Introduction
Retinoblastoma (RB) is the most common type of
malignant intraocular cancer in children <5 years old (1). In the majority of cases, particularly in
developing countries, RB is only detected at the late stages of the
cancer, which severely threatens the lives of the patients
(2). Prior to the 1990s, surgical
removal of the afflicted eyes, followed by external-beam
radiotherapy, was the major treatment method for RB; however, the
approach was associated with high morbidity and craniofacial
deformity (3). At present, the most
effective therapy against RB is chemotherapy combined with
immunotherapy, which is cytotoxic to RB cells, increasing cell
apoptosis (2). Despite its high
efficacy, the current therapy remains somewhat unsatisfactory,
owing to the adverse effects of chemotherapy. Thus, the development
of mild therapeutic modalities to improve the outcome and survival
of RB is required.
Numerous signaling molecules are relevant to the
oncogenesis and development of cancer. One of these signaling
transduction pathways is the hedgehog (HH) pathway, which governs
processes such as embryonic development, cell differentiation, cell
proliferation and tissue patterning (4). The most studied member of the vertebrate
HH family is sonic hedgehog (SHH). SHH exerts its function by
selectively activating the transcription factors of different
genes. Altered SHH pathway activation has been observed in
different types of solid and non-solid cancer (5). The selective regulation of SHH
production can influence cell apoptosis and the metastasis of
pancreatic and ovarian cancer cells through multiple mechanisms
(6–8).
These findings reveal the close connection between the SHH pathway
and other critical pathways, which include phosphoinositide-3
kinase (PI3K)/Akt and nuclear factor κ-light-chain-enhancer of
activated B cells pathways. Considering that tumorigenesis
initiates aberrant apoptotic processes, the aforementioned data
suggest that the SHH pathway may exert control over apoptosis in
cancer cells. Moreover, a recent study regarding the SHH pathway
has validated the critical role of the SHH pathway in the
progression of RB (9). From a
therapeutic point of view, it is therefore desirable to further
evaluate how regulation of the SHH pathway affects RB cell
survival.
In the current study, the expression of SHH
in the human RB WERI-Rb-1 cell line was regulated using specific
short hairpin RNAs (shRNAs). Cell viability and apoptosis were
measured to investigate the function of SHH in the survival of RB
cells. As PI3K/Akt serves such a central role in cell apoptosis,
further experiments subjecting WERI-Rb-1 cells to insulin-like
growth factor 1 (IGF-1) were conducted to assess the possible
mechanism by which SHH drives the promotion of RB. IGF-1 is a
pleiotropic polypeptide with a wide range of actions in the central
and peripheral nervous systems, which protects neurons against cell
death induced by amyloidogenic derivatives, glucose or serum
deprivation via the activation of intracellular pathways
implicating PI3K/Akt (10).
Inhibition of SHH significantly increased the apoptotic rate in the
WERI-Rb-1 cells and resulted in a decrease in cell viability.
Downregulation of PI3K/Akt is therefore required for SHH
inhibition-based anti-RB therapies. The findings in the present
study demonstrate a clear interaction between SHH and the PI3K/Akt
pathway in the survival of RB cells and could aid the development
of therapies for the prevention of this malignancy.
Materials and methods
Chemicals and cell culture
Antibodies against SHH and phosphorylated (p)-PI3K
(cat. nos. bs-1544R and bs-5538R, respectively) were purchased from
Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China).
Antibodies against cleaved caspase-3 and cleaved poly (ADP-ribose)
polymerase (PARP) (cat. nos. ab2302 and ab32561, respectively) were
purchased from Abcam (Cambridge, MA, USA). Antibodies against
B-cell CLL/lymphoma 2 (Bcl-2), Bcl-2 associated X (Bax) and PI3K
(cat. nos. BA0412, BA0315 and BA1352, respectively) were purchased
from Boster Systems, Inc. (Pleasanton, CA, USA). Antibodies against
p-Akt (Ser 473), Akt and β-actin (cat. nos. sc-8312, sc-135651 and
sc-47778, respectively) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The secondary IgG antibodies
conjugated to horseradish peroxidase (cat. nos. WLA023 and WLA024)
were purchased from Wanleibio Co., Ltd. (Shanghai, China). The
PI3K/Akt agonist IGF-1 (cat. no. 10598-R023) was obtained from Sino
Biological Inc. (Beijing, China). Transfection agents (cat. no.
c1507) were purchased from Applygen Technologies Inc. (Beijing,
China). A specific SHH-targeted shRNA
(5′-CCCGACATCATATTTAAGGAT-3′) and control shRNA
(5′-GCTGTTGGACAGCGAGACCAT-3′) were constructed by Wanlei Life
Sciences (Shenyang, China) in accordance with a previous study
(11). The human RB WERI-Rb-1 cell
line was obtained from Wanlei Life Sciences and cultured in
RPMI-1640 medium (cat. no. 31800-014; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal
bovine serum (FBS; cat. no. SH30084.03; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) and a 1% (v/v) antibiotics mixture
(penicillin/streptomycin; cat. no. E485-20ML; Amresco, LLC, Solon,
OH, USA) in an atmosphere of 95% air and 5% CO2 at
37°C.
Knockdown of SHH gene in WERI-Rb-1
cells
Sequences of different shRNAs (1.2 µg/100 µl) were
ligated into pRNAH1.1 plasmid (Sangon Biotech, Co., Ltd., Shanghai,
China) to form transfection vectors, and transfection was conducted
using HiGene transfection reagent (cat. no. C1507) from Applygen
Technologies Inc., according to the manufacturer's protocol. Cells
were divided into three groups: A control group of untransfected
WERI-Rb-1 cells; an shNC group of WERI-Rb-1 cells transfected with
a pRNAH1.1-NC (non-targeted shRNA) plasmid; and an shSHH group of
WERI-Rb-1 cells transfected with a pRNAH1.1-SHH
(SHH-targeted shRNA) plasmid. Each treatment was performed
in triplicate. Stable transfected cells were screened in RPMI-1640
medium in the presence of G418 (0.5 µg/µl) for subsequent
experiments. The knockdown efficiency of SHH by shRNA was
determined using reverse transcription-quantitative PCR (RT-qPCR)
and western blot analysis.
RT-qPCR
For the detection of mRNA, whole RNAs in cells under
different conditions were extracted using an RNA extraction kit
according to the manufacturer's instructions (cat. no. RP1201;
BioTeke Corporation, Beijing, China). β-actin was selected as the
internal reference gene. cDNA templates of SHH were attained
by reverse transcribing the RNA using an RT-PCR kit (cat. no.
PR6502; BioTeke Corporation), and the final RT-qPCR reaction
mixture of volume 20 µl contained 10 µl SYBR Green master mix, 0.5
µl of each primer (10 µM) (SHH, forward,
5′-AGCAACAGCAACGCAAAG-3′ and reverse, 5′-AATAGCCAGGAGAGGAGGA-3′;
and β-actin forward, 5′-TTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′), 1 µl of the cDNA template and 8 µl
RNase-free H2O. Thermal cycling parameters for the
amplification were as follows: A denaturation step at 95°C for 10
min, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and
72°C for 30 sec. The relative expression level of the targeted gene
was calculated with Data Assist Software version 3.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
2−ΔΔCq method (12). Each
assay was repeated ≥3 times.
Western blotting assay
The whole protein product in each group was
extracted using Whole Protein Extraction kit according to the
manufacturer's instructions (Wanleibio Co., Ltd.). β-actin was used
as an internal reference protein. Protein concentration was
determined using a bicinchoninic acid assay. Next, 40 µg protein
was subject to SDS-PAGE with a 10% gel. Following the transfer of
targeted proteins onto polyvinylidene difluoride sheets, the
membranes were washed with Tris-buffered saline plus Tween 20
(TTBS; cat. no. WLA025; Wanleibio Co., Ltd.) for 5 min and then
blocked with skimmed milk powder solution for 1 h. Primary
antibodies against SHH (1:500), cleaved caspase-3 (1:1,000),
cleaved-PARP (1:1,000), Bcl-2 (1:400), Bax (1:400), p-PI3K (1:500),
PI3K (1:400), p-Akt (1:200), Akt (1:200) or β-actin (1:1,000) were
added and the membranes were incubated at 4°C overnight. After an
additional four washes with TTBS, secondary IgG antibodies
conjugated to horseradish peroxidase (1:5,000) were incubated with
the membranes for 45 min at 37°C. Following a further six washes
with TTBS, the blots were developed using Beyo ECL Plus reagent
(Beyotime Institute of Biotechnology, Haimen, China) and the
results were recorded in the Gel Imaging System (cat. no. WD-9413B;
Beijing Liuyi Instrument Factory, Beijing, China). The relative
expression levels of SHH in different groups were calculated with
the Gel-Pro-Analyzer (Media Cybernetics, Inc., Rockville, MD,
USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
test
The viability of different groups of WERI-Rb-1 cells
was measured using an MTT assay. A total of 50 µl exponentially
growing cells (4×103 cells/ml) were seeded into a
96-well plate and cultured at 37°C for 120 h. For each group, the
cell viabilities at 24, 48, 72, 96 and 120 h were all determined
and each time point was represented by five replicates. In brief, 5
mg/ml MTT was added to each well. After incubating for an
additional 4 h at 37°C, 200 µl dimethyl sulfoxide was added and the
cell viabilities of different groups were measured with a
microplate reader at 490 nm.
Flow cytometry
WERI-Rb-1 cells in different groups were cultured in
RPMI-1640 medium supplemented with 10% FBS at 37°C and collected by
centrifugation at 626 × g for 5 min at 37°C. A flow cytometry assay
was conducted to determine the effect of SHH-knockdown on
the cell-cycle distribution of WERI-Rb-1 cells. In brief, cells
were fixed with 70% ethanol at 4°C for 2 h. A total of 500 µl
propidium iodide (PI)-fluorescein isothiocyanate (FITC) (2 µg/ml)
was then added to different samples to stain DNA in the dark at 4°C
for 30 min. After a 20-min incubation at room temperature, the DNA
contents of the cells were analyzed using a flow cytometer (Accuri
C6; BD Biosciences, Franklin Lakes, NJ, USA).
Colony formation in soft agar
The capability of cancer cells for
anchorage-independent growth was measured by colony formation
assay. Cells that had been transfected with different plasmids were
seeded into RPMI-1640 medium containing 3% methylcellulose at a
concentration of 500 cells per plate. After 3 weeks in culture, the
numbers of colonies were counted using an inverted phase contrast
microscope (cat. no. AE31; Motic Incorporation, Ltd., Causeway Bay,
Hong Kong). Colony formation rate=colony number/inoculation cell
number ×100.
Determination of the effect of
SHH-knockdown on the apoptotic process in WERI-Rb-1 cells
To determine the effect of SHH-knockdown on
the apoptotic process of WERI-Rb-1 cells, cells were first probed
with an Annexin V-FITC Apoptosis Detection kit (Wanleibio Co.,
Ltd.) to determine the apoptotic rates according to the
manufacturer's protocol. In brief, 5 µl annexin V was added to
different wells. Following incubation with annexin V for 10 min at
room temperature, the cells were resuspended with 1X binding buffer
(Annexin V-FITC Apoptosis Detection kit) and 5 µl PI was added.
Next, the apoptotic rates were analyzed using FACScan flow
cytometry (Accuri C6; BD Biosciences, Franklin Lakes, NJ, USA). The
apoptotic cell rate [upper right quadrant advanced-stage apoptosis
cell percentage (UR) + lower right quadrant prophase apoptosis cell
percentage (LR)-whole apoptosis cell percentage] was equal to the
sum of the late apoptotic rate (UR) and the early apoptotic rate
(LR). Additionally, the morphological changes of the nuclei in
different groups were detected using a Hoechst staining kit,
according to the manufacturer's instructions (Beyotime Institute of
Biotechnology) and the results were recorded using a fluorescence
microscope (IX53; Olympus Corporation, Tokyo, Japan) under ×400
magnification. Cells with nuclei stained bright blue were judged to
be Hoechst-positive and undergoing apoptosis. The expression of
apoptosis-associated molecules, including cleaved caspase-3,
cleaved PARP, Bcl-2, Bax, p-PI3K, PI3K, p-Akt and Akt, was
determined by western blot analysis, as aforementioned.
Determination of effect of
SHH-knockdown on the PI3K/Akt pathway in WERI-Rb-1 cells
To explain the mechanism driving the function of
SHH in RB, WERI-Rb-1 cells in shNC and shSHH groups were
administered with 1 µg/10 µl of the PI3K/Akt agonist IGF-1 for 48
h. In total, four groups were set up: The NC group, with
shNC-transfected WERI-Rb-1 cells; the shSHH group, with
shSHH-transfected WERI-Rb-1 cells; the NC+IGF-1 group, with
shNC-transfected cells treated with IGF-1; and the shSHH+IGF-1
group, with shSHH-transfected cells treated with IGF-1. Following
incubation, the cell viability, apoptotic rates and expression of
p-PI3K, PI3K, p-Akt, Akt, cleaved caspase-3, cleaved PARP, Bcl-2
and Bax were determined as aforementioned.
Statistical analysis
All statistical analysis and graph manipulation was
conducted using R version 3.2.1 (https://cran.r-project.org/doc/FAQ/R-FAQ.html#Citing-R).
Data are expressed as the mean ± standard deviation. Differences
between multiple groups were determined based on the
least-significant differen-ce method, with P<0.05 considered to
indicate a significant difference.
Results
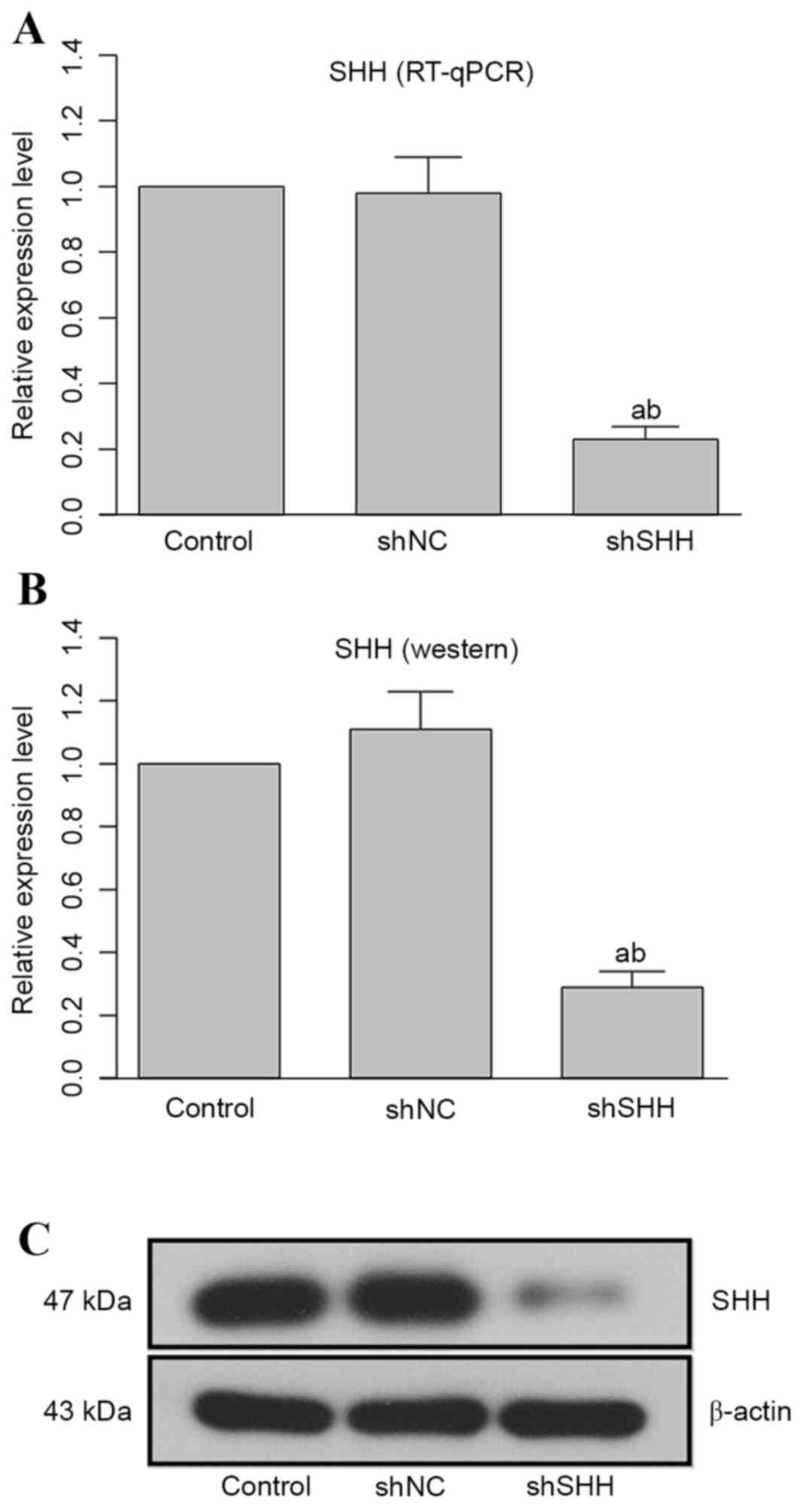
Transfection of specific shRNA reduces
the production of SHH at mRNA and protein levels
Transfection of WERI-Rb-1 cells with
SHH-specific shRNA markedly attenuated the expression of SHH
at the mRNA and protein levels, and the difference between the
shSHH group and the control or shNC groups was statistically
different (Fig. 1; both
P<0.05).
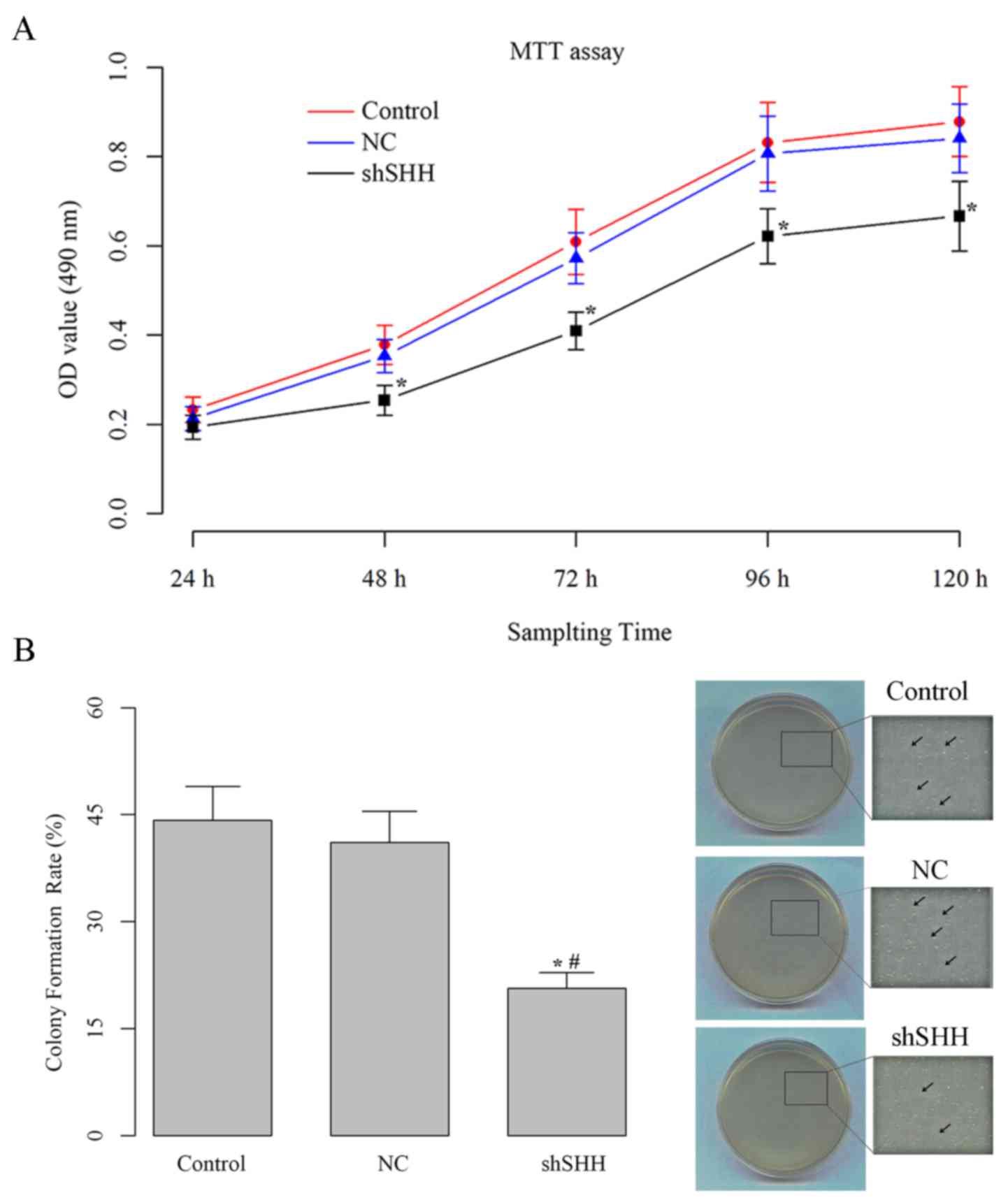
Knockdown of SHH gene decreases cell
viability and anchorage independent growth ability of WERI-Rb-1
cells
The cell viability of the different groups were
measured using an MTT assay. The transfection of
SHH-specific shRNA reduced the viability of the RB cells
(Fig. 2A). The negative effect of
shRNA transfection on cell viability could be observed from 48 h
after incubation, and the difference between the shSHH group and
the control or shNC groups was statistically different at the last
four sampling time points (all P<0.05). Additionally, the
anchorage-independent growth ability of WERI-Rb-1 cells was
markedly decreased by knockdown of SHH (Fig. 2B). The colony formation rate of cells
in the shSHH group (20.7±2.2%) was almost half of those in the
control (44.2±4.7%) and shNC (41.1±4.4%) groups, and the difference
was statistically significant (both P<0.05).
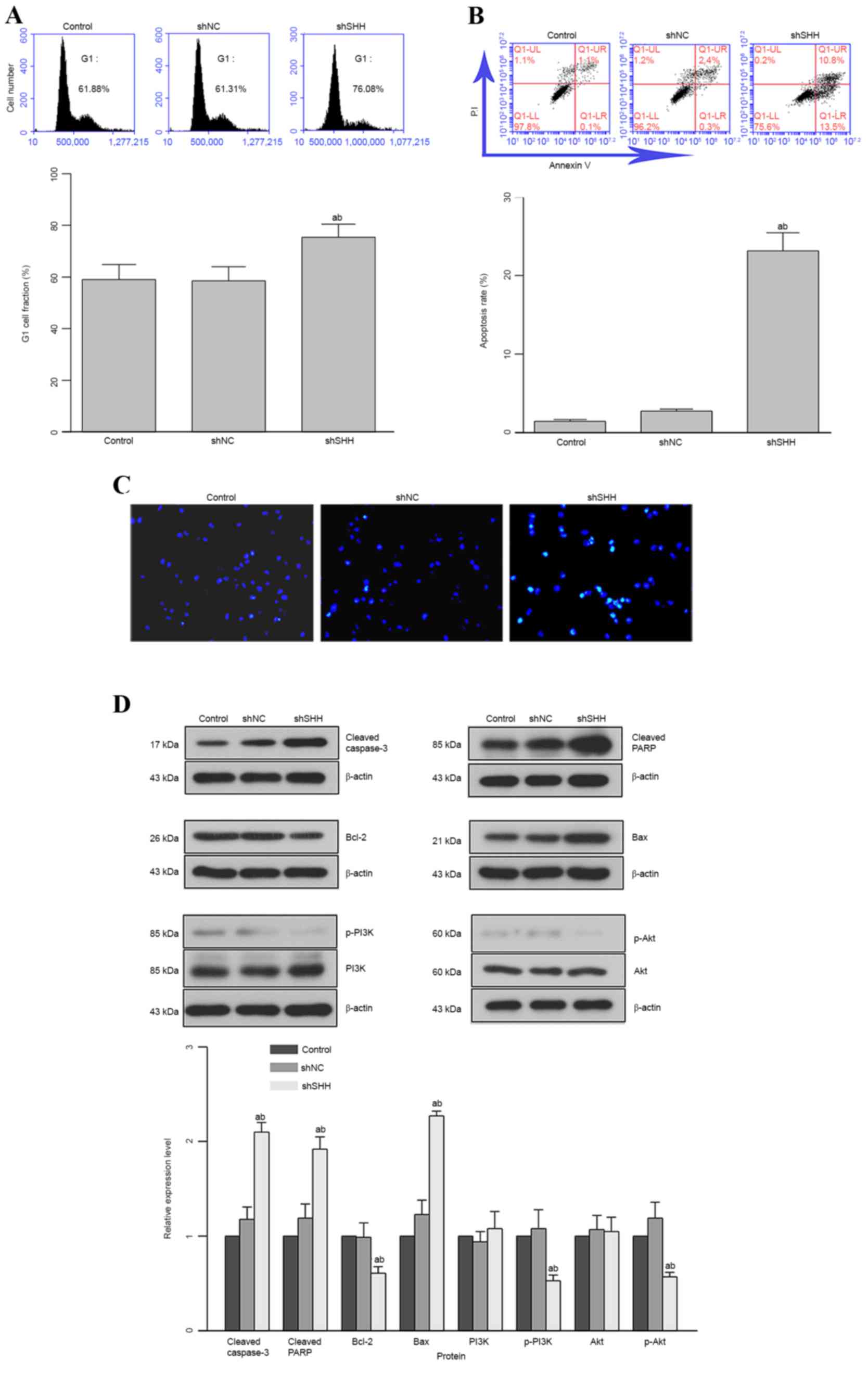
Knockdown of SHH gene induces G1 cell
cycle arrest and apoptosis in WERI-Rb-1 cells
The cell-cycle distribution and apoptosis of cells
under different conditions were analyzed with flow cytometry. The
cell-cycle distribution analysis demonstrated a G1 cell-cycle
arrest in WERI-Rb-1 cells administered with SHH-specific
shRNA (Fig. 3A). The percentage of
cells arrested in the G1 phase in the shSHH group (75.4±5.0%) was
markedly higher than those in control (58.9±5.8%) and shNC
(58.3±5.6%) groups. The downregulation of SHH also concomitantly
decreased the fraction of cells in the S phase, representing a halt
in cell proliferation through G1-phase cell-cycle arrest.
Consistent with the results of cell-cycle distribution, the cell
apoptotic rate in the shSHH group was also significantly higher
than those in the other two groups (P<0.05; Fig. 3B). The morphological alteration of
cell nuclei in different groups was observed with Hoechst staining.
More Hoechst-positive nuclei were recorded in the shSHH group than
in the control or shNC groups (Fig.
3C). To further reveal the function of SHH in the induction of
apoptosis in WERI-Rb-1 cells, the expression of indicators
associated with cell apoptosis was analyzed: It was found that the
expression of pro-apoptotic molecules (cleaved caspase-3, cleaved
PARP and Bax) was upregulated, whereas the expression of
anti-apoptotic molecules (Bcl-2, p-PI3K and p-Akt) was
downregulated (Fig. 3D). On the basis
of quantitative analyses, the difference in relative expression
level between the shSHH group and the control or shNC groups was
statistically different for these 6 groups (Fig. 3D; P<0.05).
Knockdown of SHH gene inhibits the
activation of the PI3K/Akt pathway
The effect of SHH-knockdown on the PI3K/Akt
pathway was further analyzed by administering cells in the shNC and
shSHH groups with IGF-1. IGF-1 treatment increased cell viability,
which was attenuated by transfection with shSHH (Fig. 4A). However, the difference between the
untreated shSHH and shSHH+IGF-1 groups was not statistically
significant (P>0.05). Contrary to the results of the MTT assay,
increased apoptosis induced by knockdown of SHH was markedly
inhibited by IGF-1; the difference between the untreated shSHH and
shSHH+IGF-1 groups was statistically significant (P<0.05;
Fig. 4B). Moreover, following
administration of IGF-1, the expression patterns of
apoptosis-related molecules in the shSHH+IGF-1 group were reversed
with regards to the control when compared with the untreated shSHH
group (Fig. 4C).
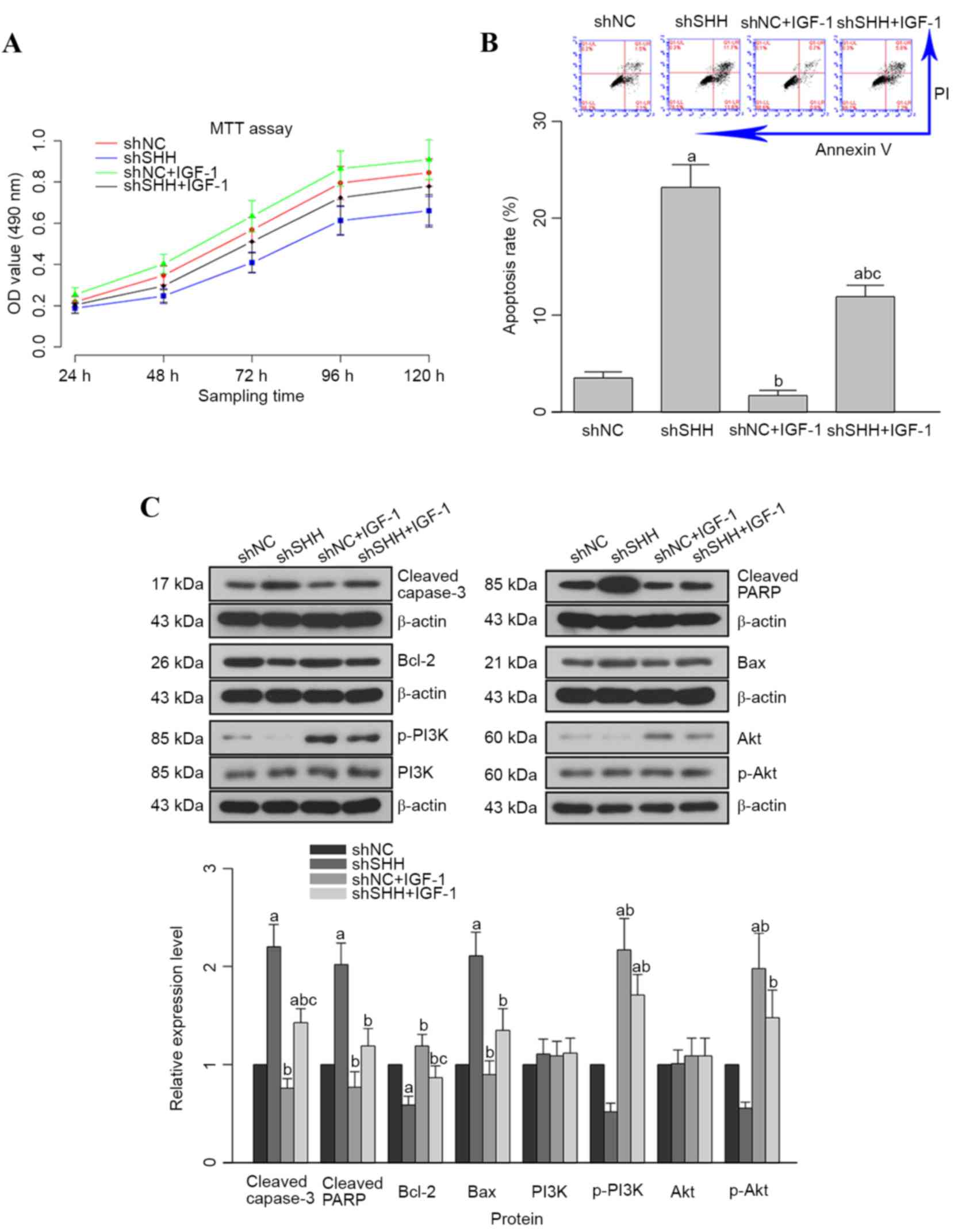 | Figure 4.Administration of PI3K/Akt agonist
IGF-1 increases cell viability and inhibits apoptosis in WERI-Rb-1
cells. (A) Quantitative analyses results of MTT assay. (B)
Representative image and quantitative analysis result of western
blotting assay; the expression of anti-apoptosis molecules (Bcl-2,
p-PI3K and p-Akt) was upregulated, while the expression of
pro-apoptosis molecules (cleaved caspase 3, cleaved PARP and Bax)
was inhibited. (C) Representative image and quantitative analysis
result of apoptosis. aP<0.05 vs. shNC group;
bP<0.05 vs. shSHH group; cP<0.05 vs.
shNC+IGF-1 group. p-PI3K, phosphorylated phosphoinositide-3 kinase;
IGF-1, insulin-like growth factor 1; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Bcl-2, B-cell chronic lymphocytic lymphoma 2; PARP, poly
(ADP-ribose) polymerase; Bax, Bcl-2-associated X; shSHH, short
hairpin RNA targeting SHH; shNC, non-targeting short hairpin RNA;
OD, optical density. |
Discussion
Recent studies have demonstrated that levels of SHH
are elevated in multiple types of human cancer (13–17), which
subsequently results in the aberrant activation of the HH signaling
cascade. Thus, the SHH-dependent initiation of the HH pathway has
been hypothesized to be a critical mechanism for the promotion of
tumor cell viability. Emerging research based on different types of
cancer has validated this hypothesis by revealing multiple
important pro-cancer pathways associated with the dysregulated
expression of SHH (7,8,18).
Consequently, the role of SHH in RB was investigated in the present
study, the major finding of which was that knockdown of SHH
decreased the proliferation of RB cells and induced apoptosis.
Moreover, the effect of SHH in RB was mediated through a
PI3K/Akt-dependent manner.
Choe et al (9)
verified the aberrant expression of SHH in patients with RB and
identified SHH as potential therapeutic target for RB treatment.
However, the study failed to provide a comprehensive mechanism to
underpin these findings. Indicating that SHH is involved in
oncogenesis and the development of RB, the expression of SHH
was downregulated by a SHH-specific shRNA in the human RB
WERI-Rb-1 cell line in the current study. Following transfection,
the cell viability and anchorage-independent growth ability were
markedly decreased. Indeed, the anchorage-independent growth
ability was representative of the oncogenicity of cells. Thus, it
can be inferred that the knockdown of SHH evidently reduces
the ability of RB to transform into a malignancy. SHH serves a
central role in embryonic development and controls key genes that
modulate cell proliferation (19)
directly and indirectly (4,20). During carcinogenesis, SHH exerts its
function in a similar pattern to normal development and organ
growth, but this aberrant signaling activity promotes the
recapitulation of the development ability in cancer cells.
In addition, in the present study, expression of
SHH not only promoted the growth of RB cells, but also
inhibited apoptosis in RB cells. As illustrated by flow cytometry,
knockdown of SHH induced G1 cell-cycle arrest and apoptosis
in the WERI-Rb-1 cell line. At the protein level, the expression of
certain apoptosis-related indicators was also detected.
Pro-apoptotic factors, including cleaved caspase-3, cleaved PARP
and Bax, were all upregulated following SHH shRNA
transfection. By contrast, the activity of anti-apoptotic factors,
including Bcl-2, PI3K and Akt, were inhibited when SHH
expression was knocked down. As aforementioned, SHH-related
pathways do not always proceed directly, but rather induce a
complex network of cascades that cross-talk with other pathways to
successfully exert a role in different biological processes
(21,22). The PI3K/Akt signal transduction
pathway is closely associated with SHH. Previous studies have shown
that SHH can promote the epithelial-mesenchymal transition (EMT) in
ovarian cancer, and promote metastasis and lymphangiogenesis in
gastric cancer in a PI3K/Akt dependent manner (8,18). Riobó
et al (23) suggested that
PI3K/Akt has a synergistic function in the SHH signaling in
embryonic development and cancer. To investigate further the
interaction between PI3K/Akt and SHH in RB, WERI-Rb-1 cells that
had been transfected with SHH-specific shRNA were treated
with IGF-1 in the present study. This treatment significantly
increased the expression of anti-apoptotic molecules, while
decreasing the apoptotic rate of SHH-knockdown RB cells.
These results clearly demonstrated that the activation of the
PI3K/Akt pathway blocked the antitumor effect of
SHH-knockdown. Prior investigation suggests that it is
possible that the effect of PI3K/Akt pathway on SHH signaling is
modulated through the enhancement of Gli-dependent transcriptional
activity (18), which requires
further study. However, the role of PI3K/Akt in regulating the SHH
pathway varies between cell types, as PI3K/Akt is critical for
SHH-mediated EMT and metastasis in multiple cancer types (18). Therefore, the suppression of the
PI3K/Akt pathway is key for the development of SHH-based anti-RB
therapies. It is thus recommended that treatment modalities
targeting SHH signaling should take into account the influence on
the PI3K/Akt pathway.
In conclusion, the results of the present study
suggest that SHH signaling plays an important role in the
development of RB, and that knockdown of SHH could decrease
cell growth and induce apoptosis in RB cells. At the molecular
level, the function of SHH was found to closely associate with the
activation of the PI3K/Akt pathway. However, the present study did
not detect a change in SHH level following administration of
IGF-1 and could not provide a detailed explanation for the response
of SHH expression to the regulation of the PI3K/Akt pathway. Thus,
further studies should be conducted to reveal further the
SHH-associated signaling transduction during RB.
References
|
1
|
Devesa SS: The incidence of
retinoblastoma. Am J Ophthalmol. 80:263–265. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Q, Wang Y, Wang H, Liu Y, Liu T and
Kunda PE: Tandem therapy for retinoblastoma: Immunotherapy and
chemotherapy enhance cytotoxicity on retinoblastoma by increasing
apoptosis. J Cancer Res Clin. 139:1357–1372. 2013. View Article : Google Scholar
|
|
3
|
Broaddus E, Topham A and Singh AD:
Survival with retinoblastoma in the USA: 1975–2004. Brit J
Ophthalmol. 93:24–27. 2009. View Article : Google Scholar
|
|
4
|
Milla LA, González-Ramírez CN and Palma V:
Sonic Hedgehog in cancer stem cells: A novel link with autophagy.
Biol Res. 45:223–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasperczyk H, Baumann B, Debatin KM and
Fulda S: Characterization of sonic hedgehog as a novel NF-kappaB
target gene that promotes NF-kappaB-mediated apoptosis resistance
and tumor growth in vivo. FASEB J. 23:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ke Z, Caiping S, Qing Z and Xiaojing W:
Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal
transition in ovarian cancer by mediating PI3K/AKT pathway. Med
Oncol. 32:3682015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choe JY, Yun JY, Jeon YK, Kim SH, Choung
HK, Oh S, Park M and Kim JE: Sonic hedgehog signalling proteins are
frequently expressed in retinoblastoma and are associated with
aggressive clinicopathological features. J Clin Pathol. 68:6–11.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng WH, Kar S, Doré S and Quirion R:
Insulin-like growth factor-1 (IGF-1): A neuroprotective trophic
factor acting via the Akt kinase pathway. J Neural Transm Suppl.
261–272. 2000.PubMed/NCBI
|
|
11
|
Hung HC, Hsiao YH and Gean PW: Sonic
hedgehog signaling regulates amygdalar neurogenesis and extinction
of fear memory. Eur Neuropsychopharmacol. 25:1723–1732. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman DM, Karhadkar SS, Hallahan AR,
Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale
J, Olson JM and Beachy PA: Medulloblastoma growth inhibition by
hedgehog pathway blockade. Science. 297:1559–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berman DM, Karhadkar SS, Maitra A, De
Montes Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y,
Eshleman JR, Watkins DN and Beachy PA: Widespread requirement for
Hedgehog ligand stimulation in growth of digestive tract tumours.
Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karhadkar SS, Bova GS, Abdallah N, Dhara
S, Gardner D, Maitra A, Isaacs JT, Berman DM and Beachy PA:
Hedgehog signalling in prostate regeneration, neoplasia and
metastasis. Nature. 431:707–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mill P, Mo R, Hu MC, Dagnino L, Rosenblum
ND and Hui CC: Shh controls epithelial proliferation via
independent pathways that converge on N-Myc. Dev Cell. 9:293–303.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eilers M and Eisenman RN: Myc's broad
reach. Genes Dev. 22:2755–2766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Javelaud D, Pierrat MJ and Mauviel A:
Crosstalk between TGF-β and hedgehog signaling in cancer. FEBS
Lett. 586:2016–2025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yanai K, Nakamura M, Akiyoshi T, Nagai S,
Wada J, Koga K, Noshiro H, Nagai E, Tsuneyoshi M, Tanaka M and
Katano M: Crosstalk of hedgehog and Wnt pathways in gastric cancer.
Cancer Lett. 263:145–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riobo K, Lu X, Ai GM, Haines CP and
Emerson CP Jr: Phosphoinositide 3-kinase, Akt are essential for
Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 103:4505–4510.
2006. View Article : Google Scholar : PubMed/NCBI
|