Introduction
Recepteur d'origine nantais (RON), a receptor
tyrosine kinase belonging to the MET proto-oncogene family
(1) shares ~60% structural homology
with the c-MET receptor (2). RON is
synthesized as a single-chain precursor, pro-RON, which is then
cleaved into a 40-kDa α-chain and a 150-kDa β-chain (3). A single disulfide bond links these two
chains to form a 180-kDa heterodimer. The α-chain is completely
extracellular, and the β-chain has extracellular, transmembrane and
intracellular regions containing a functional tyrosine kinase
segment as well as multiple regulatory elements. The ligand for RON
is macrophage-stimulating factor, also known as hepatocyte growth
factor-like protein or scatter factor-2 (4).
Altered RON expression and activation are known to
be associated with cancer progression and decreased survival in a
number of types of human cancer, including breast (5), colon (6),
gastric (7), non-small cell lung
(8), bladder (9) and ovary (10) cancer. Research into RON in pancreatic
cancer is a relatively recent development. The currently available
evidence to support the potential role of RON in carcinogenesis of
pancreatic cancer and implications for future targeted therapy in
treating pancreatic cancer was previously reviewed (11). RON has been demonstrated to serve
important roles in pancreatic cancer carcinogenesis (12–14),
epithelial-mesenchymal transition (15,16), tumor
migration (17–19), angiogenesis (20,21),
cancer stem cells (22) and apoptotic
resistance (14,23,24) as a
part of the progression of pancreatic cancer, suggesting that RON
may be a potential therapeutic target in the treatment of
pancreatic cancer. In particular, RON signaling was previously
identified to increase VEGF level and promote microtubule formation
in BxPC-3 and FG cells, suggesting an specific mechanism for the
association of RON with pancreatic cancer progression (21).
Chakedis et al (25,26)
identified a novel RON isoform in human pancreatic cancer. Partial
splicing of exons 5 and 6 (P5P6) produces a RON isoform that lacks
the first extracellular immunoglobulin-plexin-transcription domain
(25); RNA sequencing studies
revealed that the P5P6 isoform has ligand-independent activity and
induces markedly different patterns of gene expression when
compared with wild type RON, providing further understanding of RON
biology in pancreatic cancer carcinogenesis and exhibiting
potential implications for therapeutic strategy (26). RON-specific therapeutic approaches,
including tyrosine kinase inhibitors and monoclonal antibodies,
have been tested in preclinical and clinical trials to determine
their anti-cancer efficacy (27–29).
However, their therapeutic efficacy was relatively low. Great
effort has since been made to increase the efficacy of monoclonal
antibodies against RON for the treatment of pancreatic cancer
(30).
However, the data indicating potential associations
between RON expression and the clinical outcome of pancreatic
cancer are presently limited. Unless this association is confirmed,
the recent drive into RON research may be attenuated. Therefore, in
the present study, the association between VEGF expression and
clinical outcomes with RON expression was evaluated in resected
left-sided pancreatic cancer, in order to assess the potential role
of RON in the clinical setting of left-sided pancreatic cancer.
Materials and methods
Patient enrollment and review of
medical records
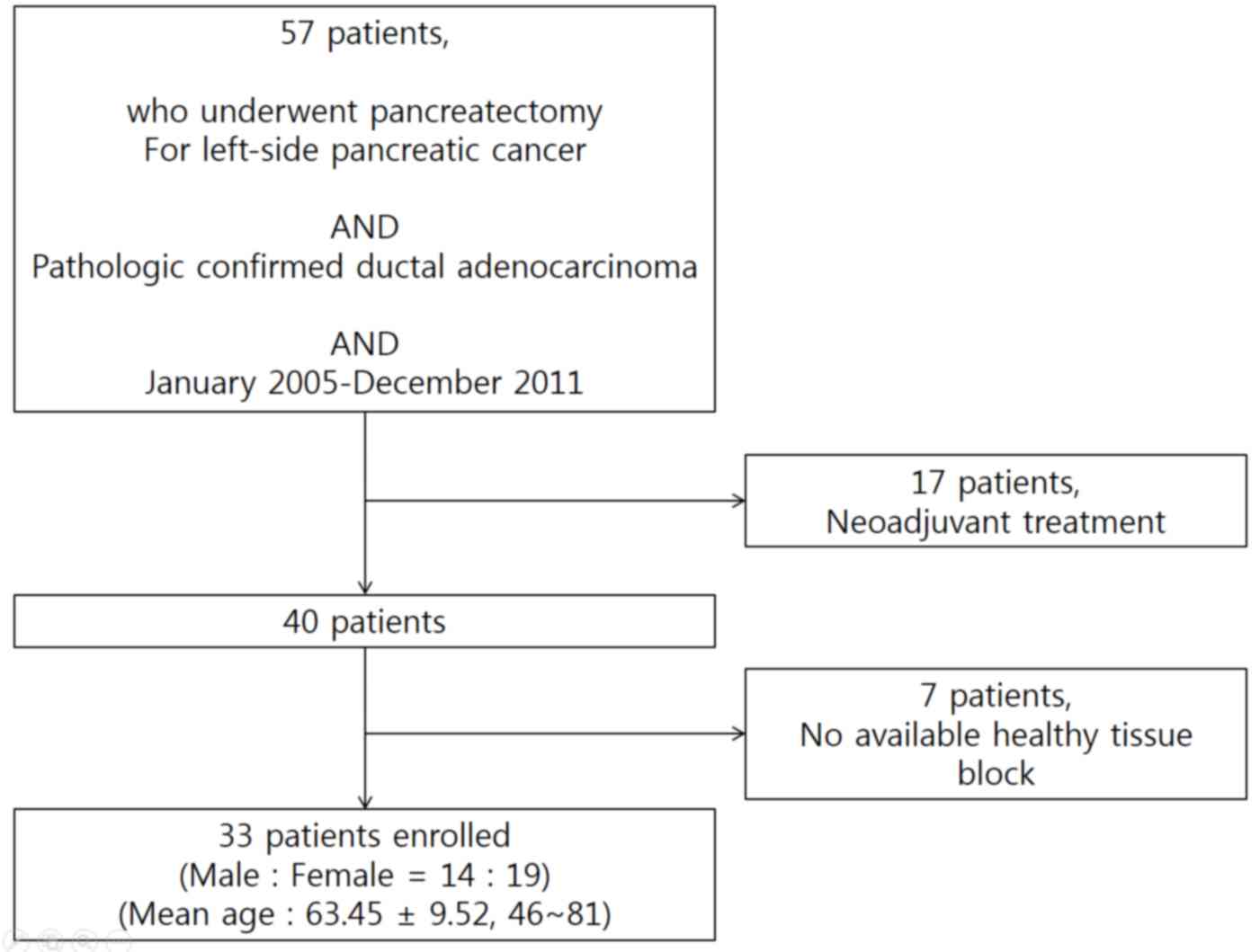
From January 2005 to December 2011, a total of 57
patients underwent radical distal pancreatosplenectomy for
left-sided pancreatic cancer at Severance hospital, Yonsei
University College of Medicine (Seoul, Korea). Ductal
adenocarcinoma was confirmed in all patients. A total of 17
patients who received preoperative neoadjuvant treatment and 7
patients for whom paraffin-embedded tissue blocks were unavailable
were excluded (Fig. 1). The patients'
clinicopathological characteristics, including age, sex, clinical
presentation, tumor size, histopathological features and follow-up
data were reviewed and recorded. The present study was approved by
the Institutional Review Board of Yonsei University College of
Medicine.
Cell lines and cell maintenance
The human pancreatic cancer cell lines ASPC-1,
BxPC-3, MiaPaCa-2 and Panc-1 were obtained from the Bioevaluation
Center (Korea Research Institute of Bioscience and Biotechnology,
Daejeon, Korea). ASPC-1 and BxPC-3 cells were maintained in RPMI
medium, Panc-1 cells were maintained in Dulbecco's modified Eagle's
medium and MiaPaCa-2 cells were maintained in minimal essential
medium. All tissue culture media were from Gibco (Thermo Fisher
Scientific, Inc.), and were supplemented with 10% fetal bovine
serum and 1% penicillin-streptomycin, unless otherwise noted. All
cells were grown at 37°C in a humidified incubator containing
CO2.
Western blot analysis
Harvested cells were lysed in cell extraction buffer
(10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM
NaF, 20 mM Na4P2O7, 2 mM
Na3VO4, 1% Triton X-100, 10% glycerol, 0.1%
SDS and 0.5% deoxycholate). A 40 µg amount of total protein, as
quantified with a Bradford assay, was treated with Laemmli sample
buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA), heated at
100°C for 5 min and then resolved by 8% SDS-PAGE. Gels were
electroblotted onto nitrocellulose membranes (GE Healthcare Life
Sciences, Chalfont, UK). Membranes were blocked with 5% non-fat dry
milk in Tris-buffered saline with Tween-20, incubated with
antibodies against total RON (cat. no., ab52927; Abcam, Cambridge,
UK) and β-actin (cat. no., ab8227; Sigma-Alrich; Merck KGaA,
Darmstadt, Germany) overnight at 4°C, and then probed with
secondary antibodies (horseradish peroxidase conjugated mouse
anti-rabbit IgG; cat. no., sc2357; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at room temperature. All antibodies were
treated with Dako Antibody Diluent (Agilent Technologies, Santa
Clara, CA, USA). The washes were repeated and the membrane was
developed using a chemiluminescent agent (GE Healthcare Life
Sciences). The whole process was performed in triplicate.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections (4
µm) thick were deparaffinized and rehydrated prior to antigen
retrieval. Deparaffinization was performed on a rack with the
following washes: Xylene for 3 min, xylene 1:1 with 100% ethanol
for 3 min, 100% ethanol for 3 min, 95% ethanol for 3 min, 70%
ethanol for 3 min, 50% ethanol with 3 min, then a final rinse with
tapwater. Immunohistochemical analysis was performed using
pre-diluted anti-RON (cat. no., ab52927; Abcam) and anti-vascular
endothelial growth factor (VEGF; cat. no., sc-152; Santa Cruz
Biotechnology, Inc.) antibodies, according to the manufacturer's
protocol. All slides were reviewed by two pathologists blinded to
the oncological outcomes and clinicopathological variables. The
intensities of RON and VEGF were scored as 0, null; 1+, positive;
and 2+, strong positive.
Statistical analysis
Statistical analysis was performed using SPSS 23
software (IBM SPSS, Armonk, NY, USA). Continuous variables are
presented as the mean ± standard deviation and categorical
variables are expressed as the frequency (%). Univariate analysis
was performed using a χ2 test, and Student's t-test was
used for statistical assessment of the association between
clinicopathological characteristics and RON overexpression.
Survival curves were obtained by the Kaplan-Meier method, and
differences in survival between groups were compared with the log
rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RON expression in pancreatic cancer
cell lines and resected left-sided pancreatic cancer
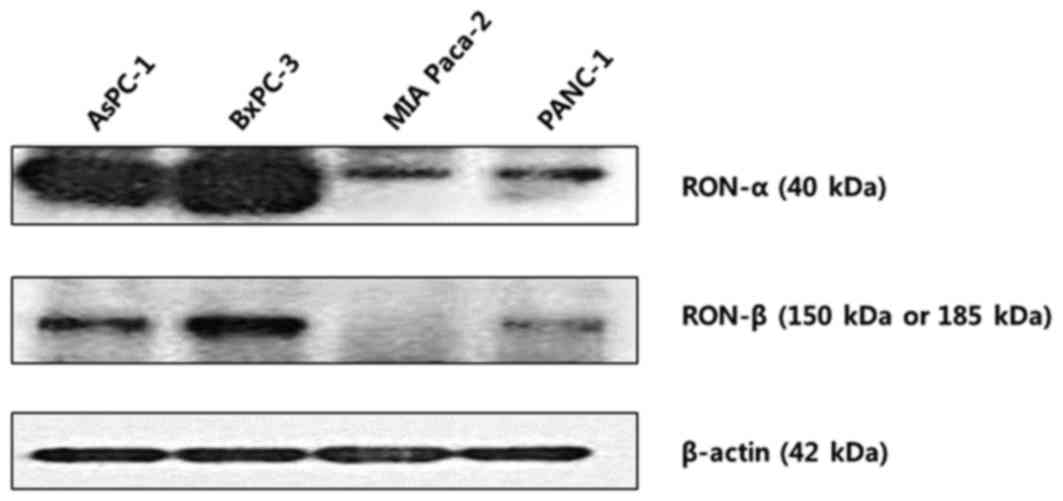
RON protein expression was determined by Western
blot analysis in pancreatic cancer cell lines (Fig. 2). All cell lines evaluated expressed
RON at various levels. In particular, AsPC-1 and BxPC-3 were
identified to exhibit increased expression of both the RON α- and
β-chains. By contrast, MiaPaCa-2 and Panc-1 cells were identified
to exhibited relatively decreased levels of RON expression. In
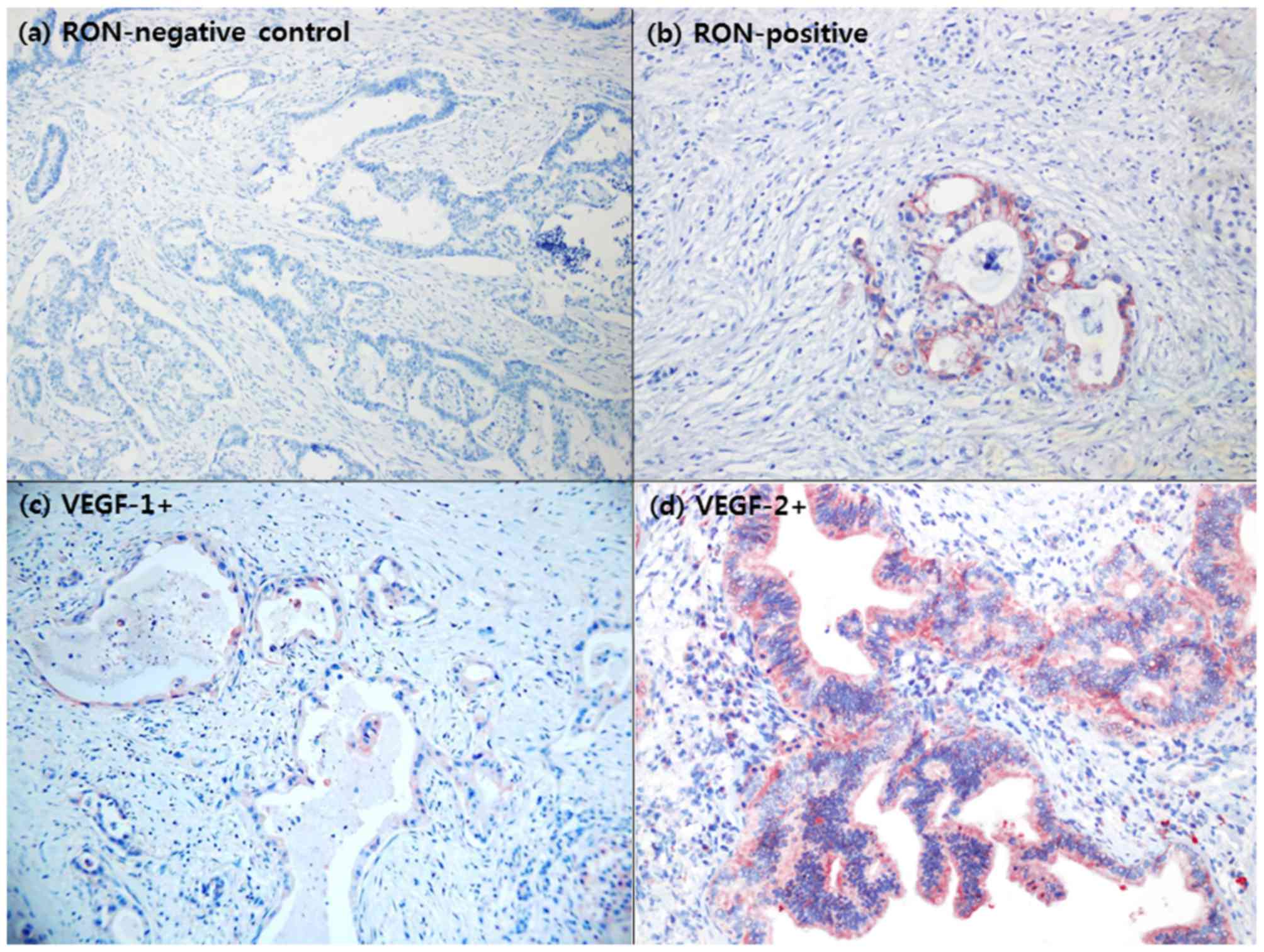
immunohistochemistry studies, 15/33 patients (45.5%) with resected
left-sided pancreatic cancer were identified to overexpress RON
(Fig. 3A and b).
RON and VEGF overexpression in
resected left-sided pancreatic cancer
Specimens from 29 patients (87.9%) exhibited VEGF
overexpression (Fig. 3C and d). No
association between RON and VEGF expression was identified in
resected left-sided pancreatic cancer (P=0.381; Table I).
 | Table I.Association between RON and VEGF
overexpression. |
Table I.
Association between RON and VEGF
overexpression.
|
|
| RON
overexpression |
|
|---|
|
|
|
|
|
|---|
| Parameter |
| 0 | 1+ | P-value |
|---|
| VEGF | 0 | 3 | 1 | 0.381 |
| overexpression | 1+ | 8 | 8 |
|
|
| 2+ | 7 | 6 |
|
Clinical validation of the oncological
role of RON expression in resected left-sided pancreatic
cancer
No association between clinical oncological
parameters and overexpression in resected left-sided pancreatic
cancer was identified (P>0.05; Table
II). In particular, no significant differences in tumor stage
(P=0.981), tumor size (P=0.2000), node stage (P=0.898), perineural
invasion (P=1.000) and lymphovascular invasion (P=0.919) were
identified.
 | Table II.Association between RON overexpression
and clinical oncological parameters. |
Table II.
Association between RON overexpression
and clinical oncological parameters.
|
| RON
overexpression |
|
|---|
|
|
|
|
|---|
| Oncological
parameter | No | Yes | P-value |
|---|
| CA19-9, U/ml ±
SD | 1304.7±4671.5 | 603.3±1475.6 | 0.581 |
| Tumor size, cm ±
SD | 3.1±0.9 | 2.7±0.9 | 0.200 |
| T stage |
|
| 0.981 |
| T1 | 0 | 0 |
|
| T2 | 1 | 1 |
|
| T3 | 16 | 13 |
|
| T4 | 1 | 1 |
|
| N stage |
|
| 0.898 |
| N0 | 8 | 7 |
|
| N1 | 10 | 8 |
|
| LNR | 0.4±1.3 | 0.1±0.1 | 0.384 |
|
Differentiation |
|
| 0.750 |
|
Well | 4 | 3 |
|
|
Moderate | 11 | 11 |
|
|
Poor | 2 | 1 |
|
|
None | 1 | 0 |
|
| LVI |
|
| 0.919 |
| No | 10 | 9 |
|
|
Yes | 6 | 5 |
|
| PNI |
|
| 1.000 |
| No | 8 | 7 |
|
|
Yes | 8 | 7 |
|
| R-status |
|
| 0.727 |
| R0 | 16 | 12 |
|
| R1 | 1 |
|
|
| R2 | 1 | 1 |
|
| Postadjuvant
chemotherapy |
|
| 0.475 |
| No | 4 | 5 |
|
|
Yes | 14 | 10 |
|
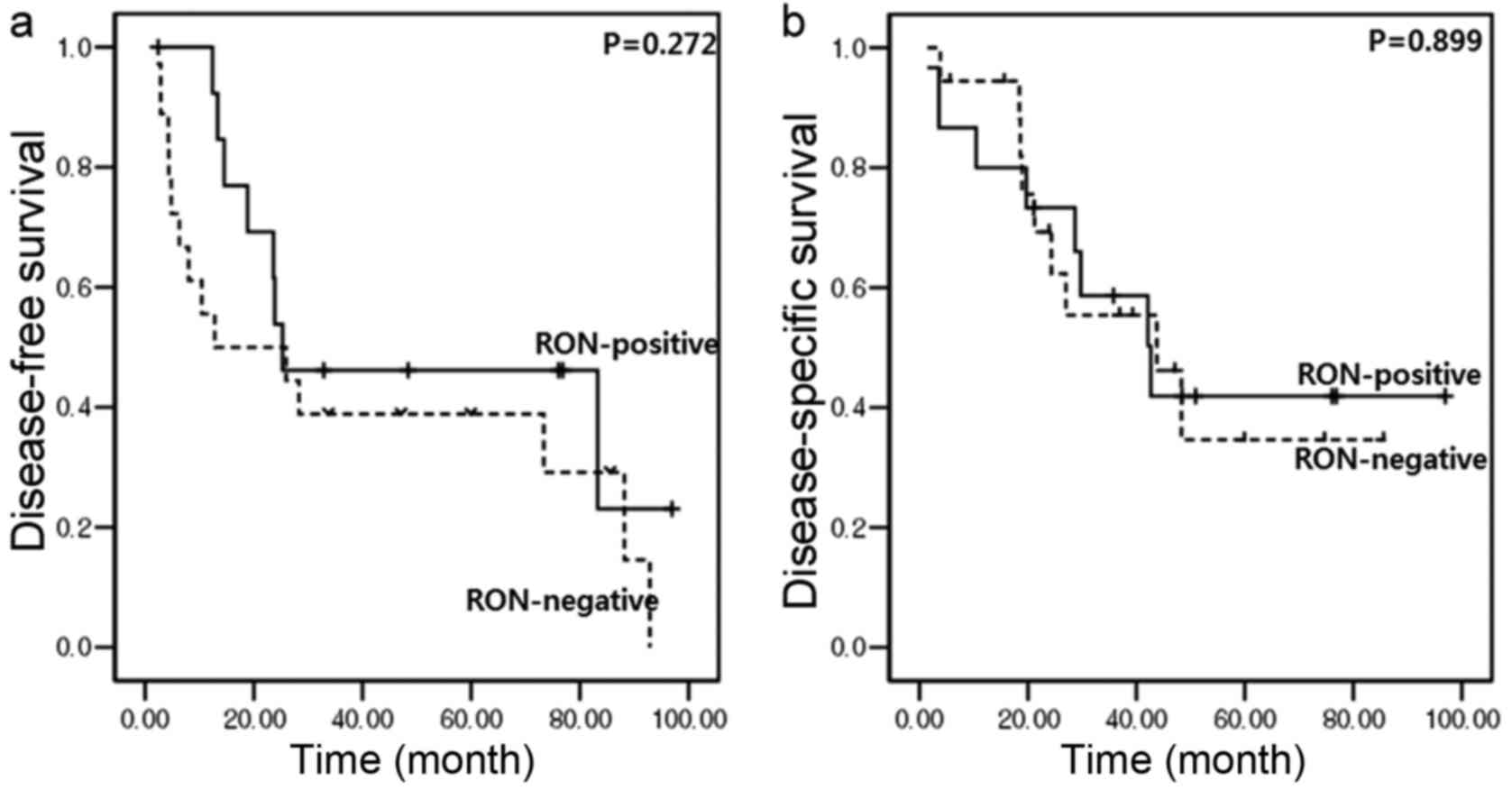
In addition, RON overexpression did not cause any
oncological effect on tumor recurrence and overall survival. In
resected left-sided pancreatic cancer, no significant differences
in disease-free survival (median, 12.8 months [95% CI (confidence
interval), 0–45.1] vs. median, 25.3 months (95% CI, 0–67.4);
P=0.272; Fig. 4A) or disease-specific
survival [median, 43.8 months (95% CI, 16.6–70.9) vs. median, 42.7
months (95% CI, 22–63.3); P=0.899; Fig.
4B] between RON-positive and RON-negative patients were
identified.
Discussion
A previous study has suggested a potential
oncological role for RON expression in pancreatic cancer
progression (10); however, there
have been limited studies concerning the oncological effect of RON
overexpression in resected pancreatic cancer. To the best of our
knowledge, only a single study has been published: Tactacan et
al (31) assessed RON expression
in a total of 492 pancreatic cancer patients and evaluated the
association between RON expression and patient outcomes and
clinicopathological variables. The study identified that increased
RON expression was a biomarker for poor prognosis in a training
set. However, the study failed to identify that RON expression was
not prognostic in the larger validation set. In addition, no
association was identified between RON expression and tumor stage
(P=0.123), tumor size (P=0.629) lymph node metastases (P=0.942),
grade (P=0.332), perineural invasion (P= 0.335) or vascular
invasion (P=0.210), leading to the conclusion that RON is not a
prognostic marker for resectable pancreatic cancer. When looking at
their patient population, >80% of the patients had pancreatic
head cancer, therefore allowing the possibility of unintended
contamination by other periampullary cancers from the ampulla of
Vater and distal bile ducts. To avoid this potential selection bias
in the present study, the study population consisted only of
patients with resected left-sided pancreatic cancer.
Thomas et al (21) demonstrated that RON signaling resulted
in mitogen-activated protein kinase-mediated VEGF secretion by
pancreatic cancer cells and in the promotion of microtubule
formation, suggesting that RON signaling may also positively
regulate an angiogenic mediator, VEGF, to promote cancer
progression in pancreatic cancer. This may explain the results of
another study where treatment with gemcitabine plus the anti-VEGF
monoclonal antibody bevacizumab failed to provide an oncological
benefit over gemcitabine treatment alone (32). However, in the present study, no
association between RON and VEGF expression in resected left-sided
pancreatic cancer was identified (P=0.381). Our understanding of
the regulation of angiogenesis in pancreatic cancer therefore
remains limited.
The clinical data of the present study also failed
to reveal an oncological role for RON in resected left-sided
pancreatic cancer. Following assessment of the potential
association of RON expression with clinicopathological
characteristics, RON overexpression was not identified to be
associated with tumor size, pathological node stage, pathological
tumor stage, tumor differentiation, perineural invasion or
lymphovascular invasion (P>0.05). In addition, there were no
significant oncological differences in terms of disease-free and
disease-specific survival.
There are a number of potential reasons for the
current missing link between RON expression and oncological outcome
in resected left-sided pancreatic cancer, as follows: i) Pancreatic
cancer harbors multiple genetic mutations and various dysregulated
signaling pathways the contribute to cancer progression. ii) It is
known that there are multiple splice variants of the RON receptor
(33–35), including RON Δ165, RON Δ160, RON Δ155,
RON Δ170, RON Δ110 and RON Δ52. To the best of our knowledge, the
presence of these variant types of the RON receptor has not been
investigated, and their individual oncogenic capability has not
been evaluated in pancreatic cancer. In addition, it is impossible
to discriminate between these variant types of RON receptors using
current immunohistochemistry techniques. In particular, a truncated
form of the RON receptor (short-form RON), lacking a majority of
the extracellular domain (36), may
not be detectable by conventional routine immunohistochemistry.
However, this short-form RON is constitutively active, leading to
pathogenesis and cancer progression in pancreatic cancer. iii) The
potential role of the microenvironment also requires consideration.
It is well-known that severe fibrosis and a desmoplastic reaction
including fibroblasts, immune cells, endothelial cells and neural
cells are associated with pancreatic cancer. Evidence from a
previous study suggests that interactions occur between the
microenvironment and pancreatic cancer cells, facilitating
pancreatic cancer pathogenesis (37).
It was not possible to replicate the potential contribution of this
‘harmony’ in the present study. iv) Finally, the present study was
based on a retrospective study design with a small number of
patients. Therefore, selection bias was unavoidable.
RON overexpression failed to result in an adverse
oncological effect in resected left-sided pancreatic cancer,
despite previous studies suggesting that RON may be a potential
target in the treatment of pancreatic cancer. Further clinical
studies validating the potential oncological role of RON are
required, and consideration of the multifactorial and heterogeneous
nature of pancreatic cancer is also required.
Acknowledgements
The present study was supported by a faculty
research grant from Yonsei University College of Medicine (grant
no, 6-2013-0041).
References
|
1
|
Ronsin C, Muscatelli F, Mattei MG and
Breathnach R: A novel putative receptor protein tyrosine kinase of
the met family. Oncogene. 8:1195–1202. 1993.PubMed/NCBI
|
|
2
|
Park M, Dean M, Kaul K, Braun MJ, Gonda MA
and Vande Woude G: Sequence of MET protooncogene cDNA has features
characteristic of the tyrosine kinase family of growth-factor
receptors. Proc Natl Acad Sci USA. 84:6379–6383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang MH, Wang D and Chen YQ: Oncogenic and
invasive potentials of human macrophage-stimulating protein
receptor, the RON receptor tyrosine kinase. Carcinogenesis.
24:1291–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang MH, Ronsin C, Gesnel MC, Coupey L,
Skeel A, Leonard EJ and Breathnach R: Identification of the ron
gene product as the receptor for the human macrophage stimulating
protein. Science. 266:117–119. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee WY, Chen HH, Chow NH, Su WC, Lin PW
and Guo HR: Prognostic significance of co-expression of RON and MET
receptors in node-negative breast cancer patients. Clin Cancer Res.
11:2222–2228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CT, Chow NH, Su PF, Lin SC, Lin PC and
Lee JC: The prognostic significance of RON and MET receptor
coexpression in patients with colorectal cancer. Dis Colon Rectum.
51:1268–1274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song YA, Park YL, Kim KY, Myung E, Chung
CY, Cho SB, Lee WS, Jung YD, Kweon SS and Joo YE: RON is associated
with tumor progression via the inhibition of apoptosis and cell
cycle arrest in human gastric cancer. Pathol Int. 62:127–136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han WL, Li WD, Hu J, Rusidanmu A, Chen LF,
Shen L and Zheng SS: Expression of the recepteur d'originenantais
receptor tyrosine kinase in non-small cell lung cancer and its
clinical significance. Chin Med J (Engl). 125:1110–1114.
2012.PubMed/NCBI
|
|
9
|
Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY,
Chang TY, Ho CL, Tzai TS and Chow NH: Co-expression of RON and MET
is a prognostic indicator for patients with transitional-cell
carcinoma of the bladder. Br J Cancer. 92:1906–1914. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrandina G, Martinelli E, Petrillo M,
Prisco MG, Zucconi A, Santaguida S, Zannoni G, Scambia G and
Ferlini C: Prognostic role of the recepteur d'origine nantais (RON)
expression in ovarian cancer patients. Gynecol Oncol. 111:237–243.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang CM, Babicky ML and Lowy AM: The RON
receptor tyrosine kinase in pancreatic cancer pathogenesis and its
potential implications for future targeted therapies. Pancreas.
43:183–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Camp ER, Yang A, Gray MJ, Fan F, Hamilton
SR, Evans DB, Hooper AT, Pereira DS, Hicklin DJ and Ellis LM:
Tyrosine kinase receptor RON in human pancreatic cancer:
Expression, function, and validation as a target. Cancer.
109:1030–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babicky ML, Maruyama K, Jaquish D and
French R: RON overexpression accelerates tumorigenesis and induces
metastasis in a KRAS mutant mouse model of pancreatic cancer. J Am
Coll Surg. 213:(Suppl). S1312011. View Article : Google Scholar
|
|
14
|
Thomas RM, Toney K, Fenoglio-Preiser C,
Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE and Lowy AM:
The RON receptor tyrosine kinase mediates oncogenic phenotypes in
pancreatic cancer cells and is increasingly expressed during
pancreatic cancer progression. Cancer Res. 67:6075–6082. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaquish DV, Yu PT, Shields DJ, French RP,
Maruyama KP, Niessen S, Hoover HA, Cheresh D, Cravatt B and Lowy
AM: IGF1-R signals through the RON receptor to mediate pancreatic
cancer cell migration. Carcinogenesis. 32:1151–1156. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajeshkumar NV, Rasheed ZA, Garcia-Garcia
E, López-Rios F, Fujiwara K, Matsui WH and Hidalgo M: A combination
of DR5 agonistic monoclonal antibody with gemcitabine targets
pancreatic cancer stem cells and results in long-term disease
control in human pancreatic cancer model. Mol Cancer Ther.
9:2582–2592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peace BE, Toney-Earley K, Collins MH and
Waltz SE: Ron receptor signaling augments mammary tumor formation
and metastasis in a murine model of breast cancer. Cancer Res.
65:1285–1293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas RM, Jaquish DV, French RP and Lowy
AM: The RON tyrosine kinase receptor regulates vascular endothelial
growth factor production in pancreatic cancer cells. Pancreas.
39:301–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang
R and Wang MH: Sustained expression of the RON receptor tyrosine
kinase by pancreatic cancer stem cells as a potential targeting
moiety for antibody-directed chemotherapeutics. Mol Pharm.
8:2310–2319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camp ER, Liu W, Fan F, Yang A, Somcio R
and Ellis LM: RON, a tyrosine kinase receptor involved in tumor
progression and metastasis. Ann Surg Oncol. 12:273–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Logan-Collins J, Thomas RM, Yu P, Jaquish
D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, et
al: Silencing of RON receptor signaling promotes apoptosis and
gemcitabine sensitivity in pancreatic cancers. Cancer Res.
70:1130–1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakedis J, French R, Babicky M, Jaquish
D, Howard H, Mose E, Lam R, Holman P, Miyamoto J, Walterscheid Z
and Lowy AM: A novel protein isoform of the RON tyrosine kinase
receptor transforms human pancreatic duct epithelial cells.
Oncogene. 35:3249–3259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakedis J, French R, Babicky M, Jaquish
D, Mose E, Cheng P, Holman P, Howard H, Miyamoto J, Porras P, et
al: Characterization of RON protein isoforms in pancreatic cancer:
Implications for biology and therapeutics. Oncotarget.
7:45959–45975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Toole JM, Rabenau KE, Burns K, Lu D,
Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M,
Gottfredsen KJ, et al: Therapeutic implications of a human
neutralizing antibody to the macrophage-stimulating protein
receptor tyrosine kinase (RON), a c-MET family member. Cancer Res.
66:9162–9170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Kaplan-Lefko PJ, Rex K, Yang Y,
Moriguchi J, Osgood T, Mattson B, Coxon A, Reese M, Kim TS, et al:
Identification of a novel recepteur d'origine nantais/c-met
small-molecule kinase inhibitor with antitumor activity in vivo.
Cancer Res. 68:6680–6687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guin S, Yao HP and Wang MH: RON receptor
tyrosine kinase as a target for delivery of chemodrugs by antibody
directed pathway for cancer cell cytotoxicity. Mol Pharm.
7:386–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao HP, Feng L, Zhou JW, Zhang RW and Wang
MH: Therapeutic evaluation of monoclonal antibody-maytansinoid
conjugate as a model of RON-targeted drug delivery for pancreatic
cancer treatment. Am J Cancer Res. 6:937–956. 2016.PubMed/NCBI
|
|
31
|
Tactacan CM, Chang DK, Cowley MJ, Humphrey
ES, Wu J, Gill AJ, Chou A, Nones K, Grimmond SM, Sutherland RL, et
al: RON is not a prognostic marker for resectable pancreatic
cancer. BMC Cancer. 12:3952012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kindler HL, Niedzwiecki D, Hollis D,
Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF,
O'Reilly E, Wozniak TF, et al: Gemcitabine plus bevacizumab
compared with gemcitabine plus placebo in patients with advanced
pancreatic cancer: Phase III trial of the Cancer and Leukemia Group
B (CALGB 80303). J Clin Oncol. 28:3617–3622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Collesi C, Santoro MM, Gaudino G and
Comoglio PM: A splicing variant of the RON transcript induces
constitutive tyrosine kinase activity and an invasive phenotype.
Mol Cell Biol. 16:5518–5526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang MH, Kurtz AL and Chen Y:
Identification of a novel splicing product of the RON receptor
tyrosine kinase in human colorectal carcinoma cells.
Carcinogenesis. 21:1507–1512. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou YQ, He C, Chen YQ, Wang D and Wang
MH: Altered expression of the RON receptor tyrosine kinase in
primary human colorectal adenocarcinomas: Generation of different
splicing RON variants and their oncogenic potential. Oncogene.
22:186–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bardella C, Costa B, Maggiora P, Patane'
S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM and Di Renzo
MF: Truncated RON tyrosine kinase drives tumor cell progression and
abrogates cell-cell adhesion through E-cadherin transcriptional
repression. Cancer Res. 64:5154–5161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Z, Pothula SP, Wilson JS and Apte MV:
Pancreatic cancer and its stroma: A conspiracy theory. World J
Gastroenterol. 20:11216–11229. 2014. View Article : Google Scholar : PubMed/NCBI
|