Introduction
Ovarian cancer is one of the three most common
malignant tumors in the female reproductive system, and its
incidence is second only to endometrial cancer (1). Various factors, including chemical,
physical and biological carcinogenic factors, genetic factors,
immune factors and poor lifestyle choices contribute to the
etiology of this disease (2).
Although surgical resection and chemoradiotherapy have gradually
improved, the survival rates for ovarian cancer remain poor
(3), and current traditional
treatments have little impact on long-term survival. This may be
due to the lack of understanding of the molecular transformations
that occur.
Transfer RNAs (tRNAs) are key to protein translation
fidelity. Growing evidence indicates that aberrant regulation of
translation is a trigger for cell transformation in cancer, and
tRNA modification enzymes may function as regulators of cancer
progression. Human tRNA methyltransferase 9-like (hTRM9L) catalyzes
tRNA wobble base modifications. TRM9 forms a complex with Trm112
and uses the methyl donor S-adenosylmethionine to catalyze the
formation of 5-methoxycarbonylmethyluridine [a precursor for
5-methoxycarbonylmethyl-2-thiouridine
(mcm5s2U) (4)
and mcm5s2U, which serve important roles in
the formation of tRNAArg and tRNAGlu
(5), respectively. In the absence of
tRNAArg and tRNAGlu (6), the resulting translational infidelity
promotes protein errors and triggers a cascade in the
retinoblastoma protein (pRB) and p53 signaling pathways (7).
In humans, the hTRM9L gene maps to the end of human
chromosome 8, a region that is commonly silenced or lost in
numerous types of cancer (8). In a
previous study by Begley et al (9), the level of hTRM9L in human cancer was
detected by a panel tissue array, which demonstrated significant
downregulation of hTRM9L in ovarian cancer, with an average change
of 1.5-fold; the downregulation of hTRM9L was most pronounced in
stage IV cancer (10). Therefore, in
the present study, it was hypothesized that hTRM9L may serve a role
in controlling ovarian cancer development. In the present study,
low hTRM9L expression was observed in ovarian cancer tissues, as
well as slowed growth of HO8910PM cells with stable transduction of
hTRM9L. By contrast, apoptosis was increased. The mechanism of
action may involve the activation of the pRB signaling pathway by
hTRM9L via LIN9, thereby inhibiting cell proliferation and driving
the p53 pathway to promote apoptosis.
Materials and methods
Patients
The present study consisted of 70 ovarian cancer
patients who had not undergone chemotherapy, radiotherapy or
hormonal therapy in the 3 months prior to surgery, between December
2013 and June 2014. The ovarian cancer specimens were obtained from
the Department of Gynecologic Surgery, University-Town Hospital of
Chongqing Medical University (Chongqing, China). Department of
Gynecologic Surgery at University-Town Hospital of Chongqing
Medical University (Chongqing, China). The use of the tissue
specimens was approved by the Medical Ethics Review Committee at
the University-Town Hospital of Chongqing Medical University and
written informed consent was obtained from all patients.
Reagents and antibodies
The human ovarian epithelial cell line HO8910PM was
purchased from American Type Culture Collection (Manassas, VA,
USA). The following antibodies were purchased for use in the
SP-9000IHCkit (Zhongshan Chemical, Beijing, China): Rabbit
anti-hTRM9L (1:100; cat. no. bs-17007RS; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China); mouse anti-LIN9 (1:200;
cat. no. sc-130571; SantaCruz Biotechnology, Inc., Dallas, TX,
USA); rabbit anti-B cell lymphoma 2 (Bcl-2; 1:100; cat. no.
bs-0032R; Beijing Biosynthesis Biotechnology Co., Ltd.)/Bcl-2
associated X protein (Bax; 1:100; cat. no. bs-0127R; Beijing
Biosynthesis Biotechnology Co., Ltd.); HRP-conjugated goat
anti-rabbit immunoglobulin (IgG; cat. no. ZB-230; OriGene
Technologies, Inc., Beijing, China); β-actin (1:5,000; cat. no.
BC007330; Wuhan Sanying Biotechnology, Wuhan, China); anti-rabbit
IgG (1:1,000; cat. no. 0007-2; Wuhan Sanying Biotechnology) and
anti-mouse IgG (1:1,000; cat. no. 00007-1; Wuhan Sanying
Biotechnology).
Immunohistochemistry (IHC)
Staining was performed with the IHC SP-9000 kit
(cat. no. SPN-9001; OriGene Technologies, Inc.) to detect hTRM9L
and LIN9 expression levels. Slides were deparaffinized, and
antigens were retrieved by heating in a microwave oven for 10 min
at 100°C in citrate buffer, followed by incubation in 3% hydrogen
peroxide for 10 min and blocking with normal goat serum (cat. no.
SPN-9001; OriGene Technologies, Inc.) for 20 min at 20°C. Slides
were incubated with primary antibody targeting hTRM9L (1:100; cat.
no. bs-17007RS; BIOSS, Beijing, China) overnight at 4°C, rewarmed
for 30 min at 37°C and then incubated with anti-rabbit IgG
secondary antibodies (1:100; cat. no. SPN-9001; OriGene
Technologies, Inc.) at 37°C for 30 min. The slides were then
incubated with streptavidin-horseradish-peroxidase (HRP) for 20 min
at 37°C, rinsed with PBS, incubated for 15 min with
3,3-diaminobenzidine (Bopei Biotech Co., Ltd., Chongqing, China)
and counterstained with hematoxylin. The staining results of the
targeted proteins were observed under a transmission light
microscope (magnifications, ×200 and ×400). Negative controls were
prepared using PBS in place of the primary antibody.
Cell culture
The cells were cultured in RPMI-1640 (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (FBS, HyClone; GE Healthcare Life Sciences) in a
humidified 5% CO2 atmosphere at 37°C. Cell growth was
observed using an inverted microscope at a magnification of ×10.
Once the cells were grown to 80–90% confluence, they were digested
with 0.125% trypsin. The medium was replaced every day, and cells
were passaged every 2–3 days. Cells in the logarithmic phase were
selected for subsequent testing.
Lentiviral overexpression of hTRM9L in
HO8910PM cells
The cDNA for hTRM9L (NM_020844) was cloned into
pIRES2 by polymerase chain reaction (PCR; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) via homologous recombination
between pIRES2 and Ubi-multiple cloning site-enhanced green
fluorescent protein (EGFP; GeneChem Co., Ltd., Shanghai, China).
Lentiviral vector plasmids for overexpressing hTRM9L were obtained
and lentivirus particles were subsequently packaged according to
the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). An empty vector lentivirus gene delivery system was used as
a negative control. HO8910PM cells were seeded onto 24-well plates
at a concentration of 1×105 perwell (50–60% confluence)
on the day prior to transfection. LV-hTRM9L was transduced into
cells at a multiplicity of infection of 50 using polybrene (10
µg/ml) and enhanced infection solution (GeneChem Co., Ltd.). A
non-target virus LV-EGFP (GeneChem Co., Ltd.) was transduced into
cells as a negative control. The enhanced infection solution was
replaced with RPMI-1640 medium supplemented with 10% FBS following
incubation for 12 h. The transfection validity was detected 72 h
following transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cultured cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The concentration and
purity of RNA were quantified using a UV spectrophotometer
(UltroSPec2100Pro; GE Healthcare Life Sciences). Total RNA was
reverse transcribed using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China) in a total volume of 20 µl,
according to the manufacturer's protocol. RT-qPCR was performed
using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) as
follows: 1 cycle at 95°C for 30 sec; 40 cycles at 95°C for 5 sec
and 60°C for 20 sec; 1 cycle at 65°C for 15 sec. GAPDH was used as
an internal control. Relative mRNA expression levels were analyzed
using the 2−ΔΔCq method (11). The primer sequences are listed in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequence |
|---|
| hTRM9L |
|
|
Forward |
5′-CCGGAGGCCAACTGATGATTT-3′ |
|
Reverse |
5′-CAGAACAGCTACACTCAGAGC-3′ |
| LIN9 |
|
|
Forward |
5′-GGAACGAAAGTTACAGCACGA-3′ |
|
Reverse |
5′-CAAGCCCTGTCCTATCAAAAGT-3′ |
| Bax |
|
|
Forward |
5′-TTGCTTCAGGGTTTCATCC-3′ |
|
Reverse |
5′-GACACTCGCTCAGCTTCTTG-3′ |
| Bcl-2 |
|
|
Forward |
5′-GGCCTCTGTTTGATTTCTCC-3′ |
|
Reverse |
5′-GCAGGCATGTTGACTTCACT-3′ |
| GAPDH |
|
|
Forward |
5′-ACCACAGTCCATGCCATCCA-3′ |
|
Reverse |
5′-TCCACCACCCTGTTGCTGTA-3′ |
Western blot analysis
Cells in the logarithmic growth phase were
harvested. Total protein was then extracted from the cells using
radio immuno precipitation assay lysis buffer according to the
manufacturer's protocol (KeyGen Biotech Co., Ltd., Nanjing, China).
Following centrifugation at 16,099 × g at 4°C for 15 min, the
supernatants of the lysates were collected for use. The protein
concentration was quantified using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China). The
indicated protein amounts (50 µg/well) were separated by 10%
SDS-PAGE and transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
for 2.5 h at room temperature and subsequently incubated with the
following primary antibodies overnight at 4°C: Rabbit anti-hTRM9L
(dilution, 1:500); rabbit anti-LIN9 (dilution, 1:100); rabbit
anti-Bcl-2/Bax (dilution, 1:500); and rabbit anti-β-actin
(dilution, 1:5,000). Subsequent to washing with TBS and 0.1% Tween
(TBST) for 15 min, the membranes were incubated with anti-rabbit
IgG secondary antibodies (dilution, 1:5,000) at 37°C for 1. 2 h.
Subsequent to washing with TBST for 15 min, detection was performed
using an enhanced chemiluminescence kit (KeyGen Biotech Co., Ltd.).
Specific bands were quantified using Quantity One 4. 6. 2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) software. Each experiment
was performed in triplicate.
Cell proliferation assay
Cells were incubated in 96-well plates at a density
of 1×103 cells per well for 24 h at 37°C. Cell
proliferation was examined on days 1, 2, 3, 4 and 5. Cell Counting
Kit-8 (CCK-8) reagent (~10 µl; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) and 90 µl of complete medium were added and
incubated for an additional 2 h at 37°C. Spectrophotometric
absorbance at 490 nm was determined with a microplate reader
(Bio-Rad Laboratories, Inc.). There were three replicates for each
sample.
Flow cytometric analysis
To detect apoptosis, cells were incubated in 6-well
plates at a density of 1×105 cells per well for 36 h and
subsequentlyre-suspended in 1,000 µl PBS. Annexin V-fluorescein
isothiocyanate and 7-amion-actinomyclin D (KeyGen Biotech Co.,
Ltd., Nanjing, China) dye were added, and samples were incubated at
room temperature in the dark for 15 min. Subsequent to passing the
cells through a mesh filter, apoptosis was detected using flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The cells were
counted using CellQuest software FCS2.0 (BD Biosciences), and the
data were analyzed using Macquit software FCS 2.0 (BD
Biosciences).
For cell cycle analysis, cells were incubated in
6-well plates at a density of 1×105 cells per well for
36 h at 37°C, and were subsequently re-suspended in 1,000 µl of 75%
alcohol overnight at −20°C. Subsequent to adding RNAase and PI dye,
the cells were incubated at room temperature in the dark for 15
min. Finally, cell cycle analysis was performed using flow
cytometry.
Statistical analysis
SPSS 19.0 (IBM SPSS, Armonk, NY, USA) was used for
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference. Differences in quantitative
data between groups were analyzed using Student's t-test, and
enumeration data was analyzed using the χ2 test.
Associations between the expression levels of hTRM9L and LIN9 were
analyzed using Pearson's correlation coefficient.
Results
Expression of hTRM9L and LIN9 in
ovarian cancer tissues
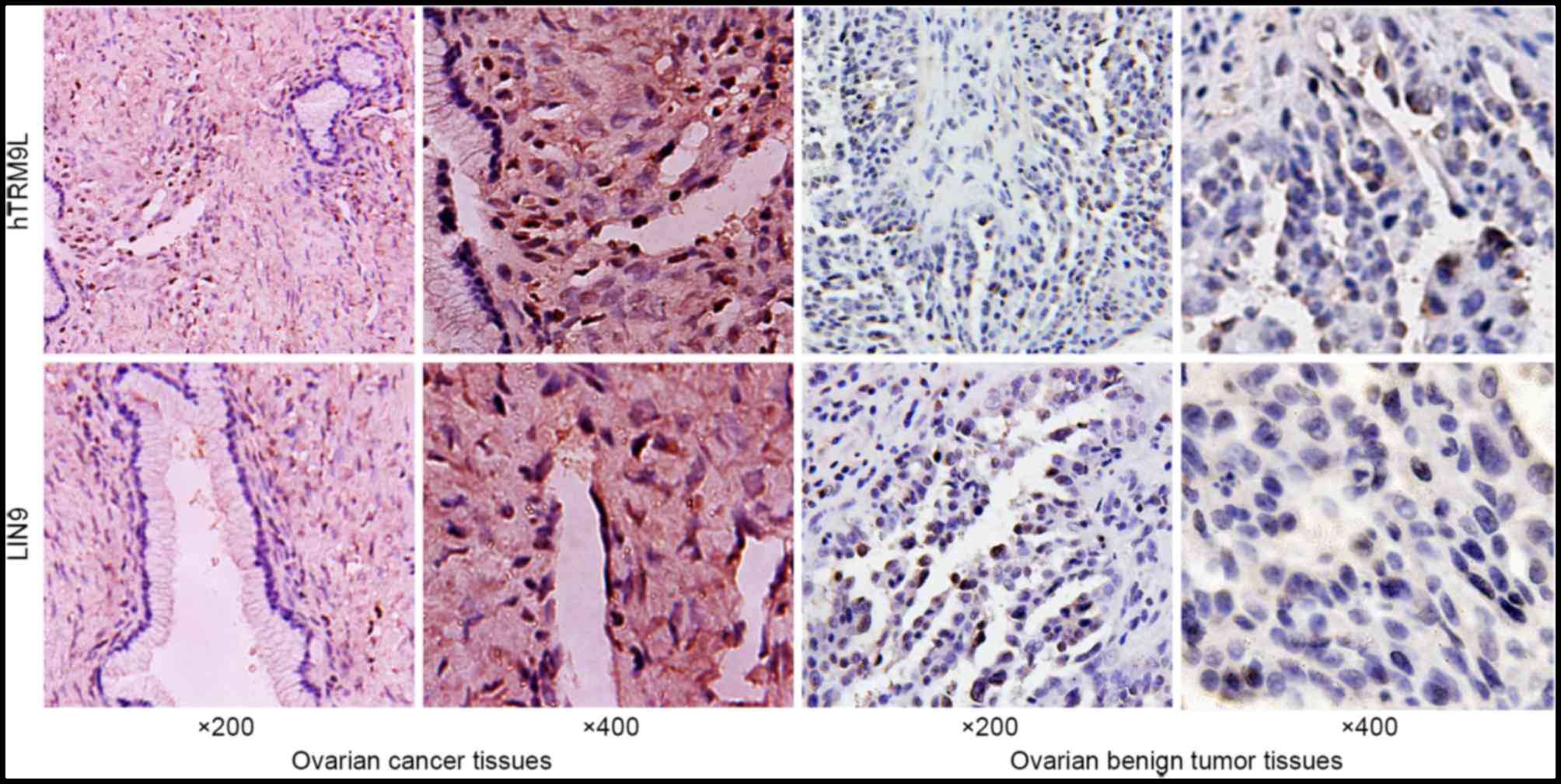
hTRM9L expression was examined in ovarian cancer
tissues by IHC. Positive staining of hTRM9L and LIN9 in ovarian
cancer tissues was identified in 38.57% (27/70) and 41.43% (29/70)
of the samples, respectively. However, the rate of positive
staining of hTRM9L and LIN9 in ovarian tumor tissues was 61.42%
(43/70; P<0.05) and 58.57% (41/70; P<0.05), respectively. The
results are shown in Fig. 1 and
Table II. A positive correlation was
observed between hTRM9L and LIN9 expression, according to the
Pearson's correlation coefficient (r=0. 406; P<0.05; Table III). The results indicated that
hTRM9L expression is associated with LIN9 expression in ovarian
cancer.
 | Table II.Expression of hTRM9L and LIN9 in 70
paired samples of ovarian cancer and ovarian tumor tissues
(χ2-test). |
Table II.
Expression of hTRM9L and LIN9 in 70
paired samples of ovarian cancer and ovarian tumor tissues
(χ2-test).
| Protein | Ovarian cancer
tissues | Ovarian tumor
tissues | P-value |
|---|
| hTRM9L |
|
| 0.002 |
|
Positive | 27 | 45 |
|
|
Negative | 43 | 25 |
|
| LIN9 |
|
| 0.028 |
|
Positive | 29 | 42 |
|
|
Negative | 41 | 28 |
|
 | Table III.Correlation between hTRM9L and LIN9
expression in ovarian cancer tissues. |
Table III.
Correlation between hTRM9L and LIN9
expression in ovarian cancer tissues.
|
| hTRM9L |
|
|
|---|
|
|
|
|
|
|---|
| LIN9 | Positive (n=27) | Negative (n=43) | Pearson's
correlation | P-value |
|---|
| Positive (n=29) | 18 | 11 | 0. 406 | 0.001 |
| Negative (n=41) | 9 | 32 |
|
|
hTRM9L promotes LIN9 and Bax
expression and inhibits Bcl-2 expression in ovarian cancer
cells
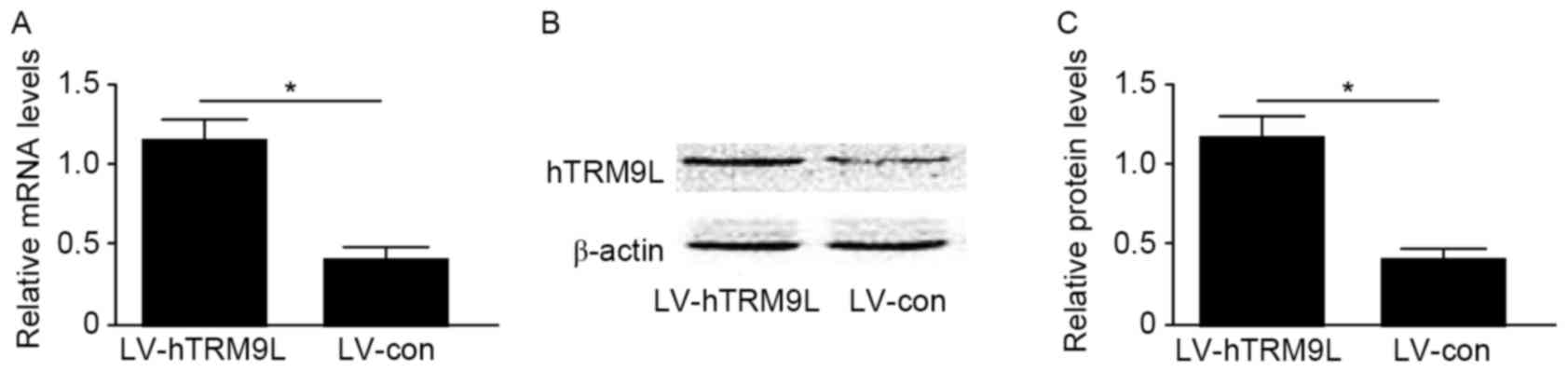
Ectopic hTRM9L-expressing ovarian cancer cells
(HO8910PM) were used to examine whether hTRM9L promotes LIN9 and
Bax expression and inhibits Bcl-2 expression in ovarian cancer
cells. hTRM9L expression was induced in HO8910PM cells using a
lentivirus (LV-hTRM9L). The expression of hTRM9L at them RNA and
protein levels in cells transfected with the LV-hTRM9L was
significantly increased compared with the negative control
(LV-con), as confirmed by RT-qPCR and western blot analysis,
respectively (P<0.05; Fig. 2). The
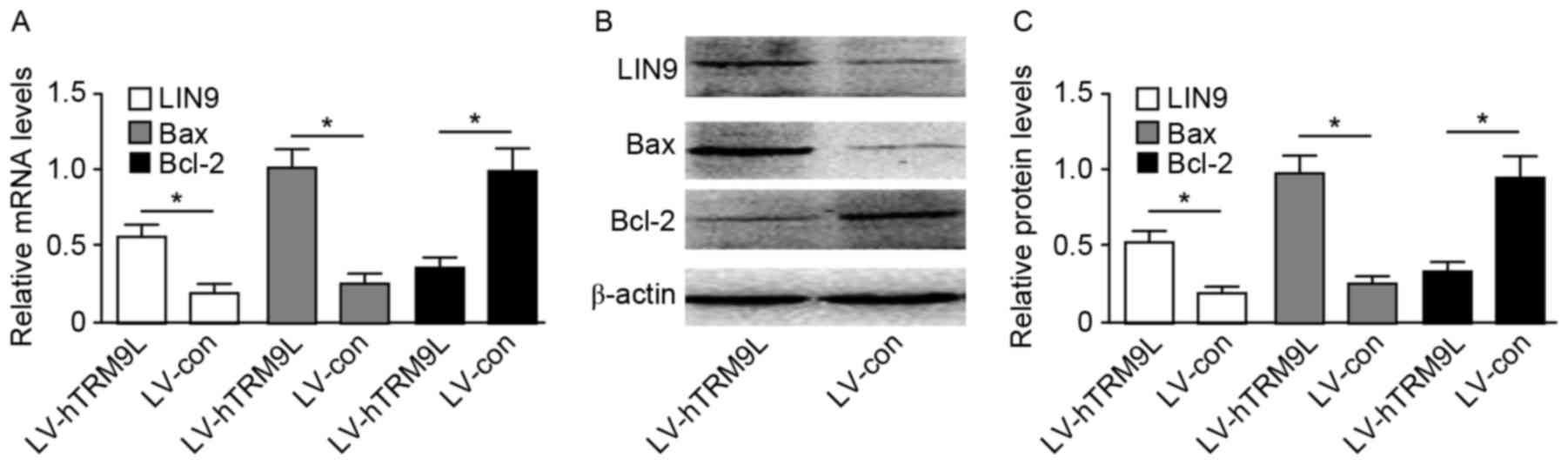
LIN9 axis performs a critical role in hTRM9L regulation of ovarian
cancer cell proliferation and apoptosis. As a result, the increase
in LIN9 expression triggered upregulation of pro-apoptotic Bax
expression and downregulation of anti-apoptotic Bcl-2 expression.
The results indicated that hTRM9L over expression accelerate
sovarian cancer cell apoptosis. The LIN9 and Bax/Bcl-2 expression
levels were assayed by RT-qPCR and western blot analysis
(P<0.05; Fig. 3).
Effect of ectopic hTRM9L expression on
apoptosis, cell cycle and growth of ovarian cancer cells
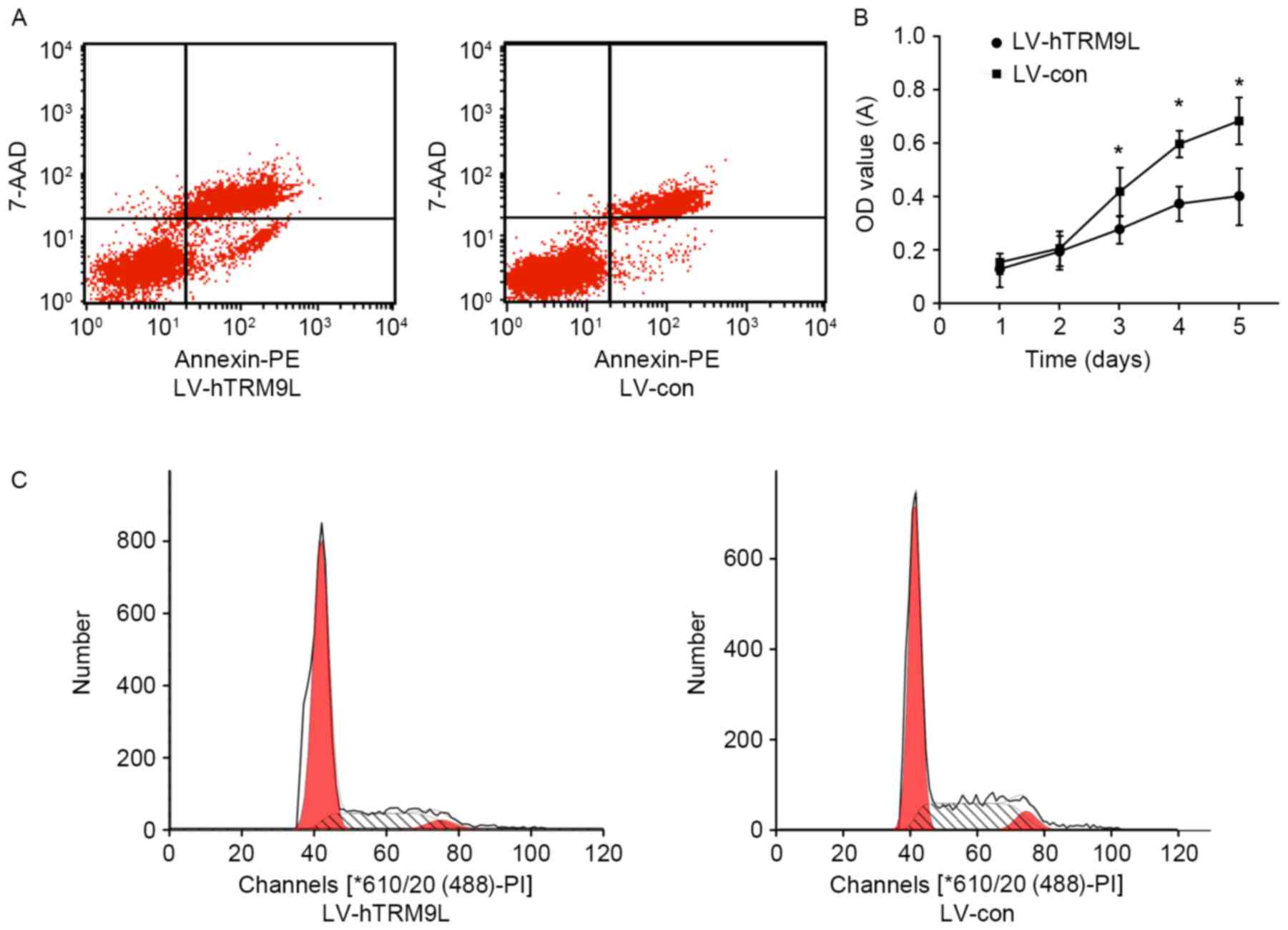
As shown by flow cytometry, the number of apoptotic
HO8910PM cells transfected with LV-hTRM9L was significantly
increased compared with the negative control group (LV-con;
P<0.05; Fig. 4A). Therefore,
hTRM9L may promote HO8910PM cell apoptosis. As shown by CCK-8, the
viability of HO8910PM cells transfected with hTRM9L was
significantly reduced compared with the negative control group
(LV-con; P<0.05; Fig. 4B). The
results indicated that hTRM9L inhibits HO8910PM cell growth. As
shown by flow cytometry, the proportion of G1 phase HO8910PM cells
transfected with LV-hTRM9L was significantly increased compared
with the negative control group (P<0.05; Fig. 4C).
Discussion
Ovarian cancer remains the primary threat to the
health of women, and its incidence increases annually (1). Therefore, it is necessary to investigate
new molecular pathways to counteract this threat. In the present
study, hTRM9L expression was depressed in ovarian cancer tissues
and significantly associated with LIN9 (r=0.406; P<0.05). To the
best of our knowledge, hTRM9L expression was detected in ovarian
cancer tissues for the first time, and it was confirmed that the
expression of hTRM9L was low.
LIN9, a member of the dimerization partner, RB-like,
E2F and multi-vulval class B (DREAM) complex, acts as a tumor
suppressor (12). The present study
validated that LIN9 transcription and protein levels were
upregulated ~2-fold in hTRM9L-expressing HO8910PM cells. The DREAM
(or LINC) complex is a master regulator of mitotic gene expression
during the cell cycle. RNA interference-mediated knockdown of the
DREAM subunits in human cells inhibits their proliferation via
inhibition of mitotic gene expression. LIN9 performs an important
role in pRB and p53 signaling to achieve cancer suppression
(13).
DREAM function involves the induction of permanent
cell cycle arrest (14,15). Cell cycle arrest in G0/G1 by LIN9 may
activate the pRB signaling pathway, as demonstrated by flow
cytometry. At the same time, the proliferation of HO8910PM cells
transfected with hTRM9L was significantly reduced compared with the
negative control group. In the present study, the number of
apoptotic HO8910PM cells transfected with LV-hTRM9L was
significantly increased compared with the negative control group,
based on flow cytometry. Additionally, pro-apoptotic Bax expression
was found to be upregulated, while anti-apoptotic Bcl-2 expression
was downregulated. Bax and Bcl-2 are important signaling molecules
in the p53 signaling pathway (16).
It was hypothesized that ectopic hTRM9L-expressing
ovarian cancer cells (HO8910PM) activate LIN9 entry into the p53
signaling pathway, thereby increasing apoptosis. Apoptosis is
considered an important fail-safe mechanism for the prevention of
tumorigenesis (17,18).
Rapid tumor cell proliferation and apoptosis
inhibition lead to increased malignancy, resulting in an escape
from immune regulation. However, hTRM9L may activate LIN9 to enter
the p53 signaling pathway, resulting in increased cell apoptosis,
as well as the pRB signaling pathway, reducing proliferation
(19).
In conclusion, the present study demonstrated that
hTRM9L may prevent tumor growth and promote apoptosis by regulating
LIN9. This may be a novel breakthrough for ovarian cancer
treatment, although additional studies investigating the mechanism
are required.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gay GM, Lim JS, Chay WY, Chow KY, Tan MH
and Lim WY: Reproductive factors, adiposity, breastfeeding and
their associations with ovarian cancer in an Asian cohort. Cancer
Causes Control. 26:1561–1573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinkelspiel HE, Champer M, Hou J, Tergas
A, Burke WM, Huang Y, Neugut AI, Ananth CV, Hershman DL and Wright
JD: Long-term mortality among women with epithelial ovarian cancer.
Gynecol Oncol. 138:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Letoquart J, van Tran N, Caroline V,
Aleksandrov A, Lazar N, van Tilbeurgh H, Liger D and Graille M:
Insights into molecular plasticity in protein complexes from
Trm9-Trm112 tRNA modifying enzyme crystal structure. Nucleic Acids
Res. 43:10989–11002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Begley U, Dyavaiah M, Patil A, Rooney JP,
DiRenzo D, Young CM, Conklin DS, Zitomer RS and Begley TJ:
Trm9-catalyzed tRNA modifications link translation to the DNA
damage response. Mol Cell. 28:860–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalhor HR and Clarke S: Novel
methyltransferase for modified uridine residues at the wobble
position of tRNA. Mol Cell Biol. 23:9283–9292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wiseman EF, Chen X, Han N, Webber A, Ji Z,
Sharrocks AD and Ang YS: Deregulation of the FOXM1 target gene
network and its coregulatory partners in oesophageal
adenocarcinoma. Mol Cancer. 14:692015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flanagan JM, Healey S, Young J, Whitehall
V, Trott DA, Newbold RF and Chenevix-Trench G: Mapping of a
candidate colorectal cancer tumor-suppressor gene to a 900-kilobase
region on the short arm of chromosome 8. Genes Chromosomes Cancer.
40:247–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Begley U, Sosa MS, Avivar-Valderas A,
Patil A, Endres L, Estrada Y, Chan CT, Su D, Dedon PC,
Aguirre-Ghiso JA and Begley T: A human tRNA methyltransferase
9-like protein prevents tumour growth by regulating LIN9 and
HIF1-α. EMBO Mol Med. 5:366–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bešević J, Gunter MJ, Fortner RT, Tsilidis
KK, Weiderpass E, Onland-Moret Charlotte N, Dossus L, Tjønneland A,
Hansen L, Overvad K, et al: Reproductive factors and epithelial
ovarian cancer survival in the EPIC cohort study. Br J Cancer.
113:1622–1631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hauser S, Ulrich T, Wurster S, Schmitt K,
Reichert N and Gaubatz S: Loss of LIN9, a member of the DREAM
complex, cooperateswith SV40 large T antigen to induce genomic
instability and anchorage-independent growth. Oncogene.
31:1859–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blais A, van Oevelen CJ, Margueron R,
Acosta-Alvear D and Dynlacht BD: Retinoblastoma tumor suppressor
protein-dependent methylation of histone H3 lysine 27 is associated
with irreversible cell cycle exit. J Cell Biol. 179:1399–1412.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hauser S, Ulrich T, Wurster S, Schmitt K,
Reichert N and Gaubatz S: Loss of LIN9, a member of the DREAM
complex, cooperates with SV40 large T antigen to induce genomic
instability and anchorage-independent growth. Oncogene.
31:1859–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stowell KM: DNA testing for malignant
hyperthermia: The reality and the dream. Anesth Analg. 118:397–406.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan KO, Nielsen AB, Meier BH and Ernst M:
Broad-band DREAM recoupling sequence. J Phys Chem Lett.
5:3366–3372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Z, Wu F, Chen L, Li Q, Wang C, Dong J
and Xie SQ: ETME, a novel β-elemene derivative, synergizes with
arsenic trioxide in inducing apoptosis and cell cycle arrest in
hepatocarcinoma cells via a p53-dependent pathway. Acta Pharm Sin
B. 4:424–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obata F, Tomioka K and Miura M:
Transcriptional profiling of apoptosis-deficient Drosophila
mutants. Genom Data. 2:254–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carper MB, Denvir J, Boskovic G, Primerano
DA and Claudio PP: RGS16, a novel p53 and pRb cross-talk candidate
inhibits migration and invasion of pancreatic cancer cells. Genes
Cancer. 5:420–435. 2014.PubMed/NCBI
|
|
19
|
Zhou Z, Flesken-Nikitin A, Corney DC, Wang
W, Goodrich DW, Roy-Burman P and Nikitin AY: Synergy of p53 and Rb
deficiency in a conditional mouse model for metastatic prostate
cancer. Cancer Res. 66:7889–7898. 2006. View Article : Google Scholar : PubMed/NCBI
|