Introduction
Diabetic retinopathy (DR) is a leading cause of
blindness and visual impairment among adults aged <40 years in
the developed world (1). It is a
sight-threatening complication of diabetes that is characterized by
an early loss of capillary pericytes and thickening of the basement
membrane, which may lead to uncontrolled endothelial proliferation
and, subsequently, angiogenesis (2).
Vascular endothelial growth factor (VEGF) is involved in the
development of proliferative DR and diabetic macular edema
(3). However, VEGF blocking is likely
to produce systemic adverse effects. Thus, it is important to
develop novel therapeutic strategies against DR by understanding
the molecular mechanisms underlying its development and
progression.
Insulin receptor substrate-1 (IRS-1) is a key
molecule in insulin signaling, and is involved in signal
transduction between the insulin receptor and phosphoinositide
3-kinase (PI3K) (4). IRS-1 is
involved in the mediation of the metabolic and mitogenic effects of
insulin in peripheral tissues, including skeletal muscle, liver and
adipose tissue, by transmitting signals from the insulin receptor
to downstream enzymes, which include PI3K,
phosphoinositide-dependent kinase 1 and protein kinase B (Akt)
(4). VEGF is an angiogenic factor is
involved in the maintenance of vascular homeostasis (5) and in pathological angiogenesis in
diabetic retinopathy (6). This
regulation maybe clinically relevant, because intensive insulin
treatment is associated with worsening of DR and is associated with
VEGF expression levels. A previous study reported that activation
of PI3K/Akt signaling pathway upregulates the expression of VEGF
through a direct interaction with the VEGF promoter (7). Regulation of PI3K/Akt activation is
essential for the long term upregulation of VEGF (8). These previous studies suggested that the
IRS-1/PI3K/Akt/VEGF pathway may be a promising molecular target for
the prevention and treatment of DR.
Vascular abnormalities and pathologies are
associated with diabetes (9). Loss of
endothelial function and poor arterial collateral formation
contribute to the morbidity and mortality of patients with diabetes
(10). This is observed in multiple
cell types, including endothelial cells (ECs) (11), vascular smooth muscle cells (12) and capillary pericytes (13). The dysfunction of the vasculature may
be associated with the loss of the direct action of insulin on
vascular cells, which has been demonstrated to be
insulin-responsive (13). Insulin
stimulates several signaling cascades in EC (8). There is selective inhibition of
insulin-induced activation of the IRS-PI3K-Akt pathway in the
micro- and macro-vessels of insulin resistant rodents (14). These reports suggested that the
regulation of IRS-PI3K-Akt regulate affected angiogenesis in
DR.
MicroRNAs (miRNAs/miRs) are an emerging class of
small, highly conserved, noncoding RNAs that act as
posttranscriptional regulators (15).
miR-126 belongs to a highly conserved miRNA family and is located
at intron 5 of EGF like domain multiple 7 (16). It may be involved in the control of
vascular integrity and angiogenesis (17) as it modulates the release of
angiogenic factors, including hypoxia-inducible factor 1-α, matrix
metalloproteinases and VEGF, which is crucial in the development of
proliferative DR (18).
The present study identified the function ofmiR-126
in the context of DR. Expression of miR-126 was significantly
decreased in ECs and retinal pericytes (RPs) compared with healthy
controls, resulting in overexpression of the target gene IRS-1,
which regulates the PI3K-Akt-VEGF pathway. Taken together, the
results suggested that miR-126 and its targeting of IRS-1 may
provide novel insights into the treatment of DR.
Materials and methods
Establishment of a DR model and cell
culture
Pericytes and their interactions with ECs in the
vessel wall are involved in the regulation of vessel formation,
stabilization and remodeling (2), so
the present study isolated ECs and RPs from a murine DR model. The
animals were obtained from the Animal Center of Dalian Medical
University. All mice were kept in an environment maintained at 21°C
with 50% humidity under a 12/12 h light/dark cycle. All mice
received food and water ad libitum. Diabetes mellitus was induced
in 30 male C57/BL6 mice of 12 weeks old, weighing 18 to 22 g by a
single intraperitoneal injection of streptozotocin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at a dose of 100 mg/kg dissolved in
100 mM citrate buffer (pH 4.5). Ten control mice were treated with
buffered saline alone. One week later, blood glucose levels were
measured, and mice with a fasting-blood glucose of N12 mmol/l were
considered diabetic and were used for further study. The DR model
was established as previously described (19). The EC and RP cells were isolated
according to the protocol as previously described (20,21). The
present study was approved by the Animal Ethical and Welfare
Committee of Dalian Medical University (Dalian, China).
The ECs and RPs were grown in minimum essential
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA), 100 U/ml of penicillin and 100 mg/ml of streptomycin. All
cells were incubated at 37°C in a humidified 21% O2, 5%
CO2 atmosphere.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using a Roche Light Cycler 480
(Roche Diagnostics, Basel Switzerland), TaqMan MicroRNA assays
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and 200 ng
total RNA extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) from 106 cells. RNA was reverse
transcribed to cDNA using stem-loop primers and the TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
obtained cDNA was amplified using a TaqMan miR-126 MicroRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The67 bp cDNA
product was amplified by PCR using the following primers: miR-126
forward, 5′-TATAAGATCTGAGGATAGGTGGGTTCCCGAGAACT-3′ and reverse,
5′-ATATGAATTCTCTCAGGGCTATGCCGCCTAAGTAC-3′ (22). The reaction mixtures were incubated at
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min according to the Stratagene RT-qPCR instrument
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
miR-126 expression was normalized to U6 mRNA expression. Gene mRNA
expression was normalized to β-actin. Primers used were as follows:
U6forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; β-actin forward,
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and reverse,
5′-ATGGAGCCACCGATCCACA-3′. Relative gene expression was quantified
using the 2−ΔΔCq method (23). Three independent experiments were
performed.
Western blot analysis
Cells (107) were lysed in lysis buffer
(50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4,
0.5 µg/ml leupeptin). Protein was quantified using a Bradford
Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Total protein (30 mg) and pre-stained molecular weight markers were
separated by 10% SDS-PAGE followed by transfer onto nitrocellulose
membranes. The membranes were blocked in TBST (Tris-buffered saline
with 0.5% Triton X-100) containing 5% nonfat milk at 4°C overnight,
and probed with primary antibodies against IRS-1 (cat. no. sc-8038;
dilution, 1:400), VEGF (cat. no. sc-57496; dilution, 1:500), PI3K
(cat. no. sc-365290; dilution, 1:400), Akt (cat. no. sc-81434;
dilution, 1:500) and β-actin (cat. no. sc-130300; dilution, 1:500)
(all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight. Then membranes were washed in TBST and incubated with a
secondary horseradish peroxidase-conjugated antibody (cat.no.
sc-516102; dilution, 1:5,000) for 2 h at room temperature.
Following incubation with secondary antibodies, membranes were
washed with TBST. An Odyssey CLx Western Blot Detection System
(LI-COR Biosciences, Lincoln, NE, USA) was used to measure the band
density. The band density of each gene was normalized to the
corresponding density of β-actin using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA). All experiments were
performed in triplicate.
Transfection assay
Recombinant plasmids (2 mg) and 200 pmol miR-126
mimic, miR-126 mimic control, miR-126 inhibitor or miR-126
inhibitor control (cat. nos. 4464066 and 4464084; Ambion; Thermo
Fisher Scientific, Inc.) were transfected into 3×106 ECs
and RPs for 48 h by electroporation, using a Nucleofector
instrument (Lonza Cologne GmbH, Cologne, Germany) according to the
manufacturer's protocol. For the IRS-1 interference experiment,
HEK293 genomic DNA was used as the PCR template and the DNA
fragment encoding mir-126 pre-miRNA (flanking upstream and
downstream 30–50 nt) was amplified and inserted into the expressing
vector pSilencer4.1CMV-puro (Ambion; Thermo Fisher Scientific,
Inc.) as described previously (24).
DNA fragments for anti-IRS-1 small interfering RNAs (siRNAs) were
generated by annealing two complementary oligonucleotides and
cloning into pSilencer4.1 CMV-puro vectors, as reported previously
(25). Following transfection, the
cells were allowed to recover by incubating for 4 h at 37°C. The
experiment was replicated three times.
Dual-luciferase reporter assay
The target gene was predicted using TargetScan
Release 3.4 (http://www.targetscan.org/) (26). DNA fragments (414 nt) of the
IRS-13′-untranslated region (3′-UTR) containing the predicted
miR-126 binding site [IRS1-wild type (wt)] were cloned into the
pGL3-promoter plasmid (Promega Corporation, Madison, WI, USA) and
the miR-126 binding sites were replaced with 4 nt fragments to
produce mutated 3′UTR pGL3-reporter plasmids (IRS1-mut) as
previously described (16). The
recombinant reporter vectors with normal and or mutated IRS-1
3′-UTRs were co-transfected with miR-126 mimic, miR-126 mimic
control, miR-126 inhibitor or miR-126 inhibitor control into EC
cells using the Trans Messenger transfection reagent (Qiagen GmbH,
Hilden, Germany). The luciferase assay was performed using the Dual
Luciferase Reporter Gene Assay kit (cat. no. RG027; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. All the experiments were performed in
triplicate. The relative luciferase activities were normalized to
that of the control cells.
Cell viability assay
Cell viability was assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, exponentially growing cells were harvested and
seeded into 96-well plates at 2×103 cells per well and
incubated in MEM medium for 24, 48, 72, 96 and 120 h, respectively.
Following this, MEM was discarded and fresh medium containing MTT
(5 mg/ml MTT in PBS; Sangon Biotech Co., Ltd., Shanghai, China) was
added, and cells were incubated for additional 4 h. Dimethyl
sulfoxide were used to dissolve the resultant formazan crystals and
the absorbance at 490 nm was measured using an ELISA reader once
every 24 h (BioTek Instruments, Inc., Winooski, VT, USA). All the
experiments were repeated 5 times.
Invasion assay
A Transwell invasion chamber (Corning Incorporated,
Corning, NY, USA) was washed with MEM, and 20 µl Matrigel (1 mg/ml)
(BD Biosciences, Franklin Lakes, NJ, USA) was added to evenly cover
the surface of the polycarbonate membrane (8 µm pore size) to
create a Matrigel membrane. The chamber was divided into upper and
lower chambers. For invasion assays, ECs and RPs (4×105)
were serum-starved overnight and seeded into MEM medium containing
10% FBSon the top chamber. The bottom chamber contained 10% FBS in
MEM, which acted as a chemoattractant. Following incubation for 48
h at 37°C, cells from the top chamber were removed using a cotton
swab and invading cells were fixed with 4% formaldehyde for 15 min
at room temperature, then stained with a 0.1% crystal violet
solution for 10 min. We randomly assessed 5 fields of view. The
invading cells were photographed using an inverted microscope. We
randomly assessed 5 fields of view, and total cell numbers were
counted and quantified using ImageJ software (version1.48; National
Institutes of Health, Bethesda, MD, USA). The results were
presented as the mean ± standard deviation, and the experiment was
repeated three times for each group.
Statistical analysis
Data were expressed as the mean ± standard deviation
of at least three independent experiments. Multiple comparisons
were conducted using a two-way analysis of variance (Tukey's test).
Differences between two groups were tested for statistical
significance using a paired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-126 and IRS-1 expression levels in
ECs and RPs
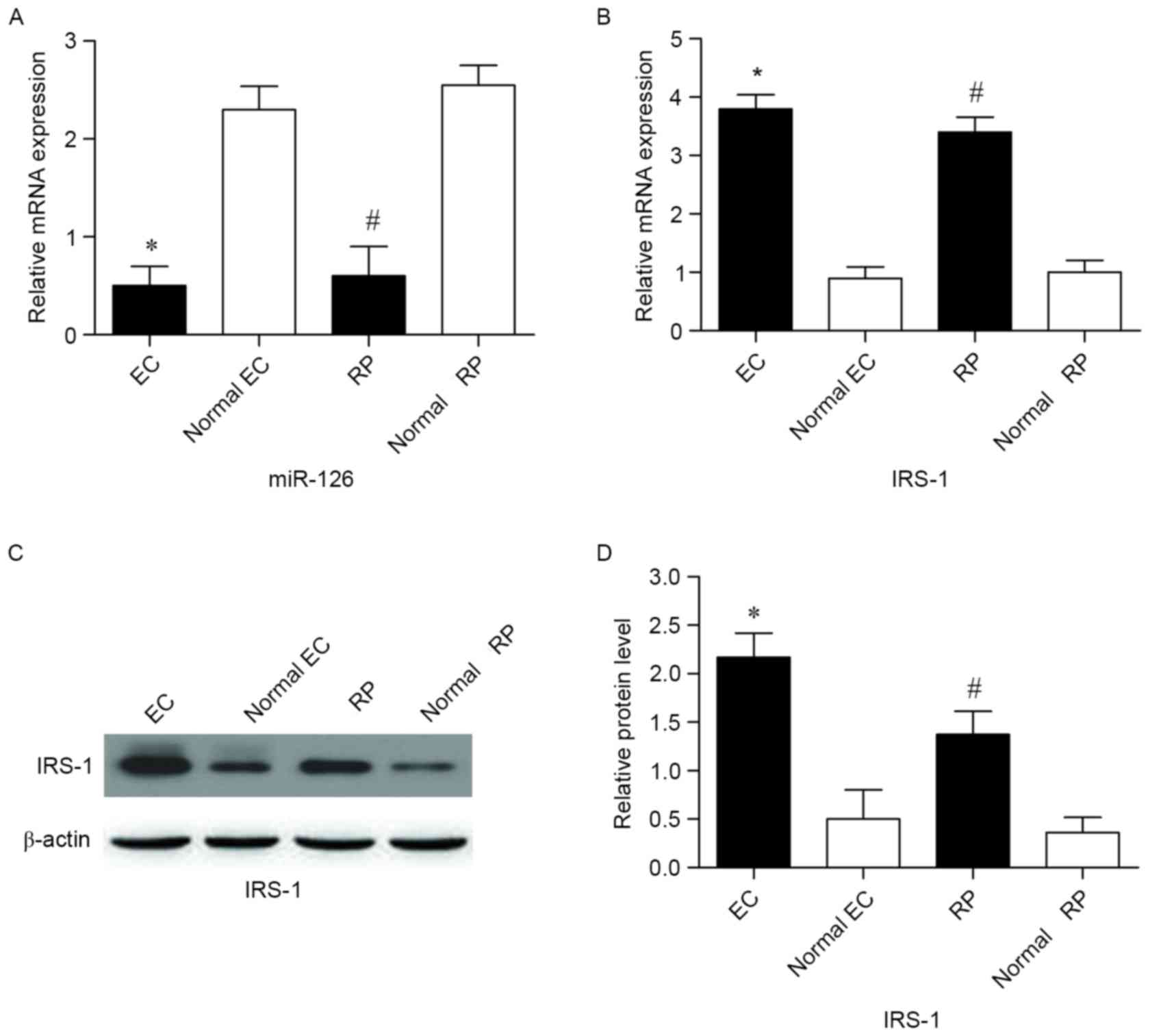
The expression level of miR-126 was detected in ECs
and RPs by RT-qPCR. The results indicated that miR-126 expression
levels were significantly lower in ECs and RPs cultured from the DR
model compared with normal controls (P<0.05; Fig. 1A). IRS-1 mRNA expression levels were
increased in ECs and RPs compared with normal controls, as detected
by RT-qPCR (P<0.05; Fig. 1B).
IRS-1 protein expression levels were examined by Western blot
analysis (Fig. 1C). The results
revealed that IRS-1 protein levels were significantly elevated in
ECs and RPs compared with normal controls (Fig. 1D).
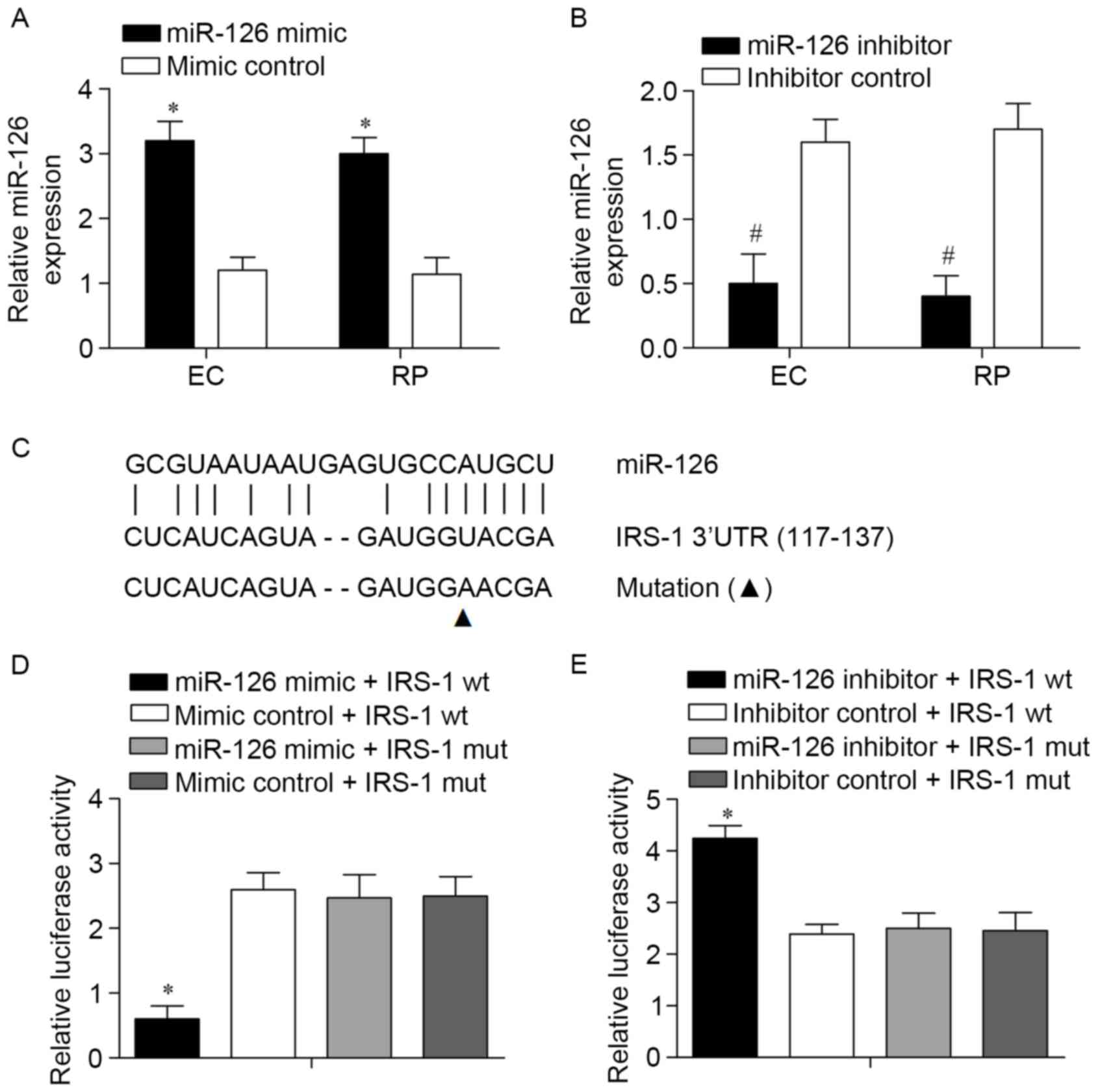
IRS-1 is targeted by miR-126 in ECs
and RPs obtained from a murine DR model
As miR-126levels were decreased in ECs and RPs from
the DR model, its involvement in the biology of the two cell types
was investigated. miR-126 mimics were used to amplify the
expression of miR-126 and a synthetic inhibitor specific to miR-126
was used to suppress the expression of endogenous miR-126 in the
ECs and RPs. The efficiency of this miR-126 mimic or inhibitor was
confirmed using a qPCR assay (Fig. 2A and
B). IRS-1 was predicted to be targeted by miR-126 by the
bioinformatic software program TargetScan. According to the
results, a potential binding target site of miR-126 was observed in
the 3′-UTR of the IRS-1 gene (Fig.
2C). To experimentally confirm IRS-1 as an authentic target of
miR-126 in ECs, IRS-1-wt or IRS-1-mut plasmids were transfected
into ECs together with miR-126 mimics or mimic controls. Following
transfection for 48 h, the results revealed that the luciferase
activity in the IRS-1-wt with miR-126 mimic group was significantly
reduced compared with the other three groups (Fig. 2D). The IRS-1-wt and IRS-1-mut
luciferase reporter vectors were co-transfected with miR-126
inhibitors or inhibitor controls into ECs. The results revealed
that transfection with the miR-126 inhibitor reversed the reduction
in the expression level of luciferase with wild-type IRS-1 3′UTR in
ECs (Fig. 2E). Taken together, these
data demonstrated that IRS-1 is a target of miR-126.
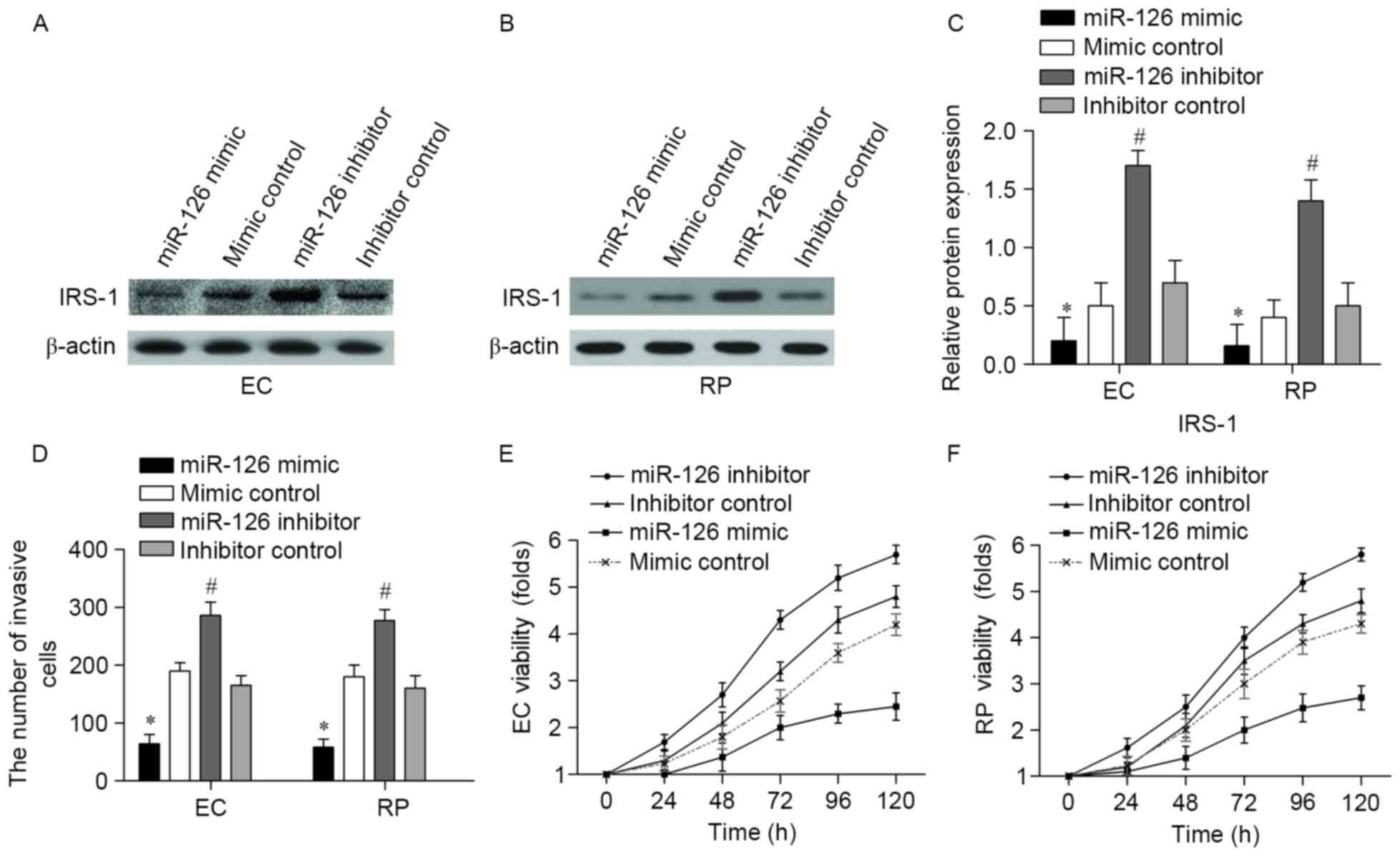
miR-126 suppresses the invasion and
viability of ECs and RPs
To further explore the impact of miR-126 in ECs and
RPs, the present study examined whether overexpression or
inhibition of miR-126 was capable of affecting cell viability. EC
and RPs were transfected with miR-126 mimic, mimic control, miR-126
inhibitor or inhibitor control. The efficiency of the miR-126 mimic
and inhibitor on the expression of IRS-1 was confirmed by western
blotting in ECs and RPs (Fig. 3A and
B, respectively). Transfection with the miR-126 mimic
significantly decreased IRS-1 protein levels compared with the
mimic control, and transfection with the miR-126 inhibitor
significantly increased IRS-1 protein levels compared with the
inhibitor control (Fig. 3C)
Transfection with the miR-126 mimic significantly decreased the
number of invasive ECs and RPs compared with the mimic control
group (P<0.05; Fig. 3D), while
transfection with the miR-126 inhibitor increased the number of
invasive ECs and RPs compared with the inhibitor control group
(P<0.05; Fig. 3D). In addition,
overexpression of miR-126 decreased the viability of ECs compared
with the mimic control group, and inhibition of miR-126 promoted EC
viability compared with the inhibitor control group (Fig. 3E). Similar MTT results were obtained
in RPs, with cell viability decreased by the overexpression of
miR-126 compared with the mimic control (Fig. 3F). Furthermore, RP viability was
increased following treatment with the miR-126 inhibitor (Fig. 3F). These results suggested that
miR-126 suppressed the invasion and viability of ECs and RPs.
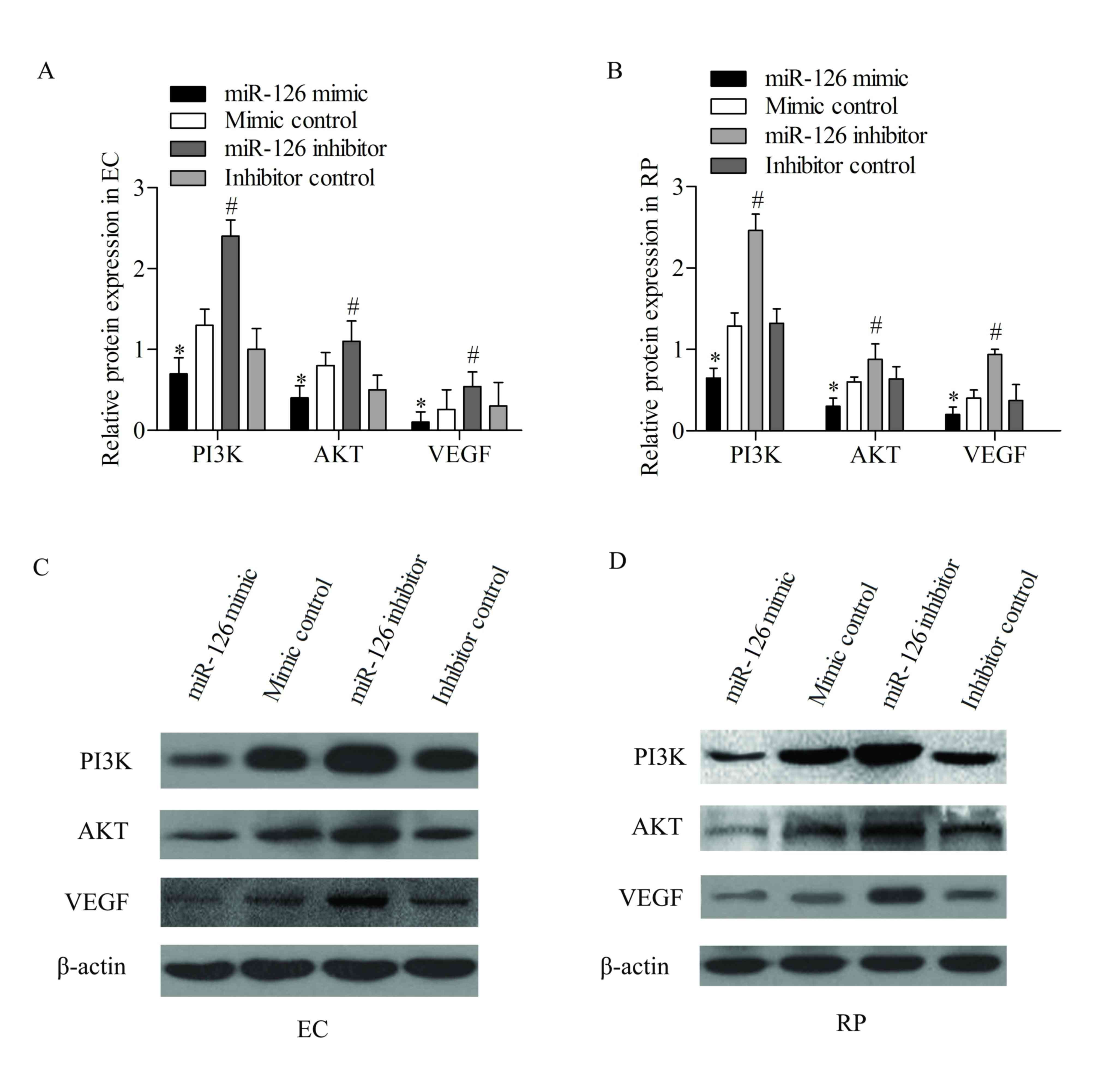
miR-126 inhibits the PI3K/Akt pathway
by downregulating IRS-1
VEGF is an angiogenic factor that is involved in the
maintenance of vascular homeostasis (27) and diabetic retinopathy (28). Insulin-induced VEGF expression has
been reported to be mediated through the activation of PI3K/Akt,
which is downstream to the insulin receptor (29).
Thus, the effect of miR-126 overexpression and
suppression on the expression of IRS-1 and PI3K/Akt pathway was
examined. The expression levels of PI3K/Akt pathway-associated
genes, including PI3K, Akt and VEGF, were detected using western
blotting analysis. The results revealed that transfection with the
miR-126 mimic effectively decreased the expression of these
proteins compared with cells transfected with the mimic control,
while transfection with the miR-126 inhibitor increased the
expression of these proteins in ECs compared with the inhibitor
control (P<0.05; Fig. 4). Western
blotting analysis was also performed in RPs, and decreased
expression of these proteins was detected following transfection
with the miR-126 mimic compared with the mimic control.
Furthermore, transfection with the miR-126 inhibitor significantly
increased the expression levels of these proteins compared with the
inhibitor control (P<0.05; Fig.
4). These data suggested that the activation of the PI3K/Akt
pathway was suppressed by miR-126 overexpression in ECs and
RPs.
siRNA interference of IRS-1 nullified
the effect of miR-126 inhibitor in ECs
To determine whether interference with IRS-1
expression counteracted the effects of miR-126 in ECs, the miR-126
inhibitor or inhibitor control were co-transfected with or without
a siRNA IRS-1 vector into ECs. Western blotting was performed to
measure the expression levels of VEGF, PI3K and Akt (Fig. 5A). Compared with the inhibitor
control, transfection with the miR-126 inhibitor increased the
expression levels of these proteins (P<0.05; Fig. 5B). For the ECs transfected with the
miR-126 inhibitor and siRNA IRS-1, the relative protein expression
levels were significantly decreased compared with the miR-126
inhibitor transfection group (P<0.01; Fig. 5B) and the expression levels of these
proteins were promoted in comparison with the siRNA IRS-1group
(P<0.05; Fig. 5B). These results
suggested that transfection with siRNA IRS-1 nullified
themiR-126-induced inhibition of VEGF pathway protein expression
levels in ECs. To further confirm the offset effect of IRS-1
silencing on miR-126 inhibition, we examined the number of invading
ECs treated with the miR-126 inhibitor or inhibitor control, with
or without the siRNA IRS-1 vector, was assessed. Interference
withIRS-1 reversed the effect of miR-126 inhibitor on cell invasion
in ECs (Fig. 5C). MTT assays were
performed to examine the viability of ECs under similar treatments.
Cell viability was increased when ECs were co-transfected with
siRNA IRS-1 and miR-126 inhibitor compared with the siRNA IRS-1
transfection group (Fig. 5D). These
results suggested that interference with IRS-1 restored the
inhibitory effect of miR-126 in ECs.
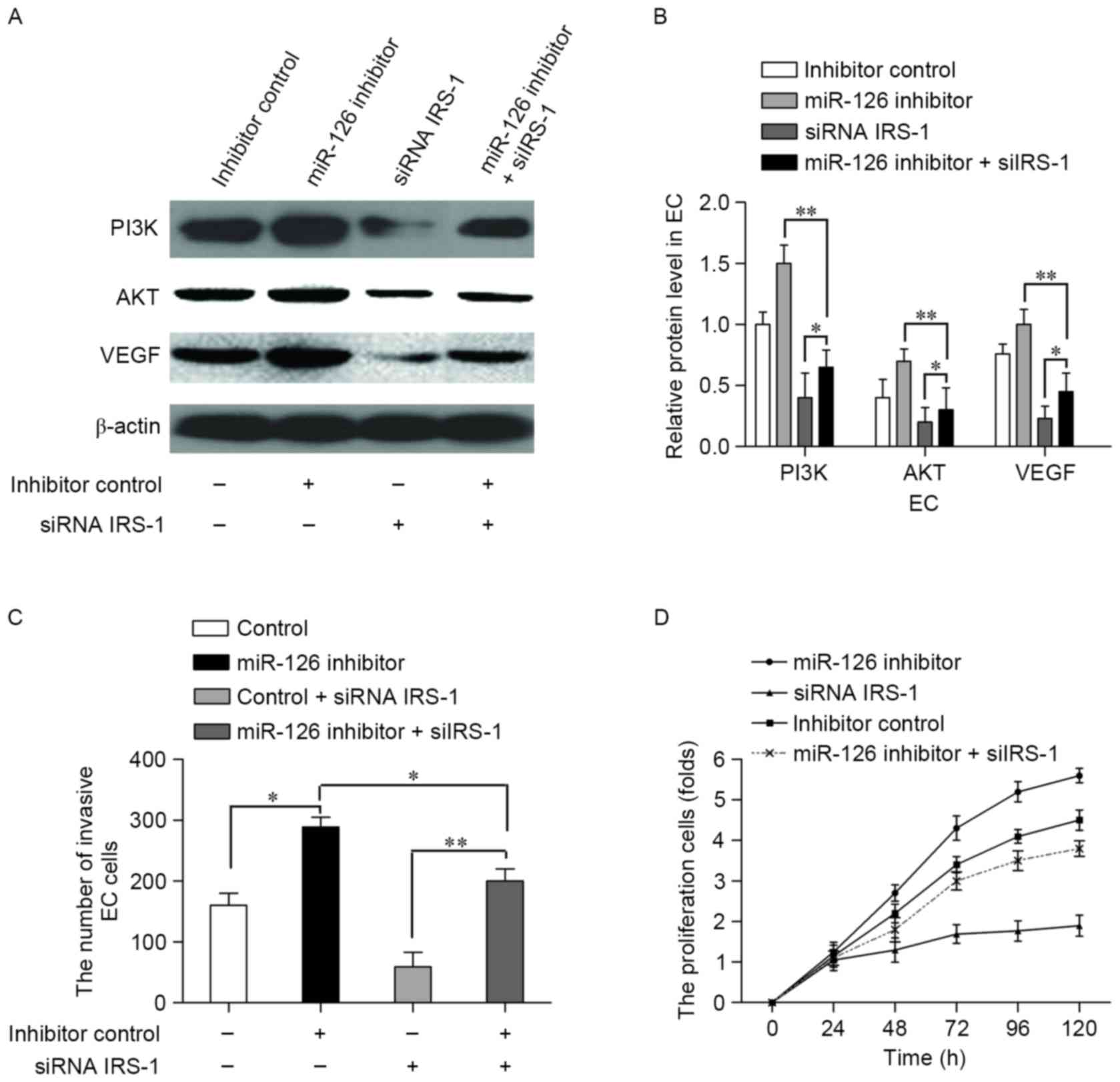 | Figure 5.siRNA targeting IRS-1 offsets the
suppression effect of miR-126 in ECs. Cells were transfected with
the miR-126 inhibitor or inhibitor control with or without a siRNA
IRS-1 vector. (A) VEGF, PI3K and Akt expression levels were
detected using western blotting. (B) Relative protein expression
levels in ECs were normalized to β-actin. (C) The number of
invading ECs. (D) The viability of ECs, assessed by MTT assay. Data
are presented as the mean ± standard deviation of three
experiments. *P<0.05 and **P<0.01, with comparisons indicated
by lines. siRNA, small interfering RNA; IRS-1, insulin receptor
substrate-1; miR-126, microRNA-126; VEGF, vascular endothelial
growth factor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase
B; EC, endothelial cell. |
Discussion
Diabetic retinopathy (DR) is characterized by the
early dropout of capillary pericytes and the resultant angiogenesis
(2). There is evidence to suggest
that the IRS-1 protein is involved in the development of type 2
diabetes (30). Consistent with this,
the present study demonstrated that the expression of IRS-1 was
significantly increased in ECs and RPs from a DR mouse model
compared with the healthy control. To examine the involvement of
IRS-1 in DR, the noncoding 3′-UTR of IRS-1 was investigated in the
present study. miRNAs are noncoding RNAs that suppress the
expression of protein-coding genes by binding to a target sequence
at the 3′-UTR of the target gene. In the present study, miR-126 was
predicted to be the miRNA that targeted IRS-1, and thus may be
involved in the pathogenesis of DR.
Expression of miR-126 is decreased in multiple types
of cancer cell, and has previously been regarded as a cell growth
suppressor that acts on IRS-1 (16)
or as a metabolic regulator in hepatocytes (31). The loss of miR-126 has been reported
in the plasma of patients with diabetes mellitus (DM). The target
connection between IRS-1 and miR-126 was predicted using
TargetScan, a bioinformatics software program. Thus, miR-126 was
selected as the miRNA targeting IRS-1. The results of the present
study revealed thatmiR-126 expression was significantly decreased
when IRS-1 expression was increased in ECs and RPs compared with
healthy controls, which was consistent with the results of a former
study in breast cancer cells (16).
To verify the targeting reaction between miR-126 and IRS-1,
IRS-1-wt and IRS-1-mutluciferase reporter vectors were constructed.
The results revealed that overexpression of miR-126 inhibited
luciferase expression when cells were transfected with the wt-IRS-1
luciferase reporter vector, but it was not inhibited in the
mut-IRS-1 group. Furthermore, inhibition of miR-126 increased
luciferase activity in the wt-IRS-1 transfection group compared
with the mut-IRS-1 group. These results demonstrated that IRS-1 was
a target gene for miR-126 in ECs and RPs from a DR mouse model.
miR-126 is enriched in endothelial cells and is
involved in maintaining endothelial homeostasis and vascular
integrity (32). Reduced levels of
miR-126 were reported to be an underlying cause of endothelial
progenitor cell (EPC) dysfunction in DM. As restoration of miR-126
expression in EPCs from patients with DM may promote EPC
proliferation and migration and inhibit their apoptosis, miR-126
may restore the ability of EPCs to be incorporated into the damaged
endothelium and work in concert with existing endothelial cells to
form blood vessels (22). Thus, to
investigate the involvement of miR-126 in EC and RP viability and
invasion via targeting IRS-1, the effect of miR-126 inhibition and
miR-126 overexpression on the viability and invasion of ECs and RPs
was explored. The results demonstrated that transfection with the
miR-126 mimic inhibited the expression of IRS-1, and inhibited cell
viability and invasion. However, under inhibition of miR-126, IRS-1
expression was significantly increased and cell viability and
invasion were promoted in ECs and RPs. As a cell growth suppressor,
mir-126 has been reported to target IRS-1 and inhibit the cell
cycle phase transition from G1/G0 to S (16). These results suggested that miR-126
negatively influenced viability and invasion in ECs and RPs
obtained from DR mice model, via targeting IRS-1.
VEGF is a key regulatory factor associated with
angiopoiesis, carcinogenesis and metastasis (33). Insulin upregulates VEGF expression,
and this action is mediated by the insulin receptor (29). The effect of insulin on VEGF
expression has been suggested to be a potential explanation for the
worsening of diabetic retinopathy in DR (28). A previous report also indicated that
insulin induces VEGF expression in vascular cells by activating the
PI3K/Akt and Ras/MAP kinase pathways (8). As predicted by the Kyoto Encyclopedia of
Genes and Genomes pathway database, VEGF is located downstream of
IRS-1/PI3K/Akt (34). The present
study revealed that expression of PI3K/Akt pathway proteins, PI3K,
Akt and VEGF, were inhibited following transfection with the
miR-126 mimic compared with the mimic control group. The results
indicated that the overexpression of miR-126 inhibited the
expression of PI3K/Akt pathway proteins by decreasing IRS-1
expression, with the inhibition of miR-126 causing the reverse
effect. Insulin has been reported to stimulate several signaling
cascades in EC and vascular smooth muscle cells following
activation of the insulin receptor via its tyrosine kinase subunit,
by phosphorylating IRS-1 and IRS-2. Tyrosine-phosphorylated IRS-1
and IRS-2 interact with several downstream cellular proteins,
including the p85 regulatory subunit of PI3K, resulting in the
activation of Akt (14). In order to
further confirm the inhibitory effect of miR-126 on the expression
of PI3K/Akt pathway proteins and on the viability and invasion of
ECs was via targeting IRS-1, interference with IRS-1 expression was
performed in ECs using siRNA. The results revealed that
transfection with siRNA targeting IRS-1 restored the inhibitory
effect of miR-126 on the PI3K/Akt pathway protein expression in
ECs. These results suggested that miR-126 suppresses the expression
of IRS-1 in ECs and RPs. This result contributes to our
understanding of the VEGF pathway regulatory network in DR. The
present study demonstrated that IRS-1 is a novel DR-associated
tumor promoting gene. Further studies are required to develop a
therapeutic strategy targeting IRS-1 for DR treatment.
In conclusion, the results of the present study
demonstrated that the overexpression of miR-126 affected the
expression of IRS-1, resulting in the downregulation of VEGF
pathway proteins, and suppressed the invasion and viability of ECs
and RPs. The present study further elucidates the pathogenesis of
DR and implicates miR-126 as a potential therapeutic target for
DR.
Glossary
Abbreviations
Abbreviations:
|
DR
|
diabetic retinopathy
|
|
IRS-1
|
insulin receptor substrate 1
|
|
miRNA/miR
|
microRNA
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
VEGF
|
vascular endothelial growth factor
|
|
3′-UTR
|
3′-untranslated region
|
References
|
1
|
Congdon NG, Friedman DS and Lietman T:
Important causes of visual impairment in the world today. JAMA.
290:2057–2060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armulik A, Abramsson A and Betsholtz C:
Endothelial/pericyte interactions. Circ Res. 97:512–523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caldwell RB, Bartoli M, Behzadian MA,
El-Remessy AE, Al-Shabrawey M, Platt DH and Caldwell RW: Vascular
endothelial growth factor and diabetic retinopathy:
Pathophysiological mechanisms and treatment perspectives. Diabetes
Metab Res Rev. 19:442–455. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saltiel AR and Pessin JE: Insulin
signaling pathways in time and space. Trends Cell Biol. 12:65–71.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 280:C1358–C1366. 2001.PubMed/NCBI
|
|
6
|
Behl T and Kotwani A: Exploring the
various aspects of the pathological role of vascular endothelial
growth factor (VEGF) in diabetic retinopathy. Pharmacol Res.
99:137–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poulaki V, Qin W, Joussen AM, Hurlbut P,
Wiegand SJ, Rudge J, Yancopoulos GD and Adamis AP: Acute intensive
insulin therapy exacerbates diabetic blood-retinal barrier
breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin
Invest. 109:805–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang ZY, He Z, King BL, Kuroki T, Opland
DM, Suzuma K, Suzuma I, Ueki K, Kulkarni RN, Kahn CR and King GL:
Characterization of multiple signaling pathways of insulin in the
regulation of vascular endothelial growth factor expression in
vascular cells and angiogenesis. J Biol Chem. 278:31964–31971.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
American Diabetes Association, .
Peripheral arterial disease in people with diabetes. Diabetes Care.
26:3333–3341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waltenberger J: Impaired collateral vessel
development in diabetes: Potential cellular mechanisms and
therapeutic implications. Cardiovasc Res. 49:554–560. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carlsson PO and Jansson L: Disruption of
insulin receptor signaling in endothelial cells shows the central
role of an intact islet blood flow for in vivo β-cell function.
Diabetes. 64:700–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tardif K, Hertig V, Dumais C, Villeneuve
L, Perrault L, Tanguay JF and Calderone A: Nestin downregulation in
rat vascular smooth muscle cells represents an early marker of
vascular disease in experimental type I diabetes. Cardiovasc
Diabetol. 13:1192014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SW, Yun JH and Kim JH, Kim KW, Cho CH
and Kim JH: Angiopoietin 2 induces pericyte apoptosis via α3β1
integrin signaling in diabetic retinopathy. Diabetes. 63:3057–3068.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang ZY, Lin YW, Clemont A, Feener EP,
Hein KD, Igarashi M, Yamauchi T, White MF and King GL:
Characterization of selective resistance to insulin signaling in
the vasculature of obese Zucker (fa/fa) rats. J Clin Invest.
104:447–457. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Figliolini F, Cantaluppi V, De Lena M,
Beltramo S, Romagnoli R, Salizzoni M, Melzi R, Nano R, Piemonti L,
Tetta C, et al: Isolation, characterization and potential role in
beta cell-endothelium cross-talk of extracellular vesicles released
from human pancreatic islets. PLoS One. 9:e1025212014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bandello F, Lattanzio R, Zucchiatti I and
Del Turco C: Pathophysiology and treatment of diabetic retinopathy.
Acta Diabetol. 50:1–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang D, Zhong P, Hu J, Lin F, Qian Y, Xu
Z, Wang J, Zeng C, Li X and Liang G: EGFR inhibition protects
cardiac damage and remodeling through attenuating oxidative
stressin STZ-induced diabetic mouse model. J Mol Cell Cardiol.
82:63–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su X, Sorenson CM and Sheibani N:
Isolation and characterization of murine retinal endothelial cells.
Mol Vis. 9:171–178. 2003.PubMed/NCBI
|
|
21
|
Buzney SM, Massicotte SJ, Hetu N and
Zetter BR: Retinal vascular endothelial cells and pericytes.
Differential growth characteristics in vitro. Invest Ophthalmol Vis
Sci. 24:470–480. 1983.PubMed/NCBI
|
|
22
|
Meng S, Cao JT, Zhang B, Zhou Q, Shen CX
and Wang CQ: Downregulation of microRNA-126 in endothelial
progenitor cells from diabetes patients, impairs their functional
properties, via target gene Spred-1. J Mol Cell Cardiol. 53:64–72.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cesarone G, Garofalo C, Abrams MT,
Igoucheva O, Alexeev V, Yoon K, Surmacz E and Wickstrom E:
RNAi-mediated silencing of insulin receptor substrate 1 (IRS-1)
enhances tamoxifen-induced cell death in MCF-7 breast cancer cells.
J Cell Biochem. 98:440–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of humangenes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoeppner LH, Sinha S, Wang Y, Bhattacharya
R, Dutta S, Gong X, Bedell VM, Suresh S, Chun C, Ramchandran R, et
al: RhoC maintains vascular homeostasis by regulating VEGF-induced
signaling in endothelial cells. J Cell Sci. 128:3556–3568. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simó R, Sundstrom JM and Antonetti DA:
Ocular anti-VEGF therapy for diabetic retinopathy: The role of VEGF
in the pathogenesis of diabetic retinopathy. Diabetes Care.
37:893–899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
King GL, Goodman AD, Buzney S, Moses A and
Kahn CR: Receptors and growth-promoting effects of insulin and
insulinlike growth factors on cells from bovine retinal capillaries
and aorta. J Clin Invest. 75:1028–1036. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerouz NJ, Hörsch D, Pons S and Kahn CR:
Differential regulation of insulin receptor substrates-1 and-2
(IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in
liver and muscle of the obese diabetic (ob/ob) mouse. J Clin
Invest. 100:3164–3172. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu HS, Park SY, Ma D, Zhang J and Lee W:
The induction of microRNA targeting IRS-1 is involved in the
development of insulin resistance under conditions of mitochondrial
dysfunction in hepatocytes. PLoS One. 6:e173432011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|