Introduction
Despite decreased incidence and mortality rates in
previous decades, gastric cancer remains the fourth most common
malignancy and the third leading cause of cancer-associated
mortality worldwide (1,2). Due to the mild and atypical symptoms at
the early stage, >80% of the patients are clinically diagnosed
at an advanced stage, which generally indicates a poor outcome
(1,3).
For the stratification of patient risk, the underlying molecular
and cellular processes during gastric carcinogenesis are ignored in
the widely-used Union for International Cancer Control/American
Joint Committee on Cancer (UICC/AJCC) Tumor Node Metastasis (TNM)
staging systems (4), while previous
evidence has demonstrated its heterogeneity, with an unpredictable
clinical outcome (4). There is an
urgent requirement to illuminate the molecular events involved in
the development and progression of gastric cancer, making it
possible to improve disease prognosis and provide novel therapeutic
targets for treatment.
Since the 19th century, cancer has been associated
with inflammation. Emerging evidence has revealed that inflammation
serves an important role in the initiation, development and
progression of human malignancy (5,6). As the
most abundant immune cell in the tumor microenvironment,
macrophages have received attention for their pro-tumoral role by
facilitating neoangiogenesis in the primary tumor site, and
promoting metastasis in distant sites (7–9).
Macrophages that infiltrated into the tumor microenvironment were
primed to adopt a pro-tumoral M2-phenotype rather than a
tumoricidal M1-phenotype (10). In
the process of the polarization and activation of macrophages, a
variety of chemokines were identified, of which
granulocyte-macrophage colony-stimulating factor (GM-CSF) may be
the essential orchestrator (10,11). The
role of GM-CSF, also termed CSF-2, in the tumor microenvironment is
controversial. Certain studies revealed that GM-CSF promoted
tumorigenesis via stimulating the epithelial release of VEGF
(12), while others stated that
GM-CSF released by tumor cells was associated with improved
survival (13) in colorectal cancer.
In breast cancer, GM-CSF was identified to inhibit cancer growth
and metastasis (14), and GM-CSF
triggered and maintained the alternative activation of
tumor-associated macrophages (TAM), and promoted tumor growth and
angiogenesis in glioma (15).
However, the clinical significance of intratumoral GM-CSF and its
prognostic value in gastric cancer remains obscure.
The prognostic role of diametrically polarized TAMs
in gastric cancer has been identified in our previous study
(16). The present study aimed to
investigate the expression of GM-CSF in gastric cancer and its
correlation with the clinicopathological characteristics and
clinical outcomes, including overall survival (OS) and disease-free
survival (DFS) times. In addition, nomograms were generated to
evaluate the 3- and 5-year DFS and OS rates for the patients with
gastric cancer following surgery.
Patients and methods
Clinical specimens
A total of 408 patients diagnosed with gastric
cancer at Zhongshan Hospital, Fudan University (Shanghai, China)
from January 1, 2008 to December 31, 2008 were enrolled in the
present study. The male:female ratio was 2.37 and the median age of
the patients was 60 (range, 27–88) years old. Written informed
consent from each patient was obtained, and the use of human
specimens was approved by the Clinical Research Ethics Committee of
Zhongshan Hospital. All the patients received a radical resection
(R0) with a D2 lymphadenectomy from the same surgical team and the
resultant formalin-fixed paraffin-embedded surgically resected
specimens were used in the present study. The specimens were fixed
in 10% formalin for 12 h at room temperature and were embedded in
paraffin for 4 h at 60°C. The section width was 5 µm. No patients
had received any anti-cancer therapy prior to surgery. The
clinicopathological and baseline demographic characteristics of the
patients were retrospectively collected, including age, sex, tumor
size, tumor histological classification (17), Lauren's classification (18) and TNM stage (4). A total of 2 independent gastroenterology
pathologists from the Department of Pathology of Zhongshan Hospital
provided reassessments for the tumor stage according to the 7th
Edition of the UICC/AJCC TNM staging system (4). DFS was defined as the time from the date
of surgery to the date of recurrence or the last visit. OS was
defined as the time from the date of surgery to the date of death
from all causes or the last visit.
Tissue microarray and
immunohistochemistry
The construction of the tissue microarray and the
immunohistochemical protocols were as previously described
(19). An anti-GM-CSF antibody
(dilution, 1:100 at 5 µg/ml; cat. no. ab9741, Abcam, Cambridge, MA,
USA) was used as the primary antibody in the immunohistochemical
analysis. The semi-quantitative H-score, which ranged from 0 to
300, was calculated by multiplying the staining intensities (0,
negative; 1, weak; 2, moderate; 3, strong) by the distribution
areas (percentage of positive staining cancer cells, 0–100%) at
each intensity level for each sample. A total of 2 independent
observers who were blinded to the patient outcomes and
clinicopathological characteristics provided the evaluation of the
immunostaining.
Statistical analysis
The cut-off point for the definition of high/low
expression subgroups was determined by X-tile plot analysis
(20). SPSS 19.0 (IBM Corp., Armonk,
NY, USA) was used to perform the analyses. The Pearson
χ2 test or Kruskal-Wallis test was used to compare
categorical variables. Continuous variables were analyzed with an
unpaired Student's t-test. Survival estimates were conducted with
Kaplan-Meier curves, and statistical significance was determined
using the log-rank test. Multivariable Cox proportional hazards
models were used to identify the independent prognosticator. A
nomogram was generated by R software v3.2.2 with ‘rms’ package (R
Foundation for Statistical Computing, Vienna, Austria). Calibration
plots for 3- and 5-year survival rates were constructed to examine
the performance characteristics of the generated nomograms. The
prognostic accuracy was measured by calculating the Harrell's
concordance indices (c-indices). All statistical analyses were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Associations between GM-CSF
immunohistochemical expression and the clinicopathological
features
Immunohistochemical staining analysis was performed
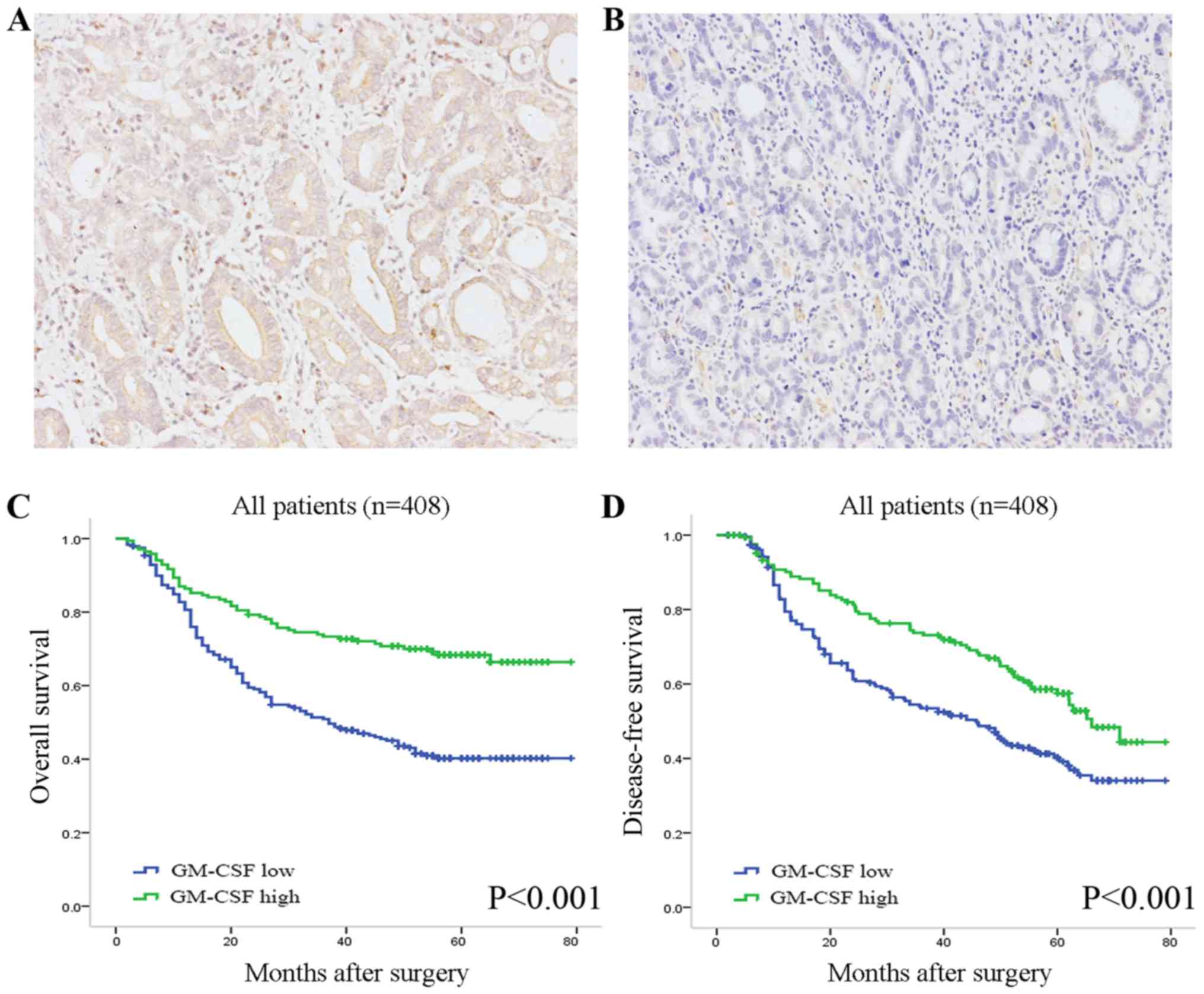
in 408 clinical specimens resected from primary tumor sites. GM-CSF
staining greatly varied in intensity in the tumor tissues (Fig. 1A and B). The positive staining of
GM-CSF was observed primarily in the cytoplasm and/or on the
membrane of neoplastic epithelia, and partially in the stroma.
According to the semi-quantitative H-score, 239 (58.6%) cases were
included in the GM-CSF low expression group. The
clinicopathological features of the patients dichotomized by
intratumoral GM-CSF expression are summarized in Table I. No significant association was
identified between GM-CSF expression patterns and the
clinicopathological features.
 | Table I.Correlations between GM-CSF expression
and clinicopathological features in patients with gastric cancer
(n=408). |
Table I.
Correlations between GM-CSF expression
and clinicopathological features in patients with gastric cancer
(n=408).
|
|
| GM-CSF
expression |
|---|
|
|
|
|
|---|
| Characteristics | All patients | Low | High | P-valuea |
|---|
| Age, years |
|
|
| 0.358 |
| Mean ±
SD | 60.0±11.7 | 60.5±11.8 | 59.4±11.5 |
|
| Sex |
|
|
| 0.178 |
| Male | 287 | 162 | 125 |
|
|
Female | 121 | 77 | 44 |
|
| Tumor size, cm |
|
|
| 0.073 |
| Mean ±
SD | 3.81±2.16 | 3.98±2.23 | 3.59±2.05 |
|
| Differentiation |
|
|
| 0.811 |
| Well
differentiated | 17 | 9 | 8 |
|
|
Moderately differentiated | 150 | 88 | 62 |
|
| Poorly
differentiatedb | 241 | 142 | 99 |
|
| Lauren's
classification |
|
|
| 0.998 |
|
Intestinal | 261 | 153 | 108 |
|
|
Diffuse | 96 | 56 | 40 |
|
|
Mixed | 51 | 30 | 21 |
|
| Depth of
invasion |
|
|
| 0.081 |
| T1 | 70 | 34 | 36 |
|
| T2 | 57 | 34 | 23 |
|
| T3 | 75 | 43 | 32 |
|
| T4 | 206 | 128 | 78 |
|
| Lymph node
metastasis |
|
|
| 0.280 |
| N0 | 153 | 84 | 69 |
|
| N1 | 45 | 26 | 19 |
|
| N2 | 78 | 49 | 29 |
|
| N3 | 132 | 80 | 52 |
|
| pTNM stage |
|
|
| 0.113 |
| I | 97 | 51 | 46 |
|
| II | 93 | 53 | 40 |
|
|
III | 218 | 135 | 83 |
|
| Adjuvant
chemocherapyc |
|
|
| 0.916 |
|
Yes | 245 | 143 | 102 |
|
| No | 163 | 96 | 67 |
|
Prognostic evaluation of GM-CSF
expression in patients with gastric cancer
The Kaplan-Meier method and log-rank tests were
performed to assess the association between GM-CSF expression and
clinical outcome in patients with gastric cancer. At the last
follow-up, the mean duration of OS was 40.2 months (median, 44.5
months) and DFS was 37.9 months (median, 41.0 months). Patients
with low GM-CSF expression were more likely to exhibit poorer
survival [Hazard ratio (HR), 2.26; 95% confidence interval (CI),
1.65–3.11; P<0.001; Fig. 1C] and
suffer from earlier recurrence (HR, 1.68; 95% CI, 1.26–2.26;
P=0.001; Fig. 1D) compared with those
with high expression. The median DFS and OS times for the GM-CSF
low expression subgroup were 52 and 55 months, respectively, while
those for the high expression subgroup were 30.1 and 34 months,
respectively.
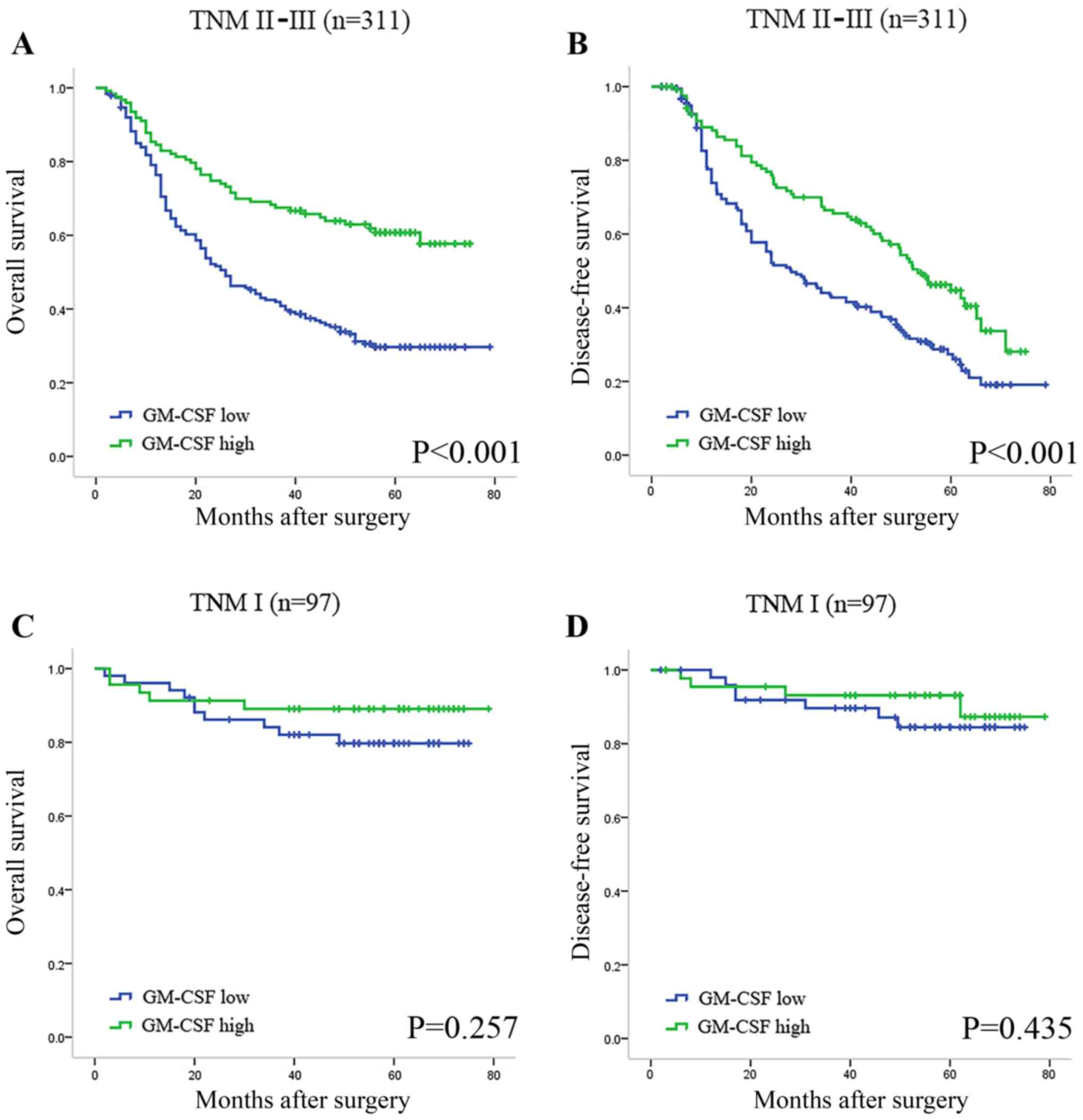
In order to eliminate the effects of tumor stage on
prognosis, Kaplan-Meier analysis was also applied to compare OS
according to GM-CSF expression in different TNM stages. A
statistically significant difference was identified in advanced
stages of tumors when stratified by GM-CSF expression levels
(Fig. 2A and B), while the DFS and OS
of the patients with TNM I stage tumors were not significantly
different (Fig. 2C and D).
In the univariate Cox regression analysis of OS,
intratumoral GM-CSF expression was defined as a prognostic factor
(P<0.001). Multivariable Cox proportional hazards models that
included depth of tumor invasion, lymph node metastasis, GM-CSF
expression and Lauren's classification as co-variables were
constructed. For OS, depth of tumor invasion (P<0.001), lymph
node metastasis (P<0.001), adjuvant chemotherapy (P<0.001)
and GM-CSF expression (P<0.001) were identified to be
independent prognostic factors for patients with gastric cancer,
while for DFS, depth of tumor invasion (P<0.001), lymph node
metastasis (P<0.001), Lauren's classification (P=0.029),
adjuvant chemotherapy (P=0.001) and GM-CSF expression (P=0.009)
were identified to be independent prognostic factors (Table II).
 | Table II.Multivariate analysis for OS and DFS
in patients with gastric cancer (n=408). |
Table II.
Multivariate analysis for OS and DFS
in patients with gastric cancer (n=408).
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| Depth of tumor
invasion (T3+4 vs. T1+2) | 3.674 | 2.292–5.889 | <0.001 | 4.704 | 2.846–7.774 | <0.001 |
| Lymph node
metastasis (N2+3 vs. N0+1) | 2.827 | 1.982–4.033 | <0.001 | 3.128 | 2.199–4.449 | <0.001 |
| Lauren
classification (diffused + mixed vs. intestinal) | 1.201 | 0.897–1.608 | 0.218 | 1.376 | 1.032–1.834 | 0.029 |
| GM-CSF expression
(low vs. high) | 2.109 | 1.531–2.904 | <0.001 | 1.631 | 1.130–2.354 | 0.009 |
| Adjuvant
chemotherapyb (no vs.
yes) | 2.297 | 1.656–3.186 | <0.001 | 1.630 | 1.214–2.190 | 0.001 |
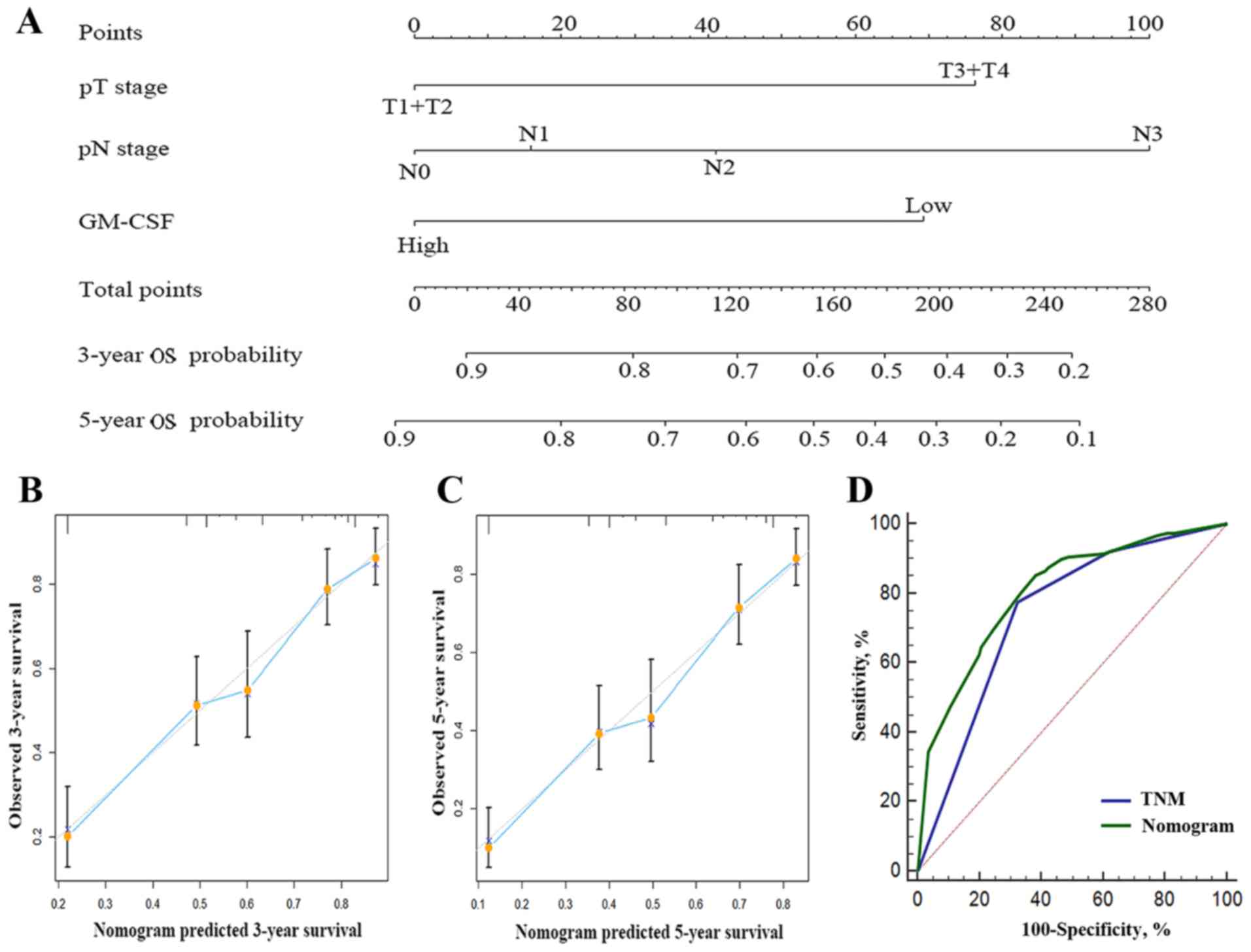
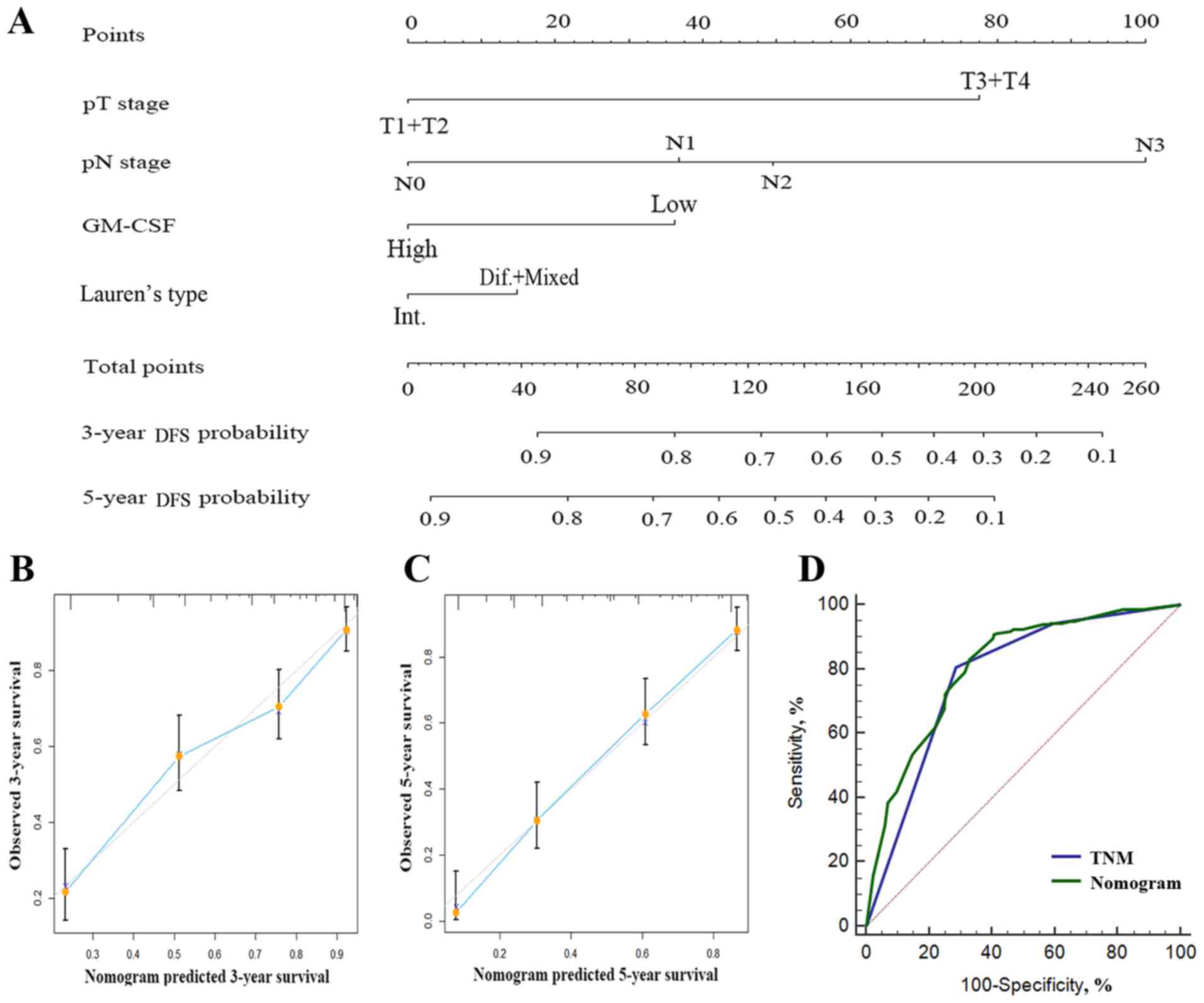
Predictive nomogram for OS in gastric
cancer patients
In order to provide a quantitative assessment for
outcomes of patients with gastric cancer, 2 nomograms were
constructed to provide a more sensitive prognostic model (Figs. 3A and 4A). The factors incorporated in the nomogram
were independent factors for OS selected subsequent to multivariate
analysis, with the exception of adjuvant chemotherapy, to generate
a model that only included the characteristics of the tumor without
artificial interventions. A higher number of total points predicted
a poorer prognosis. The total point was raised by the addition of
the score of each factor for each patient correspondingly.
Calibration curves for the internal validation of the nomogram
predictions of 3- and 5-year survival rate were constructed, and
the nomograms performed well with the ideal model (Fig. 3B and C; Fig.
4B and C). Harrell's c-index for the generated nomogram was
0.714 (95% CI, 0.679–0.749) for OS and 0.743 for DFS (95% CI,
0.708–0.778). The area under the receiver operating curve of the
generated nomogram for OS was 0.804, which was significantly larger
compared with that of TNM stage (0.742; P<0.001; Fig. 3D). The area under the receiver
operating curve of the generated nomogram for DFS was 0.807, which
was also larger compared with that of TNM stage (0.779; P=0.015;
Fig. 4D). These data indicated that
the nomograms performed well in predicting the OS of the
patients.
Discussion
Previous studies have revealed the important role of
tumor-associated macrophages in the process of tumor development
and progression (10,21). Our previous study also demonstrated
the prognostic value of infiltrated polarized macrophages in
gastric cancer (16). However, the
role of cytokines that are involved in the orchestration of the
polarization of TAM remains controversial. To the best of our
knowledge, the present study is the first study that identified
intratumoral GM-CSF expression as an independent prognostic factor
for patients following gastrectomy. In addition, the generated
nomograms performed better compared with the TNM staging system in
predicting the DFS and OS for the patients.
Much attention has been paid to TAMs for their
crucial role in the carcinogenesis of various tumors (10). For gastric cancer, Ohno et al
(22) stated that the aggregation of
TAMs within the tumor nest demonstrated a tumoricidal effect, while
Ishigami et al (23)
identified that the presence of TAMs in tumor tissue was correlated
with an adverse prognosis. However, our previous study revealed
that the infiltration of M2-polarized TAM in tumor tissue was
associated with a favorable outcome, while M1-TAM infiltration
exhibited the opposite effect (16).
These raised lead to the investigation of the cytokines that are
involved in the polarization of TAMs. In a previous study, a high
expression of C-C motif chemokine 2 (CCL-2) in the tumor tissue of
gastric cancer was revealed to be associated with the poor OS of
the patients (24). The present study
identified that high intratumoral expression of GM-CSF was
correlated with an improved clinical outcome. Therefore, it is
conceivable that increased GM-CSF may promote M2 to M1 polarization
of macrophages, which would impede infiltration and invasion of the
primary tumor, while CCL-2 possibly directed the opposite
polarization.
Since Lauren's classification was introduced in 1965
(18), debates have continued on
whether this may provide risk stratification in patients with
gastric cancer. Lauren diffuse-type gastric cancer is frequently
associated with a mutation of the Cadherin 1 (CDH1) gene (25–27).
Mutation or loss and methylation of CDH1 leads to the aberrant
expression of E-cadherin, disturbing the normal cell-cell adhesion
(27). Therefore, gastric cancer
cells of diffuse-type may be more likely to disperse and
disseminate. This may provide a potential explanation for the
results of the present study, which suggest that Lauren's
classification was an independent prognostic factor for DFS, but
not for OS.
The prognostic value of GM-CSF expression in gastric
cancer, particularly in advanced tumors, was investigated in the
present study. According to intratumoral GM-CSF expression,
patients were separated into two subgroups. GM-CSF expression was
demonstrated to be an independent adverse prognosticator for OS and
DFS in patients with gastric cancer. Furthermore, nomograms were
constructed by integrating GM-CSF expression, depth of tumor
invasion and lymph node metastasis status to provide a prediction
for the 3- and 5-year OS and DFS rates of the patients. Calibration
plots and c-indices indicated that the generated nomograms
performed better than the TNM staging system in terms of
discriminating between patients with different clinical outcomes.
However, a limitation exists that the study design is retrospective
in nature and the number of patients enrolled was relatively small.
A large, multi-center, prospective study is required to validate
these results.
It is known that anticancer therapies, including
cytotoxic drugs, radiotherapy and targeted agents, depend on the
activation of anticancer immune responses (28). Studies on the reversion of M1/M2
polarization, the prognostic value of TAMs (29,30) and
GM-CSF expression, as in the present study, have raised the
possibility that by altering the level of cytokines, for example,
increasing the concentration of GM-CSF in the tumor
microenvironment, the reversal of the polarization of TAMs may
provide a novel target for the treatment of gastric cancer.
In conclusion, intratumoral expression of GM-CSF in
gastric cancer has been identified as an independent prognostic
factor for OS and DFS. Furthermore, intratumoral GM-CSF expression
may be integrated with the depth of tumor invasion and lymph node
metastasis status to provide improved risk stratification for
patients with gastric cancer with different prognosis, particularly
in advanced stages.
Acknowledgements
The present study was funded by grants from the
National Key Projects for Infectious Diseases of China (grant nos.
2012ZX10002012-007 and 2016ZX10002018-008), the National Natural
Science Foundation of China (grant nos. 31100629, 31270863,
81372755, 31470794, 81401988, 81402082, 81402085, 81471621,
81472227, 81472376, 31570803, 81501999 and 81572352) and the
Program for New Century Excellent Talents in University (grant no.
NCET-13-0146).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayer RJ, Venook AP and Schilsky RL:
Progress against GI cancer during the American society of clinical
oncology's first 50 years. J Clin Oncol. 32:1521–1530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WQ, Zheng RS, Zhang SW, Zeng HM and
Zou XN: The incidences and mortalities of major cancers in China,
2010. Chin J Cancer. 33:402–405. 2014.PubMed/NCBI
|
|
4
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Xu JB, He YL, Peng JJ, Zhang XH,
Chen CQ, Li W and Cai SR: Tumor-associated macrophages promote
angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol.
106:462–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pollard JW: Trophic macrophages in
development and disease. Nat Rev Immunol. 9:259–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Han G, Wang K, Liu G, Wang R, Xiao
H, Li X, Hou C, Shen B, Guo R, et al: Tumor-derived GM-CSF promotes
inflammatory colon carcinogenesis via stimulating epithelial
release of VEGF. Cancer Res. 74:716–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nebiker CA, Han J, Eppenberger-Castori S,
Iezzi G, Hirt C, Amicarella F, Cremonesi E, Huber X, Padovan E,
Angrisani B, et al: GM-CSF production by tumor cells is associated
with improved survival in colorectal cancer. Clin Cancer Res.
20:3094–3106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eubank TD, Roberts RD, Khan M, Curry JM,
Nuovo GJ, Kuppusamy P and Marsh CB: Granulocyte macrophage
colony-stimulating factor inhibits breast cancer growth and
metastasis by invoking an anti-angiogenic program in tumor-educated
macrophages. Cancer Res. 69:2133–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sielska M, Przanowski P, Wylot B,
Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J,
Vinnakota K, Kettenmann H, et al: Distinct roles of CSF family
cytokines in macrophage infiltration and activation in glioma
progression and injury response. J Pathol. 230:310–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Wang X, Shen Z, Xu J, Qin J and
Sun Y: Infiltration of diametrically polarized macrophages predicts
overall survival of patients with gastric cancer after surgical
resection. Gastric Cancer. 18:740–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sica A, Schioppa T, Mantovani A and
Allavena P: Tumour-associated macrophages are a distinct M2
polarised population promoting tumour progression: Potential
targets of anti-cancer therapy. Eur J Cancer. 42:717–727. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda
S, Tachibana M, et al: The degree of macrophage infiltration into
the cancer cell nest is a significant predictor of survival in
gastric cancer patients. Anticancer Res. 23:5015–5022.
2003.PubMed/NCBI
|
|
23
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Okumura H, Matsumoto M, et al: Tumor-associated macrophage (TAM)
infiltration in gastric cancer. Anticancer Res. 23:4079–4083.
2003.PubMed/NCBI
|
|
24
|
Liu H, Shen Z, Wang X, Zhang H, Qin J, Qin
X, Xu J and Sun Y: Increased expression of C-C motif ligand 2
associates with poor prognosis in patients with gastric cancer
after gastrectomy. Tumor Biol. 37:3285–3293. 2016. View Article : Google Scholar
|
|
25
|
Oda T, Kanai Y, Oyama T, Yoshiura K,
Shimoyama Y, Birchmeier W, Sugimura T and Hirohashi S: E-cadherin
gene mutations in human gastric carcinoma cell lines. Proc Natl
Acad Sci USA. 91:1858–1862. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshiura K, Kanai Y, Ochiai A, Shimoyama
Y, Sugimura T and Hirohashi S: Silencing of the E-cadherin
invasion-suppressor gene by CpG methylation in human carcinomas.
Proc Natl Acad Sci USA. 92:7416–7419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hansford S, Kaurah P, Li-Chang H, Woo M,
Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K,
Zogopoulos G, et al: Hereditary diffuse gastric cancer syndrome:
CDH1 mutations and beyond. JAMA Oncol. 1:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galluzzi L, Senovilla L, Zitvogel L and
Kroemer G: The secret ally: Immunostimulation by anticancer drugs.
Nat Rev Drug Discov. 11:215–233. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guiducci C, Vicari AP, Sangaletti S,
Trinchieri G and Colombo MP: Redirecting in vivo elicited tumor
infiltrating macrophages and dendritic cells towards tumor
rejection. Cancer Res. 65:3437–3446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saccani A, Schioppa T, Porta C, Biswas SK,
Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A and Sica A:
P50 nuclear factor-kappaB overexpression in tumor-associated
macrophages inhibits M1 inflammatory responses and antitumor
resistance. Cancer Res. 66:11432–11440. 2006. View Article : Google Scholar : PubMed/NCBI
|