Introduction
Currently, the number of patients with morbid
obesity is increasing in Japan, as the Japanese population has
encountered a fast and radical change in their way of life, among
which are dietary changes that include the consumption of a
high-fat diet. It is well known that morbid obesity is associated
with insulin resistance and can increase the risk for developing
diabetes mellitus type 2 (1,2). Consistent with these changes, the rates
of pancreatic, colon, prostate and breast cancers, which are more
frequent in Western nations, have increased in Japan (2–4). The World
Cancer Research Fund and American Institute for Cancer Research
assessed causal associations between certain variables and each
type of cancer based on systematic reviews of epidemiological
evidence, and it was suggested that body fat levels exceeding
recommended values markedly increases the risk of colorectal,
postmenopausal breast, esophageal, endometrial, pancreatic and
kidney cancers (5). Epidemiological
studies have previously demonstrated that obesity increases the
risk of colon cancer by 1.5–2.0-fold, with obesity-associated colon
cancer accounting for 14–35% of the total incidence (6). Overall, the risk of colorectal neoplasia
is increased in individuals with a body fat level that exceeds
recommended values, insulin resistance and type 2 diabetes compared
with individuals without metabolic disorders (7).
The gut incretin hormone glucagon-like peptide-1
(GLP-1) is secreted from enteroendocrine cells in response to
ingested nutrients, and is a crucial determinant of blood glucose
homeostasis due to its capacity to slow gastric emptying, increase
the secretion of insulin by the pancreas, and suppress the
secretion of glucagon by the pancreas (8–10). Since
GLP-1 is quickly degraded and deactivated by dipeptidyl peptidase-4
(DPP-4), DPP-4 inhibitors, including sitagliptin (STG),
vildagliptin and saxagliptin, have been developed as therapeutic
options for the treatment of type 2 diabetes (11,12). The
processing product of proglucagon GLP-2 also acts as a substrate of
DPP-4. GLP-2 is a pleiotropic hormone that has an effect on
numerous aspects of intestinal physiology, including epithelial
development, barrier function, digestion, absorption, motility and
blood flow (13,14). Potent anti-apoptotic effects are
exerted by GLP-2 in the gastrointestinal tract, resulting in GLP-2
being a promising focus when investigating novel therapies for the
treatment of intestinal diseases. Since DPP-4 rapidly degrades and
deactivates GLP-2, DPP-4 inhibitors are viewed as a novel method
for treating of intestinal inflammation (15,16). It
has previously been shown that DPP-4 inhibitors have a protective
effect on indomethacin-induced intestinal damage in rats (17,18).
However, in terms of colon carcinogenesis, exogenous and endogenous
GLP-2 has been identified as a factor that increases colon
carcinogenesis in azoxymethane-treated mice (19). Furthermore, since DPP-4 also
inactivates other chemokines that participate in tumor progression,
including C-X-C motif chemokine ligand (CXCL) 5 and CXCL12/stromal
cell-derived factor-1 (SDF-1), long-term use of a DPP-4 inhibitor
may accelerate tumorigenesis in the intestine (20). However, at present, the effect of
DPP-4 inhibitors on intestinal tumors has not been determined.
Materials and methods
Animals and diets
Six-week-old male
C57BL/6J-ApcMin/+/J mice weighing 18–22 (Charles
River Laboratories Japan, Inc., Yokohama, Japan) were used in the
present study. The animals were maintained in an animal colony with
controlled temperature (23°C) and light (12/12-h light-dark cycle)
at the Osaka Medical College (Osaka, Japan). Mice had free access
to chow pellets and tap water. The diets used in the present study
were standard rodent chow (normal diet; ND) containing 10 kcal% fat
and a high-fat diet (HFD) containing 60 kcal% fat (Charles River
Laboratories Japan, Inc.). Ethical approval for the experiments in
the present study was granted by the Animal Ethical Committee of
the Osaka Medical College (Osaka, Japan) and all procedures were
conducted according to the guidelines of the Institute for
Laboratory Animal Research at Osaka Medical College.
Protocol for experimental procedures
and tumor counts
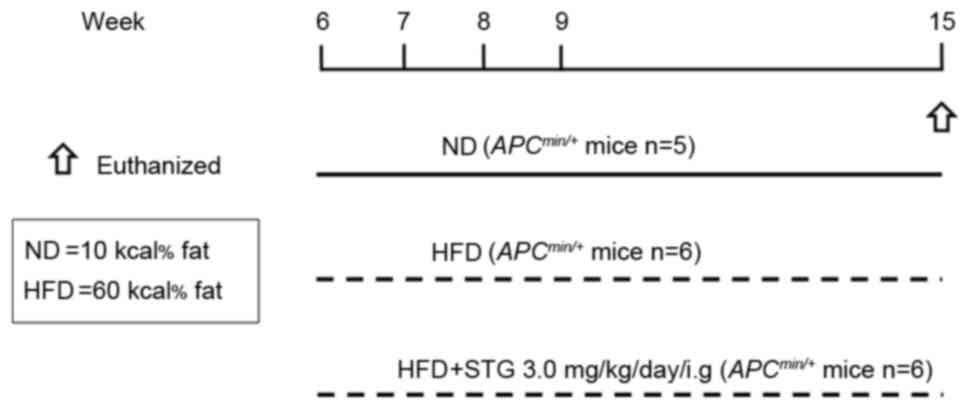
The following three groups of mice were used in the
present study: ND; HFD; and HFD + STG (HFDS). The experimental
design is shown in Fig. 1. The effect
of STG (0.3–3 mg/kg; intragastric gavage administration) on mucosal
DPP activity was previously investigated, and it was reported that
oral administration of STG dose-dependently suppressed mucosal DPP
activity (17). Therefore, in the
present study, the DPP-4 inhibitor STG phosphate monohydrate (3
mg/kg; Carbosynth Limited, Compton, UK) was administered to mice by
oral gavage every day during the experimental period. All mice were
sacrificed at 15 weeks of age. Following sacrifice, the entire
small intestine from the pylorus to the ileocecal junction was
excised, opened longitudinally, and rinsed with ice-cold PBS. The
entire small intestine was rolled up longitudinally using a wooden
stick, starting from the ileum and working toward the duodenum,
with the mucosa outwardly exposed, and fixed in a 10% formalin
solution. Tissue sections (4 µm) were hematoxylin and eosin stained
and analyzed by K.F. and T.I., who were blinded to the treatment
groups. The number, size and localization of tumors were
determined.
Measurement of plasma and mucosal
GLP-2, plasma CXCL5 and SDF-1
Mucosal scraped tissues were collected in lysis
buffer (TBS containing protease inhibitors; Complete Mini: Roche
Diagnostics, Indianapolis, IN, USA) with the DPP-4 inhibitor K-579
(Tocris Bioscience, Bristol, UK). Blood samples were collected into
chilled Eppendorf tubes containing 1 µl each of 0.5 M EDTA and 100
µM K-579. The samples were immediately centrifuged at 3,000 × g for
5 min and their supernatants and plasma were kept at −80°C until
measurement. Plasma and mucosal total GLP-2 was measured utilizing
a GLP-2 (mouse) ELISA kit (ALPCO Diagnostics, Salem, NH, USA)
according to the manufacturer's protocol. Plasma concentration of
CXCL5 and SDF-1 was also determined using a Mouse LIX Quantikine
ELISA kit and a Mouse CXCL12/SDF-1 alpha Quantikine ELISA kit
(R&D Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer's protocol.
Analysis of chemokines
A Mouse Chemokine Array kit (R&D Systems, Inc.)
was used to simultaneously detect the relative expression levels of
25 different chemokines. The array was performed according to the
manufacturer's protocol. Blots were developed using enhanced
chemiluminescence and a Fujifilm Imaging System (LAS-3000;
Fujifilm, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). The data represent five to six animals in each
treatment group. Comparisons were performed using one-way analysis
of variance or Kruskal-Wallis followed by Fisher's protected least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of chronic administration of
STG on body weight, plasma glucose level and number of small
intestinal tumors
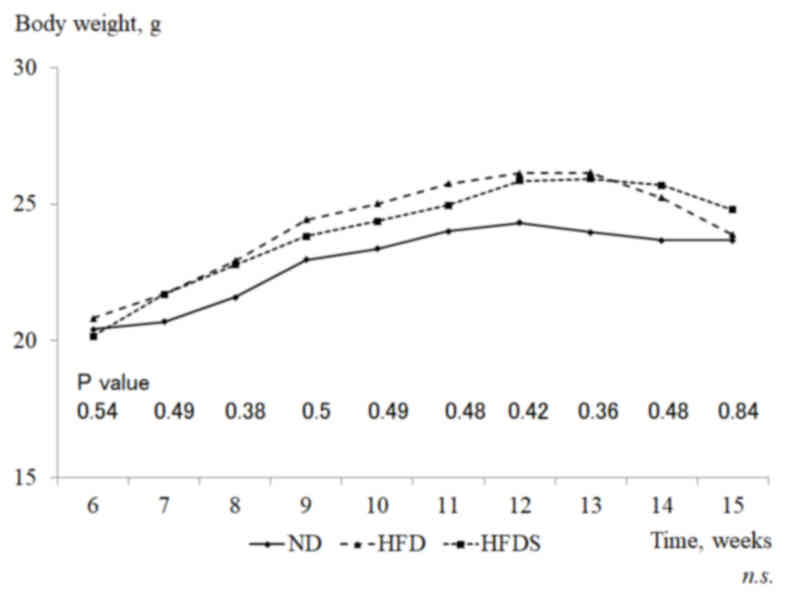
As shown in Fig. 2,
mice fed the HFD had increased mean gains in body weight compared
with mice fed the ND (ND, 20.5±0.4 to 24.3±1.2 g; HFD, 20.8±1.5 to
26.1±3.0 g), although the gains were not significantly different.
The administration of STG did not affect the body weight in the
mice fed the HFD. Mean body weights prior to euthanasia were
23.7±3.7, 23.8±3.5 and 24.8±3.2 g in the ND, HFD and HFDS groups,
respectively. Plasma glucose levels measured pre-sacrifice were
264.4±51.4, 252.8±5.9 and 264.8±29.5 mg/dl in the ND, HFD and HFDS
groups, respectively, and were unaffected by the administration of
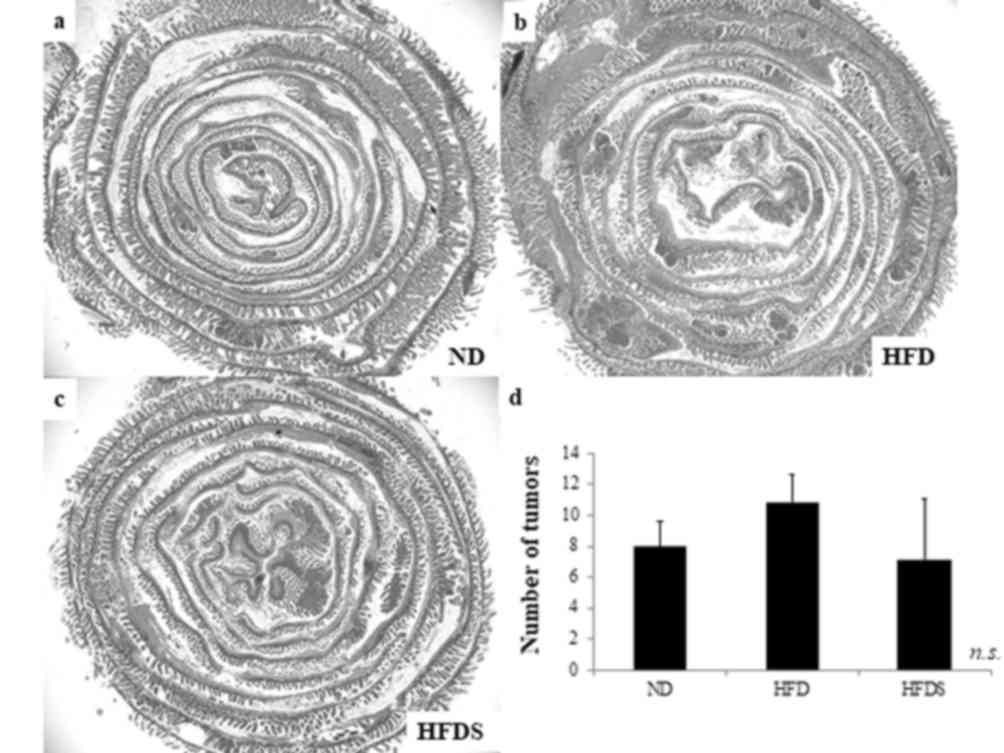
STG in mice fed the HFD. According to a previous study,
ApcMin/+ mice developed polyps mainly in the
small intestine (21). The mean
number of small intestinal tumors was 8.0±1.6, 10.8±1.8 and 7.2±3.9
in the ND, HFD and HFDS groups, respectively. The administration of
STG decreased the actual mean number of small intestinal tumors,
although not to the point of statistical significance (P=0.086;
Fig. 3).
Concentration of plasma and mucosal
GLP-2
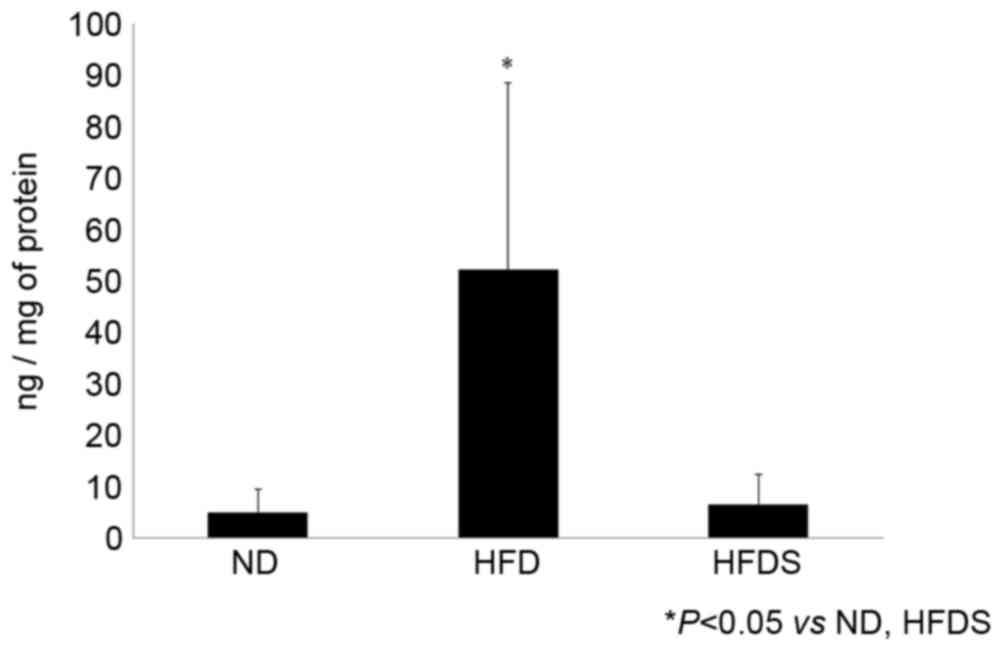
No significant difference was identified in plasma
GLP-2 concentrations among the three treatment groups, whereas the
concentration of mucosal GLP-2 was significantly elevated in the
mice fed the HFD. In the mice fed the HFDS, the concentration of
mucosal GLP-2 was significantly decreased (Fig. 4).
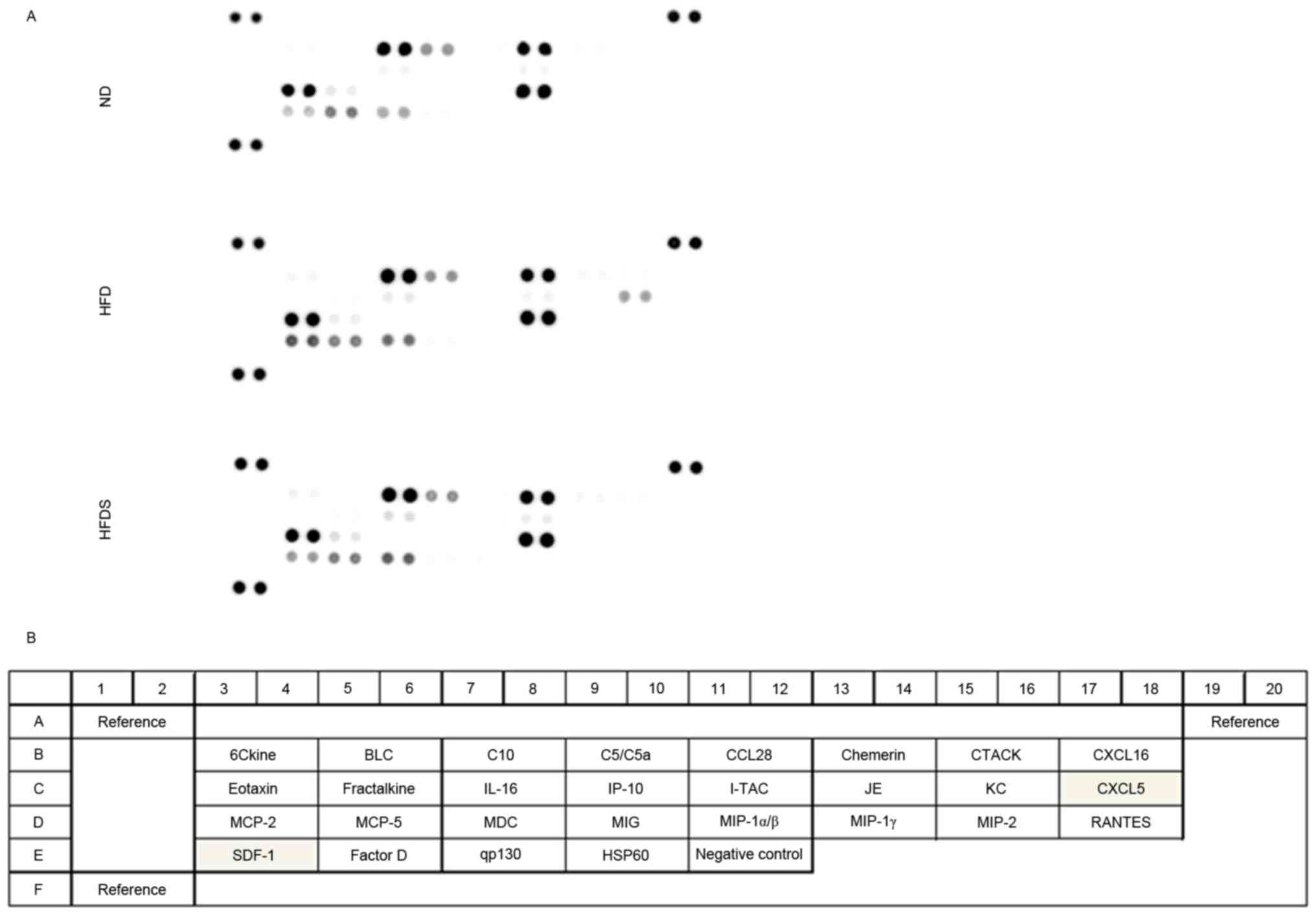
Analysis of chemokines
The chemokines evaluated using the chemokine array
(Fig. 5) were quantified using ELISA
(Fig. 6). As shown in Fig. 5, the chemokine array suggested
elevated concentrations of plasma CXCL5 and SDF-1 in the HFD group,
and chemokines in the HFDS group were decreased relative to those
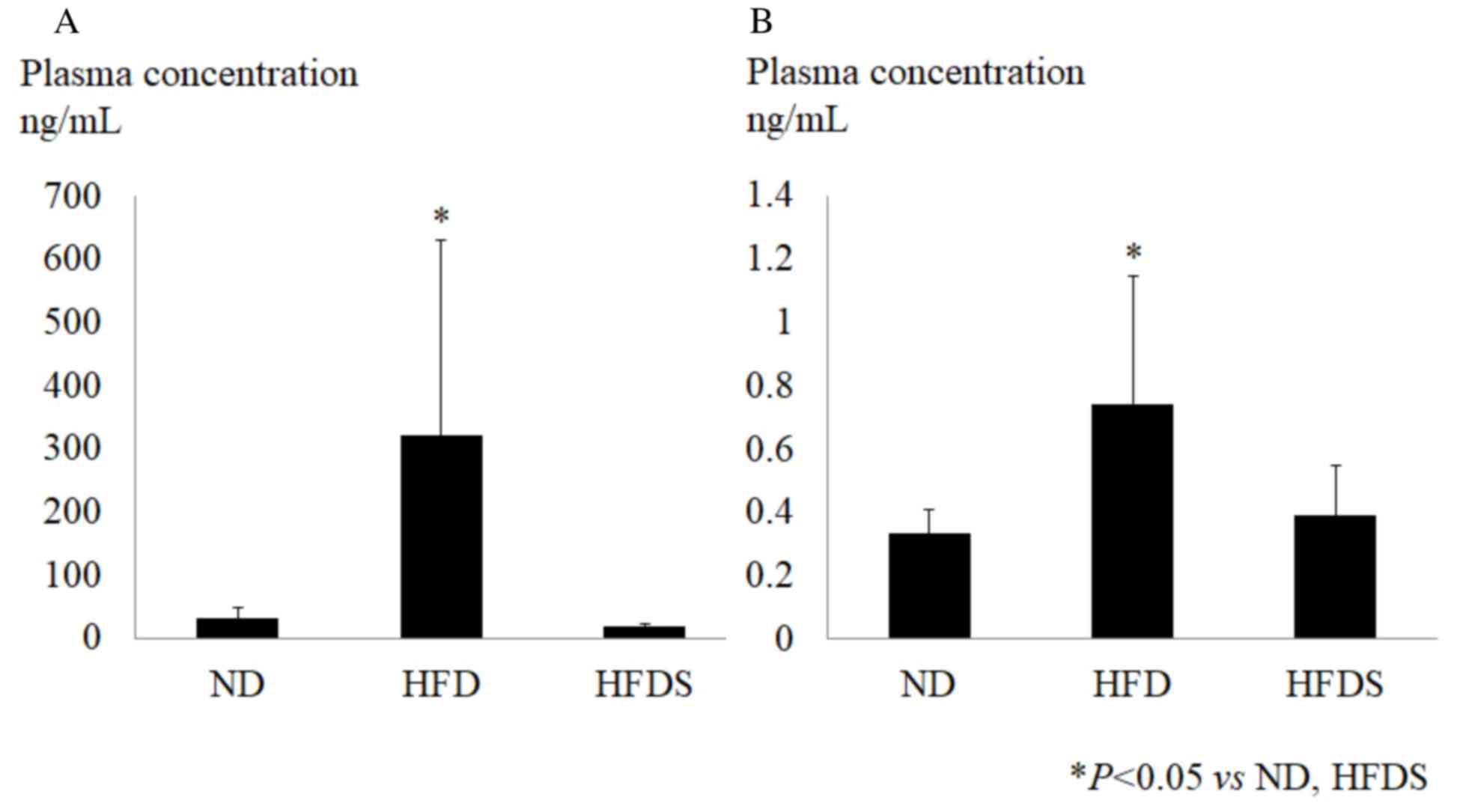
in the HFD group. Following the chemokine array, the plasma
concentrations of CXCL5 and SDF-1 were determined by ELISA. These
chemokines were significantly elevated in the HFD group and the
long-term administration of STG significantly suppressed the plasma
concentration of CXCL5 and SDF-1 (Fig.
6). This indicated that the long-term administration of STG
improved metabolic status, even though the body weight and feeding
plasma glucose level were not changed.
Discussion
It is well known that obesity, insulin resistance
and diabetes are associated with an increased risk of colon cancer
(5). Since DPP-4 inhibitors stabilize
incretin hormones, in previous years they have been widely used to
treat patients with type 2 diabetes mellitus. In addition, DPP-4
inhibitors stabilize hormones and cytokines, including GLP-2, SDF-1
and CXCL5, which are associated with tumor progression subsequent
to long-term use (20). At present,
few studies have examined the connection between DPP-4 inhibitors
and colon carcinogenesis. In the present study, the concentration
of mucosal GLP-2, plasma SDF-1 and plasma CXCL5 were markedly
increased in mice fed a HFD compared with mice fed a ND. Notably,
the long-term administration of STG significantly suppressed the
concentration of those hormones and chemokines and did not increase
the number of intestinal tumors in ApcMin/+
mice.
Previously, colon tumors were induced in
leptin-deficient (ob/ob) mice using the colon carcinogen
1,2-dimethylhydrazine and dextran sulfate sodium (22). In this model, the long-term
administration of STG significantly suppressed the mucosal
expression of IL-6, which was upregulated in the colons of ob/ob
mice, and significantly suppressed the number of colon tumors.
Ob/ob mice are obese as they harbor a mutation that causes them to
eat excessively; the administration of STG did not alter their
metabolic status, including their insulin resistance. Additionally,
DPP-4 is mainly expressed in the small intestine, but not in the
colon, and so DPP-4 inhibitors do not affect the mucosal DPP
activity in the colon (17,23). Therefore, in the previous study, the
effect of a DPP-4 inhibitor on tumorigenesis in the intestines of
mice could not be evaluated via assessment of the changes in
mucosal DPP and GLP, even though the results provided insights
about the role of a DPP-4 inhibitor (22).
CXCL5 is secreted from adipose tissue during states
of obesity and mediates insulin resistance (24). CXCL5 has a well-established role in
stimulating the chemotaxis of angiogenic neutrophils, particularly
in response to inflammation (25).
SDF-1 is also secreted from adipose tissue (26). Since SDF-1 secretion is positively
associated with the body mass index in humans and induces insulin
resistance, SDF-1 is considered one of the important contributing
risk factors for type 2 diabetes (27). Chemokines such as these are recognized
as important angiogenic factors and are reported to be associated
with cancer development (28). Since
they undergo N-terminal truncation by DPP-4, DPP-4 inhibition may
affect tumorigenesis in the intestine via increased concentrations
of CXCL5 and SDF-1. The SDF-1 concentration was reported to be
increased in Dpp-4−/− mice, but whether SDF-1 is
primarily regulated by DPP or not remains controversial (29,30). In
the present study, the number of small intestinal tumors in mice
fed a HFD to induce obesity were compared with mice fed a ND diet,
and the effects with and without STG were assessed. STG suppressed
the mucosal concentration of CXCL5 and SDF-1, which were
significantly increased in the mice fed the HFD. Since CXCL5 and
SDF-1 are substrates for DPP-4, the administration of STG is
hypothesized to increase the concentrations of CXCL5 and SDF-1.
However, in the present study, long-term treatment with STG in
conditionally obese mice paradoxically suppressed chemokines
associated with obesity. Since it has been reported that the
administration of SPP-4 inhibitor improved insulin resistance and
lipid metabolism (31,32), it was hypothesized that the long term
administration of STG improved the metabolic status of mice fed a
HFD, and as a result the expression of these chemokines was
decreased.
The mucosal concentration of GLP-2 in the small
intestine was significantly increased in mice fed the HFD compared
with the mice fed the ND in the present study. Since luminal
nutrients, including fatty acids, stimulate the production and
secretion of GLP-2 in the intestinal mucosa, a HFD may contribute
to intestinal tumorigenesis via increased mucosal GLP-2 (33,34).
Chronic exposure to a HFD significantly increases the concentration
of plasma GLP-2 and intestinal crypt-villus height (35). In the present study, similar to CXCL5
and SDF-1, mucosal GLP-2 increased in mice fed the HFD and was
significantly decreased by the long-term administration of STG.
These results suggest the possibility that the long-term
administration of STG may be a treatment option for patients who
are obese or have type 2 diabetes and are considered to have an
increased risk of developing colonic polyps and cancer. However,
the administration of STG generally increases the concentration of
GLPs, and the mechanism of the suppressive effect of long-term STG
administration on the mucosal concentration of GLP-2 in mice fed a
HFD remains to be elucidated. Therefore, additional studies are
required.
In conclusion, consistent with previous studies, the
present preclinical study demonstrated that chronic exposure to a
HFD increased the mucosal concentration of GLP-2, and the
concentrations of CXCL5 and SDF-1 in the plasma. However, the
long-term administration of STG in combination with HFD suppressed
the expression of GLP-2 and the evaluated chemokines. Since the
number of mice in the present study was small, the suppressive
effect of the DPP-4 inhibitor STG on intestinal carcinogenesis was
not determined. However, the long-term administration of STG did
not increase intestinal tumors in mice fed a HFD. Pending clinical
trials, the present results suggest that STG may be safe and
suitable treatment option for patients with type 2 diabetes who are
at increased risk of developing colonic polyps and cancer.
References
|
1
|
Tsugane S and Inoue M: Insulin resistance
and cancer: Epidemiological evidence. Cancer Sci. 101:1073–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville SE, Boye KS, Montgomery WS,
Iwamoto K, Okamura M and Hayes RP: Diabetes in Japan: A review of
disease burden and approaches to treatment. Diabetes Metab Res Rev.
25:705–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katanoda K, Matsuda T, Matsuda A, Shibata
A, Nishino Y, Fujita M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report of the trends in cancer incidence and mortality in
Japan. Jpn J Clin Oncol. 43:492–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vargas AJ and Thompson PA: Diet and
nutrient factors in colorectal cancer risk. Nutr Clin Pract.
27:613–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang XF and Chen JZ: Obesity, the
PI3K/Akt signal pathway and colon cancer. Obes Rev. 10:610–616.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berster JM and Göke B: Type 2 diabetes
mellitus as risk factor for colorectal cancer. Arch Physiol
Biochem. 114:84–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reimer MK, Holst JJ and Ahrén B: Long-term
inhibition of dipeptidyl peptidase IV improves glucose tolerance
and preserves islet function in mice. Eur J Endocrinol.
146:717–727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nadkarni P, Chepurny OG and Holz GG:
Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl
Sci. 121:23–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frezza EE, Wachtel MS and
Chiriva-Internati M: The multiple faces of glucagon-like
peptide-1-obesity, appetite, and stress: What is next? A review.
Dig Dis Sci. 52:643–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takasaki K, Takada H, Nakajima T, Ueno K,
Ushiki J and Higo K: Involvement of the active metabolites in the
inhibitory activity of K579 on rat plasma dipeptidyl peptidase IV.
Eur J Pharmacol. 505:237–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takasaki K, Nakajima T, Ueno K, Nomoto Y
and Higo K: Effects of combination treatment with dipeptidyl
peptidase IV inhibitor and sulfonylurea on glucose levels in rats.
J Pharmacol Sci. 95:291–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubé PE and Brubaker PL: Frontiers in
glucagon-like peptide-2: Multiple actions, multiple mediators. Am J
Physiol Endocrinol Metab. 293:E460–E465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drozdowski L and Thomson AB: Intestinal
hormones and growth factors: Effects on the small intestine. World
J Gastroenterol. 15:385–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartmann B, Thulesen J, Kissow H, Thulesen
S, Orskov C, Ropke C, Poulsen SS and Holst JJ: Dipeptidyl peptidase
IV inhibition enhances the intestinotrophic effect of glucagon-like
peptide-2 in rats and mice. Endocrinology. 141:4013–4020. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boushey RP, Yusta B and Drucker DJ:
Glucagon-like peptide 2 decreases mortality and reduces the
severity of indomethacin-induced murine enteritis. Am J Physiol.
277:E937–E947. 1999.PubMed/NCBI
|
|
17
|
Fujiwara K, Inoue T, Yorifuji N, Iguchi M,
Sakanaka T, Narabayashi K, Kakimoto K, Nouda S, Okada T, Ishida K,
et al: Combined treatment with dipeptidyl peptidase 4 (DPP4)
inhibitor sitagliptin and elemental diets reduced
indomethacin-induced intestinal injury in rats via the increase of
mucosal glucagon-like peptide-2 concentration. J Clin Biochem Nutr.
56:155–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue T, Higashiyama M, Kaji I, Rudenkyy
S, Higuchi K, Guth PH, Engel E, Kaunitz JD and Akiba Y: Dipeptidyl
peptidase IV inhibition prevents the formation and promotes the
healing of indomethacin-induced intestinal ulcers in rats. Dig Dis
Sci. 59:1286–1295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iakoubov R, Lauffer LM, Trivedi S, Kim YI
and Brubaker PL: Carcinogenic effects of exogenous and endogenous
glucagon-like peptide-2 in azoxymethane-treated mice.
Endocrinology. 150:4033–4043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim JB and Chung HW: Serum ENA78/CXCL5,
SDF-1/CXCL12, and their combinations as potential biomarkers for
prediction of the presence and distant metastasis of primary
gastric cancer. Cytokine. 73:16–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson RL and Fleet JC: Animal models of
colorectal cancer. Cancer Metastasis Rev. 32:39–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yorifuji N, Inoue T, Iguchi M, Fujiwara K,
Kakimoto K, Nouda S, Okada T, Kawakami K, Abe Y, Takeuchi T and
Higuchi K: The dipeptidyl peptidase-4 inhibitor sitagliptin
suppresses mouse colon tumorigenesis in type 2 diabetic mice. Oncol
Rep. 35:676–682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakanaka T, Inoue T, Yorifuji N, Iguchi M,
Fujiwara K, Narabayashi K, Kakimoto K, Nouda S, Okada T, Kuramoto
T, et al: The effects of a TGR5 agonist and a dipeptidyl peptidase
IV inhibitor on dextran sulfate sodium-induced colitis in mice. J
Gastroenterol Hepatol. 30:(Suppl 1). S60–S65. 2015. View Article : Google Scholar
|
|
24
|
Chavey C and Fajas L: CXCL5 drives obesity
to diabetes, and further. Aging (Albany NY). 1:674–677. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nunemaker CS, Chung HG, Verrilli GM,
Corbin KL, Upadhye A and Sharma PR: Increased serum CXCL1 and CXCL5
are linked to obesity, hyperglycemia, and impaired islet function.
J Endocrinol. 222:267–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim D, Kim J, Yoon JH, Ghim J, Yea K, Song
P, Park S, Lee A, Hong CP, Jang MS, et al: CXCL12 secreted from
adipose tissue recruits macrophages and induces insulin resistance
in mice. Diabetologia. 57:1456–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blogowski W, Serwin K, Budkowska M, Salata
D, Dolegowska B, Lokaj M, Prowans P and Starzynska T: Clinical
analysis of systemic and adipose tissue levels of selected
hormones/adipokines and stromal-derived factor-1. J Biol Regul
Homeost Agents. 26:607–615. 2012.PubMed/NCBI
|
|
28
|
Kitahara CM, Trabert B, Katki HA,
Chaturvedi AK, Kemp TJ, Pinto LA, Moore SC, Purdue MP, Wentzensen
N, Hildesheim A and Shiels MS: Body mass index, physical activity,
and serum markers of inflammation, immunity, and insulin
resistance. Cancer Epidemiol Biomarkers Prev. 23:2840–2849. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yazbeck R, Howarth GS and Abbott CA:
Dipeptidyl peptidase inhibitors, an emerging drug class for
inflammatory disease? Trends Pharmacol Sci. 30:600–607. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Busso N, Wagtmann N, Herling C,
Chobaz-Péclat V, Bischof-Delaloye A, So A and Grouzmann E:
Circulating CD26 is negatively associated with inflammation in
human and experimental arthritis. Am J Pathol. 166:433–442. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakagami H, Pang Z, Shimosato T, Moritani
T, Kurinami H, Koriyama H, Tenma A, Shimamura M and Morishita R:
The dipeptidyl peptidase-4 inhibitor teneligliptin improved
endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp
rat model of metabolic syndrome. Hypertens Res. 37:629–635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li CL, Zhao LJ, Zhou XL, Wu HX and Zhao
JJ: Review on the effect of glucagon-like peptide-1 receptor
agonists and dipeptidyl peptidase-4 inhibitors for the treatment of
non-alcoholic fatty liver disease. J Huazhong Univ Sci Technolog
Med Sci. 35:333–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang JH, Inoue T, Higashiyama M, Guth PH,
Engel E, Kaunitz JD and Akiba Y: Umami receptor activation
increases duodenal bicarbonate secretion via glucagon-like
peptide-2 release in rats. J Pharmacol Exp Ther. 339:464–473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Inoue T, Wang JH, Higashiyama M, Rudenkyy
S, Higuchi K, Guth PH, Engel E, Kaunitz JD and Akiba Y: Dipeptidyl
peptidase IV inhibition potentiates amino acid- and bile
acid-induced bicarbonate secretion in rat duodenum. Am J Physiol
Gastrointest Liver Physiol. 303:G810–G816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baldassano S, Amato A, Cappello F, Rappa F
and Mulè F: Glucagon-like peptide-2 and mouse intestinal adaptation
to a high-fat diet. J Endocrinol. 217:11–20. 2013. View Article : Google Scholar : PubMed/NCBI
|