA expression
analysis is not yet common, convenient or economic enough to put
into daily clinical use. Thus, the present study aimed to
investigate additional clinical and pathological characteristics
that are associated with pCR status as a means to predict the
response to chemotherapy using information that is already readily
available in daily clinical practice. If the effects could be
predicted prior to actually performing chemotherapy on patients
with TNBC, eventually unnecessary severe side effects caused by
ineffective chemotherapy could be avoided.
Patients and methods
Patients
Of the 1,773 patients with operable primary breast
cancer on record between January 2007 and January 2016 in the
National Hospital Organization Tokyo Medical Center (Tokyo, Japan),
a total of 40 were diagnosed with TNBC by needle biopsy, classified
clinically as Stage II (12) or
higher and required NAC. All patients were female, and no patient
was excluded from the present analysis. The characteristics of the
40 patients are described in Table I.
The effectiveness of chemotherapy following surgery was
pathologically evaluated. The patients were divided into two groups
according to their response: pCR (n=12) and non-pCR (n=28). The
clinical and pathological data of the patients were retrieved from
medical records and retrospectively reviewed and analyzed according
to these groupings. Ethical approval for the present study was
provided by the Ethics Committee at the National Hospital
Organization Tokyo Medical Center, and the study was performed in
accordance with the appropriate ethical standards.
 | Table I.Clinical features of patients with
TNBC according to pCR status (n=40). |
Table I.
Clinical features of patients with
TNBC according to pCR status (n=40).
| Characteristic | pCR | non-pCR | P-value |
|---|
| Total, n | 12 | 28 |
|
| Age, years |
|
| 0.974 |
| Mean | 53 | 53 |
|
|
Range | 27–82 | 32–74 |
|
| Menopausal status, n
(%) |
|
| 0.471 |
|
Premenopausal | 6 (50) | 12 (43) |
|
|
Postmenopausal | 6 (50) | 16 (57) |
|
| Obesity
ratea, n (%) |
|
| 0.570 |
|
Obese | 3 (25) | 8 (29) |
|
|
Non-obese | 9 (75) | 20 (71) |
|
| Blood serum
T-cholesterol, mg/dl |
|
| 0.200 |
| Mean | 195 | 208 |
|
|
Range | 168–249 | 122–277 |
|
| cT prior to NAC, n
(%) |
|
| 0.009 |
| cT1 | 1 (8) | 0 (0) |
|
| cT2 | 10 (83) | 13 (46) |
|
| cT3 | 1 (8) | 4 (14) |
|
| cT4 | 0 (0) | 11 (39) |
|
| cN prior to NAC, n
(%) |
|
| 0.436 |
|
cN0 | 7 (58) | 11 (39) |
|
|
cN1 | 4 (33) | 14 (50) |
|
|
cN2 | 1 (8) | 1 (4) |
|
| cStage prior to
NAC, n (%) |
|
| 0.030 |
| cStage
II | 11 (92) | 13 (46) |
|
| cStage
III | 1 (8) | 12 (43) |
|
| cStage
IV | 0 (0) | 3 (11) |
|
| NAC regimen, n
(%) |
|
| 1.000 |
| Ant
only | 0 (0) | 1 (4) |
|
| Tax
only | 0 (0) | 2 (7) |
|
|
Ant/Tax | 12 (100) | 25 (89) |
|
Examinations prior to NAC
Prior to NAC, imaging studies, blood tests and
physical measurements were performed. Digital mammography was
performed for 38 patients; mammography was not possible for 2
patients in the non-pCR group due to skin ulceration or bleeding of
the tumor. Mammograms were performed in the mediolateral oblique
and craniocaudal views for each patient; additional views were
performed when the lesion was not clearly identified by these 2
views. The mammograms were evaluated according to the criteria
established by the Japan Central Organization on Quality Assurance
of Breast Cancer Screening (13), by
≥2 radiologists or surgeons certified by the aforementioned
organization. The mammographic findings were described as masses,
calcifications (typically benign calcifications were excluded) or
other (including focal asymmetric densities and architectural
distortions).
The clinical Tumor-Node-Metastasis (TNM)
classification (14) and stage
(12) were evaluated and determined
prior to the administration of NAC. The tumor size and nodal status
were evaluated by ultrasonography, magnetic resonance imaging or
computed tomography. Distant metastasis was detected with computed
tomography.
The height and body weight of the patients were
recorded, and body mass index (BMI) was calculated, with BMI ≥25
kg/m2 defined as obese. The blood tests performed
included regular measurement of total cholesterol level and 3
breast cancer tumor markers (carbohydrate antigen 15–3,
carcinoembryonic antigen and National Cancer Center-Stomach-439) as
described previously (15–17).
Treatment
The patients were treated with chemotherapy
regimens, including anthracyclines (5-fluorouracil, 500
mg/m2; epirubicin, 100 mg/m2; or
cyclophosphamide, 500 mg/m2; triweekly for 4 cycles)
and/or taxanes (docetaxel, 70 mg/m2; triweekly for 4
cycles; or nab-paclitaxel, 100 mg/m2; weekly for 12
cycles). Subsequent to NAC, the patients underwent mastectomy or
partial mastectomy with axillary lymph node dissection or sentinel
lymph node biopsy.
Histological assessment of tissue
specimens
All microscopy slides were independently evaluated
by ≥2 senior pathologists. Cases of breast cancer with negative ER,
PgR and HER2 were diagnosed as TNBC by core needle biopsy prior to
all treatment.
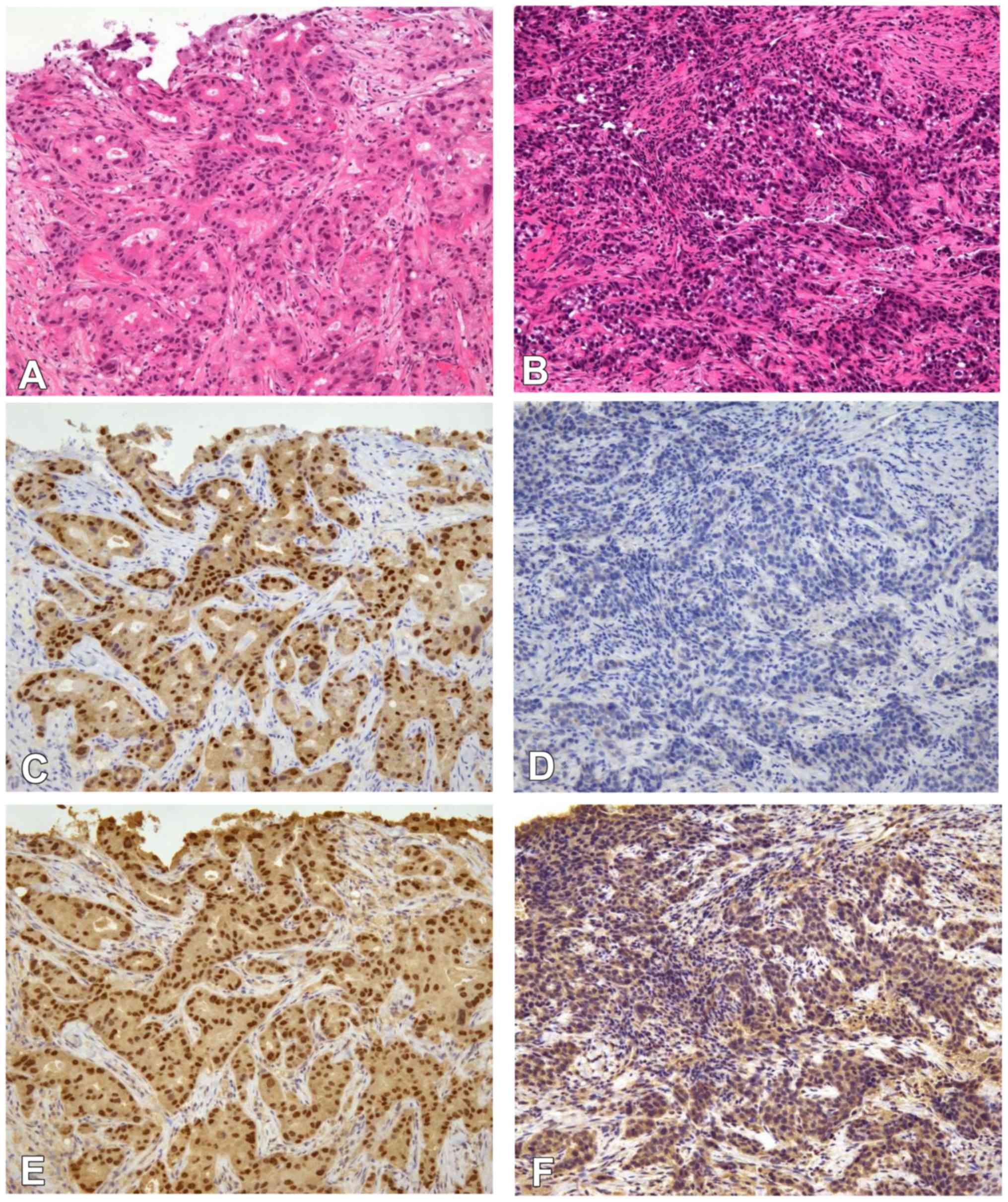
The expression of ER, PgR, Ki-67, androgen receptor
(AR) and forkhead-box A1 (FOXA1) were evaluated using
semiquantitative immunohistochemistry (IHC) scoring of the
percentage of cells with positive nuclear staining (1–100%)
(Fig. 1). The following antibodies
were used for IHC: ER mouse monoclonal antibody (clone, 1D5SP1;
pre-diluted kit; cat. no. 790-4323; Ventana Medical Systems, Inc.,
Innovation Park Drive, Tucson, AZ, USA), PgR mouse monoclonal
antibody (clone, 1E2; pre-diluted kit; cat. no. 790-2223; Ventana
Medical Systems, Inc.) AR mouse monoclonal antibody (clone, AR441;
dilution, 1:50; cat. no. M3562; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA), HER2 mouse monoclonal antibody (clone, 4B5;
pre-diluted kit; cat. no. 790-2991; Ventana Medical Systems, Inc.),
FOXA1 goat polyclonal antibody (clone, ab5089; dilution, 1:50; cat.
no. GR120766-17; Abcam, Cambridge, UK) and Ki-67 mouse monoclonal
antibody (MIB-1; dilution, 1:100; cat. no. M7240; Dako; Agilent
Technologies, Inc.). Positivity for ER, PgR, AR or FOXA1 was
defined as nuclear staining in ≥1% of tumor cells. Ki-67 expression
was considered low when ≤50% and high when >50% stained cells
were observed.
HER2 status was assessed using IHC and/or
fluorescence in situ hybridization. HER2 expression was
scored as 0 to 3+ by IHC based on ASCO/CAP recommendations
(18), and HER2 positivity was
defined by an IHC score of 3+ or by the identification of HER2 gene
amplification from fluorescence in situ hybridization.
Surgical specimens were used to evaluate the
pathological response of NAC. pCR was defined as the absence of any
residual invasive cancer observed following hematoxylin and eosin
(H&E) staining of the resected breast specimen. Residual ductal
carcinoma in situ was included in the pCR category.
The specimens were fixed in 10% neutral buffered
formalin immediately following resection for 24–48 h at 20°C.
Subsequent to the specimens being cut in 5 mm slices, an automatic
H&E stain was applied for 40 min at 25°C. The H&E specimens
were examined at magnifications, ×40–100 views, and also at
magnifications ×200–400 when pathologists required more detailed
information of each cell to make a diagnosis. Immunostaining
specimens were studied at magnifications, ×100 or ×200.
Statistical analysis
For comparison of the sample means, an independent
sample t-test was performed. Associations between pCR and
clinicopathological characteristics were assessed with a
χ2 test, Fisher's exact test or Mann-Whitney U-test, as
appropriate. Clinical features of patients with TNBC according to
pCR status were assessed by χ2 test or Fisher's exact
test. Mammographic features in patients with triple negative breast
cancer according to pCR status were assessed by Fisher's exact
test, and AR positive rate was additionally assessed by Man-Whitney
U test. All analyses were performed using SPSS statistical software
(version 23.0; IBM Corp., Armonk, NY, United States). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical findings
There were significant differences in the likelihood
of pCR according to TNM classification and clinical stage; patients
in the pCR group presented with a less advanced T classification
(P=0.009) and clinical stage (P=0.030) compared with non-pCR
patients (Table I). The pCR group
tended to have lower serum total cholesterol levels compared with
the non-pCR group, although the difference was not statistically
significant (195±40 vs. 208±20 mg/dl; P=0.200). There were no
significant differences between the pCR and non-pCR groups with
regard to mean age, menopausal status, obesity, NAC regimen or
tumor marker status.
Mammography findings
There was no difference between the pCR and non-pCR
groups in the rate of the mammographic presentation of masses (67
vs. 77%; P=0.694). However, there was a significant difference in
the presentation of mammographic calcification; pCR patients were
less likely to exhibit calcification in mammograms compared with
non-pCR patients (17 vs. 58%; P=0.034; Table II).
 | Table II.Mammographic features in patients
with triple negative breast cancer according to pCR status
(n=38). |
Table II.
Mammographic features in patients
with triple negative breast cancer according to pCR status
(n=38).
| Characteristic | pCR | non-pCR | P-value |
|---|
| Total, n | 12 | 26 |
|
| Presence of mass, n
(%) |
|
| 0.694 |
|
Yes | 8 (67) | 20 (77) |
|
| No | 4 (33) | 6 (23) |
|
| Presence of
calcifications, n (%) |
|
| 0.034 |
|
Yes | 2 (17) | 15 (58) |
|
| No | 10 (83) | 11 (42) |
|
Pathological findings
The histological types of the two groups tended to
differ, although the difference was not significant (P=0.079); All
cases in the pCR group (12/12, 100%) were IDC-NOS, whereas the
non-pCR group included less cases of IDC-NOS (20/28, 71%) and more
special histological types, including mucinous, metaplastic,
medullary and apocrine carcinomas.
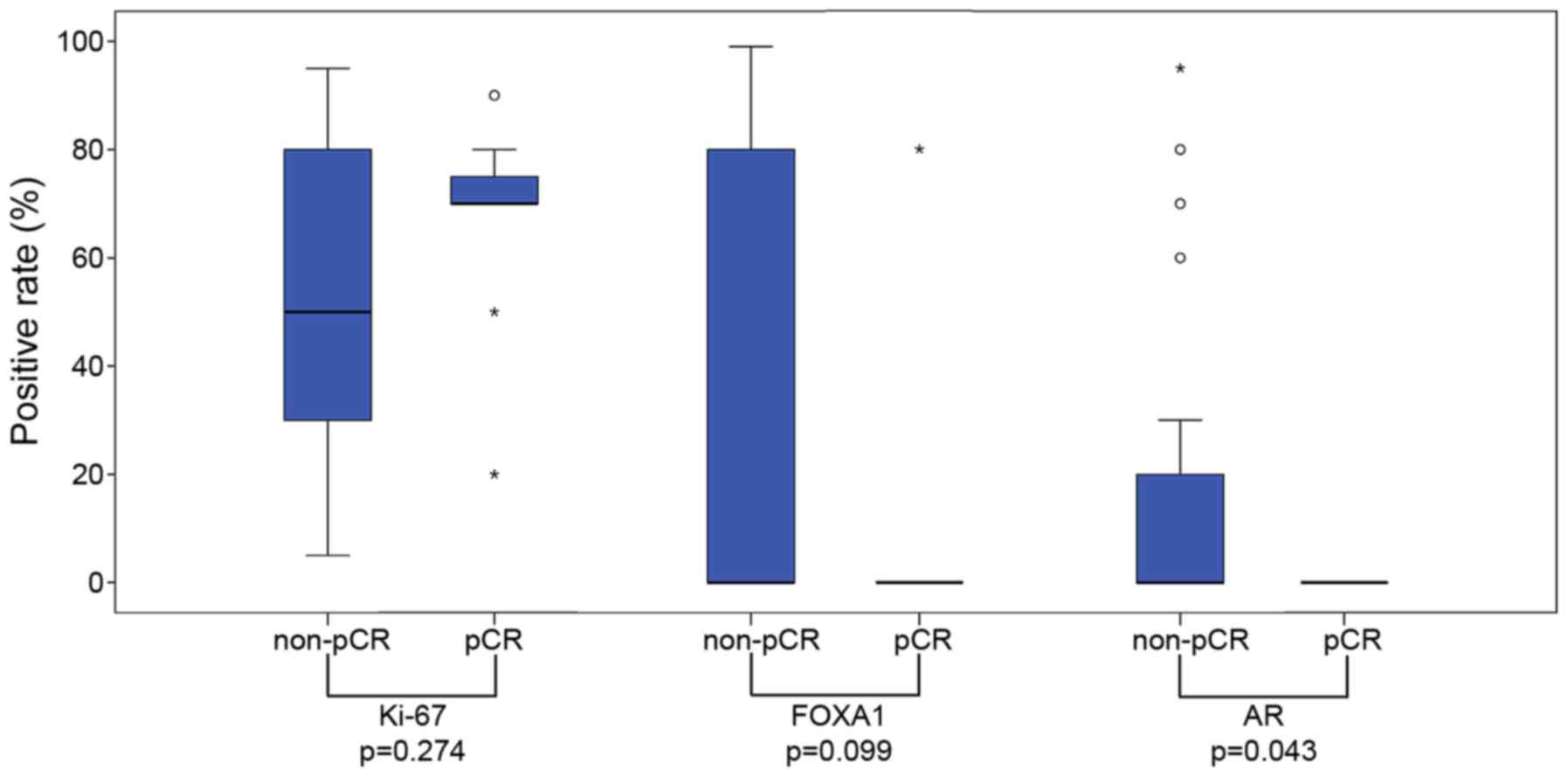
The positive rates for AR and FOXA1 were lower in
the pCR group, although no significant difference was observed (AR
positive rate, 0 vs. 29%, P=0.079; FOXA1 positive rate, 8 vs. 29%;
P=0.233), as assessed with a Fisher's exact test;, AR positivity
was significantly less common in the pCR group when considered with
a Mann-Whitney U test (P=0.043; Fig.
2). Ki-67 scores were significantly higher in the pCR group
than in the non-pCR group, as assessed with a Fisher's test
(P=0.041; Table III).
 | Table III.Pathological features in TNBC
patients according to pCR status (n=40). |
Table III.
Pathological features in TNBC
patients according to pCR status (n=40).
| Characteristic | pCR | non-pCR | P-value |
|---|
| Total, n | 12 | 28 |
|
| Histological type,
n (%) |
|
| 0.079 |
|
IDC-NOS | 12 (100) | 20 (71) |
|
|
Specific type | 0 (0) | 8 (29) |
|
| Ki-67
scorea, n (%) |
|
| 0.041 |
|
Low | 2 (17) | 15 (54) |
|
|
High | 10 (83) | 13 (46) |
|
| Androgen receptor
status, n (%) |
|
| 0.079 |
|
Positive | 0 (0) | 8 (29) |
|
|
Negative | 12 (100) | 20 (71) |
|
| Forkhead-box A1
status, n (%) |
|
| 0.233 |
|
Positive | 1 (8) | 8 (29) |
|
|
Negative | 11 (92) | 20 (71) |
|
Discussion
The results of the present study suggested that
chemotherapy-sensitive patients with TNBC present clinically less
advanced tumors with less frequent mammographic calcifications,
negative AR status and higher Ki-67 scores.
Nwaogu et al (7) demonstrated that lower clinical stage and
negative lymph node involvement were associated with pCR in all
breast cancer subtypes. In accordance with these findings, in the
pCR group of patients with TNBC in the present study, the primary
tumor status, lymph node status and clinical stage were less
advanced. It is simple and reasonable to hypothesize that the tumor
burden influences the treatment efficiency. It may be of note that
the T4 category, indicating invasion of the skin or chest wall, was
particularly frequent in non-pCR patients in the present study.
Thus, not only the size or volume of the tumor, but also its robust
invasive characteristics may influence the treatment effectiveness.
Additionally, Lehmann et al (5) reported that BL1 tumors, which are more
responsive to chemotherapy, presented at a lower clinical stage
than BL2 and LAR tumors.
Mammographic calcifications reflect the intraductal
component of tumor cells (19).
Considering the results of the present study, this may suggest that
tumors with a rich intraductal component may be less sensitive to
chemotherapy. Furthermore, Li et al (20) reported that ER-, PgR- or HER2-positive
tumors, which are less sensitive to chemotherapy than TNBCs,
present with more mammographic calcifications than TNBCs.
Bae et al (21)
further reported that mammographic calcifications are significantly
associated with AR-positive TNBC, compared with AR-negative TNBC,
and that AR-positive TNBC was more likely to have a ductal
carcinoma in situ component. As previously mentioned, the
LAR subgroup exhibited poor sensitivity to chemotherapy in the
study by Masuda et al (11).
Furthermore, Asano et al (22)
recently reported that the rate of pCR following NAC was
significantly lower in patients with AR-positive compared with
AR-negative TNBC. Among the 7 AR-positive TNBC patients who
underwent mammography in the present study, 6 presented with
mammographic calcifications. In other words, of the 17 patients who
presented with mammographic calcifications in the study, 6 were
AR-positive. Taken together, it can be concluded that typical
patients with AR-positive TNBC tend to present with calcifications
on mammography and are less likely to achieve pCR, resembling the
characteristics of ER/PgR-positive tumors.
To the best of our knowledge, this is the first
study to demonstrate that mammographic calcification of TNBC prior
to chemotherapy may indicate the efficacy of treatment. Although
further investigations are required, it can be presumed that tumors
with a rich intraductal component, which often present with
mammographic calcifications, are more likely to be
chemotherapy-resistant. Furthermore, TNBCs present with few
calcifications overall, with AR-positive tumors being more likely
to present with calcifications, indicating the rich intraductal
component and poor response to chemotherapy of such tumors.
Although not statistically significant, certain
results from the present study also indicated that
chemotherapy-sensitive patients with TNBC exhibit IDC-NOS tumors
rather than specific histological types of tumors. Certain
researchers have assumed and recognized that special histological
type tumors react poorly to chemotherapy. Hennessy et al
(23) described metaplastic breast
cancer as chemotherapy-resistant. The IM, M, MSL and LAR subtypes
which were defined by Lehmann et al (4) were identified to correspond a specific
histological type. These subtypes were reported to be a poor
response for chemotherapy (4). The
aforementioned data correspond with the result from the present
study that the pCR group did not include special histological type
tumors. Therefore, the present study hypothesized that special
histological type tumors are more likely to be
chemotherapy-resistant, whereas IDC-NOS type tumors are more likely
to be sensitive to chemotherapy.
Denkert et al (24) reported that the expression of Ki-67 is
predictive for the response to NAC in the majority of breast cancer
subtypes, including TNBC. Accordingly, the BL1 subtype, the most
chemotherapy-sensitive subgroup in the study by Masuda et al
(11), presents with high Ki-67
expression. This can be explained by the observation that highly
proliferative tumors exhibit an improved response to NAC; these
data are in agreement with the results of the present study showing
that the pCR group presented with higher Ki-67 scores than the
non-pCR group.
FOXA1 is a key determinant of ER function and
endocrine response; FOXA1 functions as an important pioneer factor
for the interactions between ER or AR with chromatin (25). Among the 8 AR-positive patients in the
present study, all patients were FOXA1-positive, supporting a
hypothesis of a correlation between AR and FOXA1 status. It is
assumed that FOXA1-positive tumors share characteristics with ER-
or AR-positive tumors. Sasahara et al (26) reported that apocrine carcinomas were
often positive for AR and FOXA1 in IHC, further indicating a
correlation between AR and FOXA1.
Previous studies have suggested that
tumor-infiltrating lymphocytes (TILs) are associated with pCR after
NAC among patients with TNBC (27–30).
Miyashita et al (27) reported
that tumors with a higher histological grade and a smaller size may
present with higher cluster of differentiation 8+ TIL
levels; this may support the result from the present study that
less advanced tumors are more likely to be chemotherapy-sensitive.
However, the associations between TILs and the other clinical
factors investigated in the present study, including AR,
calcification and histological types, remain unclear. The present
study had a number of limitations. Firstly, the sample size was
relatively small, potentially limiting the statistical power. In
order to further confirm the conclusions, studies with larger
sample sizes are required. Secondly, the patients received three
types of chemotherapy regimen. Although the regimens were performed
at similar rates in each of the groups, it may remain difficult to
precisely compare the effectiveness.
In conclusion, the results of the present study
suggest that patients with TNBC who present with clinically less
advanced tumors and less frequent mammographic calcification are
more likely to respond to chemotherapy. From a pathological aspect,
the histological type IDC-NOS, negative AR expression and higher
Ki-67 scores were demonstrated to indicate chemotherapy
sensitivity. It is necessary to be aware that TNBC comprises a
heterogeneous group of cancer types and that not all TNBCs are
truly chemotherapy-sensitive. The results indicate that, from
standard clinical information, it may be possible to predict the
effectiveness of chemotherapy and avoid the severe side effects
caused by ineffective treatment. Furthermore, patients with
chemotherapy-resistant TNBC should be distinguished from other
patients with TNBC to enable the application of novel treatment
approaches, including AR-targeted therapy.
The authors wish to declare the following conflicts
of interest: Dr Akira Matsui received research funding from Chugai
Pharmaceutical (Tokyo, Japan), Daiichi Sankyo (Tokyo, Japan), Eisai
(Tokyo, Japan), Takeda Pharmaceutical, (Osaka, Japan), Taiho
Pharmaceutical (Tokyo, Japan); also, Dr Aiko Nagayama holds stock
in Chugai Pharmaceutical.
Acknowledgements
The authors thank the technicians of the Clinical
Examination Department for sample preparation.
References
|
1
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palma G, Frasci G, Chirico A, Esposito E,
Siani C, Saturnino C, Arra C, Ciliberto G, Giordano A and D'Aiuto
M: Triple negative breast cancer: Looking for the missing link
between biology and treatments. Oncotarget. 6:26560–26574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nwaogu I, Fayanju O, Jeffe D and
Margenthaler J: Predictors of pathological complete response to
neoadjuvant chemotherapy in stage II and III breast cancer: The
impact of chemotherapeutic regimen. Mol Clin Oncol. 3:1117–1122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawood S, Broglio K, Kau SW, Green MC,
Giordano SH, Meric-Bernstam F, Buchholz TA, Albarracin C, Yang WT,
Hennessy BT, et al: Triple receptor-negative breast cancer: The
effect of race on response to primary systemic treatment and
survival outcomes. J Clin Oncol. 27:220–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The Japanese Breast Cancer Society:
General Rules for Clinical and Pathological Recording of Breast
Cancer. 17th. Kanehara & Co., Ltd.; Tokyo, Japan: 2012
|
|
13
|
Japan Radiology Society Japanese Society
of Radiologic Technology: Mammography GuidelineJapan Central
Organization on Quality Assurance of Breast Cancer Screening (ed).
3rd. IGAKU-SHOIN Ltd.; Tokyo, Japan: 2010
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer: TNM Classification of
Malignant Tumors. 7th. Wiley-Blackwell; New York: 2011
|
|
15
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
16
|
Dnistrian AM, Schwartz MK, Greenberg EJ,
Smith CA and Schwartz DC: Evaluation of CA M26, CA M29, CA 15-3 and
CEA as circulating tumor markers in breast cancer patients. Tumour
Biol. 12:82–90. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ichihara S and Aoyama H: Intraductal
carcinoma of the breast associated with high levels of circulating
tumor-associated antigens (CA 15-3 and NCC-ST-439). Cancer.
73:2181–2185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenwood HI, Heller SL, Kim S, Sigmund
EE, Shaylor SD and Moy L: Ductal carcinoma in situ of the breasts:
Review of MR imaging features. Radiographics. 33:1569–1588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Chen C, Gu Y, Di G, Wu J, Liu G and
Shao Z: The role of mammographic calcification in the neoadjuvant
therapy of breast cancer imaging evaluation. PLoS One.
9:e888532014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae MS, Park SY, Song SE, Kim WH, Lee SH,
Han W, Park IA, Noh DY and Moon WK: Heterogeneity of
triple-negative breast cancer: Mammographic, US and MR imaging
features according to androgen receptor expression. Eur Radiol.
25:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asano Y, Kashiwagi S, Onoda N, Kurata K,
Morisaki T, Noda S, Takashima T, Ohsawa M, Kitagawa S and Hirakawa
K: Clinical verification of sensitivity to preoperative
chemotherapy in cases of androgen receptor-expressing positive
breast cancer. Br J Cancer. 114:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hennessy BT, Gonzalez-Angulo AM,
Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J,
Sahin A, Agarwal R, Joy C, et al: Characterization of a naturally
occurring breast cancer subset enriched in
epithelial-to-mesenchymal transition and stem cell characteristics.
Cancer Res. 69:4116–4124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkert C, Loibl S, Müller BM, Eidtmann H,
Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et
al: Ki67 levels as predictive and prognostic parameters in
pretherapeutic breast cancer core biopsies: A translational
investigation in the neoadjuvant GeparTrio trial. Ann Oncol.
24:2786–2793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tokunaga E, Hisamatsu Y, Tanaka K,
Yamashita N, Saeki H, Oki E, Kitao H and Maehara Y: Molecular
mechanisms regulating the hormone sensitivity of breast cancer.
Cancer Sci. 105:1377–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasahara M, Matsui A, Ichimura Y, Hirakata
Y, Murata Y and Marui E: Overexpression of androgen receptor and
forkhead-box A1 protein in apocrine breast carcinoma. Anticancer
Res. 34:1261–1267. 2014.PubMed/NCBI
|
|
27
|
Miyashita M, Sasano H, Tamaki K, Chan M,
Hirakawa H, Suzuki A, Tada H, Watanabe G, Nemoto N, Nakagawa S, et
al: Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in
triple-negative breast cancer: Its correlation with pathological
complete response to neoadjuvant chemotherapy. Breast Cancer Res
Treat. 148:525–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ono M, Tsuda H, Shimizu C, Yamamoto S,
Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, et
al: Tumor-infiltrating lymphocytes are correlated with response to
neoadjuvant chemotherapy in triple-negative breast cancer. Breast
Cancer Res Treat. 132:793–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang K, Xu J, Zhang T and Xue D:
Tumor-infiltrating lymphocytes in breast cancer predict the
response to chemotherapy and survival outcome: A meta-analysis.
Oncotarget. 7:44288–44298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|