Introduction
Langerhans cell histiocytosis (LCH) is a rare
disorder affecting adults and children, characterised by an
abnormal accumulation of epidermal Langerhans-like cells in various
sites (1–3). The aetiology and pathogenesis of LCH
remains to be established, but it is hypothesized to be a clonal
disorder (4,5). A diagnosis of LCH is made following the
detection of lesional cluster of differentiation (CD)1a+
Langerhans-like cells, whereas non-Langerhans histiocytoses,
including Erdheim Chester disease, are CD1a−. LCH is a
heterogeneous disease, affecting all ages and ethnicities, and
disease may take a variety of courses: Certain disease cases may
spontaneously remit, while other cases may lead to fatality. Risk
factors include the time to diagnosis and the site of lesions
(6).
A study from the Histiocyte Society recognised a
disparity in the site, age of onset and incidence of LCH between
adults and children (7). The age of
onset in children is between 1–3 years, whereas adult disease is
more heterogeneous, with a higher incidence in young (18–30 years)
and older adults (>70 years) (7).
The reported incidence of LCH ranges from 0.5–5.4 cases per million
persons per year (8–10), but these figures are largely
associated with childhood disease. The incidence of adult disease
is likely to be underreported, as LCH is frequently treated
according to the affected system, without a secondary referral.
Childhood disease is more likely to be referred to an oncologist,
providing more accurate statistics.
Although childhood LCH lesions tend to predominate
in bone (11), adult LCH is more
widespread, commonly presenting in the skin, bone and lung.
Table I lists the common sites of LCH
in both adults and children reported in several previous studies
(6,7,12). This
heterogeneity, coupled with relative scarcity, renders it
challenging to implement randomized control trials in adults or
children to optimize therapy. Notwithstanding the Histiocyte
Society's LCH protocol that provides a therapeutic framework,
treatment for LCH is variable across centres (13).
 | Table I.Most common sites of Langerhans cell
histiocytosis in children and adults with their frequency, where
reported. |
Table I.
Most common sites of Langerhans cell
histiocytosis in children and adults with their frequency, where
reported.
|
| Frequency in adults,
% | Frequency in
children, % |
|---|
|
|
|
|
|---|
| Site | SS | MS | SS | MS |
|---|
| Bone | 52 | 77 | 70–80 |
|
| Skin | 6 | 65 | 10 | 50 |
| Ear, nose and
throat |
|
| >15 |
|
| Pituitary | 0.9 | 44 | 5–50 |
|
| Orbits |
|
| <20 |
|
| Mouth |
|
| 6 |
|
| Gastrointestinal
tract |
|
| 2–13 |
|
| Lungs | 40 | 43 | <5 | 12 |
| Liver |
| 5 | 4 |
|
| Lymph nodes |
|
| <10 |
|
| Thyroid |
| 9 |
|
|
Despite an unknown aetiology, the misguided myeloid
dendritic cell precursor model provides one underlying mechanism to
explain the abnormal localization and accumulation of dendritic
cells (14,15), and this is consistent with the
treatment of LCH as a haematological disease (at least in
paediatric cases). Haematopoietic forms of cancer occur as a
consequence of arrest at a discrete stage of an ordered
developmental pathway, frequently associated with distinct patterns
of mutation. This is in contrast to primary and metastatic solid
tumours, which tend to exhibit a higher level of heterogeneity at
the genetic level (16). The most
significant recent advance in the understanding of LCH has been
provided by studies describing the high incidence of
BRAFV600E mutations in childhood disease (17–19). This
observation, along with the immature phenotype of LCH cells, is
consistent with the discrete patterns of mutation and arrested
development observed in haematopoietic types of cancer. In order to
further investigate whether this mutation demonstrated a discrete
pattern in LCH, the current study aimed to investigate the
BRAFV600E status in a broader range of LCH cases (with
respect to age and site) in order to establish whether there is
commonality in the aetiology of a disease with heterologous
presentation. In addition, the assessment of numerous biopsies from
patients with LCH presenting at multiple sites may provide further
evidence for a clonal origin of this disorder.
Materials and methods
Meta-analysis
PubMed (http://www.ncbi.nlm.nih.gov/pubmed) was searched for
manuscripts referencing LCH biopsies that had been subject to
BRAFV600E screening by direct sequencing, reverse
transcription-polymerase chain reaction (PCR) or
immunohistochemistry. Relevant patient information (disease
classification and BRAF status) from 10 published manuscripts were
isolated for meta-analysis (Table
II) (15,17–25). The
search criteria used were ‘BRAFV600E’ and ‘Langerhans cell
histiocytosis’. Results were filtered for adults (>18 years) and
paediatric disease (<18 years), and secondly according to
disease site, including bone or skin.
 | Table II.Summary of Langerhans cell
histiocytosis published data used for meta-analysis. |
Table II.
Summary of Langerhans cell
histiocytosis published data used for meta-analysis.
| Authors, year | Age, years (no. of
patients) | Total no. of
patients | Classification |
BRAFV600E screening | V600E no. of
patients | WT no. of
patients | P-value | Refs. |
|---|
| Bates et al,
2013 | <18 (1) | 1 | SS (0) | Pyrosequencing | – | – |
| (20) |
|
| >18 (N/A) |
| MS (1) |
| 1 | – |
|
|
| Yousem et
al, 2013 | <18 (N/A) | 5 | SS (5) | Next generation
sequencing and Sanger sequencing | 2 | 3 |
|
|
|
| >18 (5) |
| MS (−) |
| – | – |
|
|
| Satoh et al,
2012 | <18 (16) | 16 | SS (9) | Next generation
pyrosequencing | 6 | 3 |
0.615 | (21) |
|
| >18 (N/a) |
| MS (7) |
| 3 | 4 |
|
|
| Badalian-Very et
al, 2010 | <18 (27) | 52 | SS (44) | Pyrosequencing | 27 | 17 | 0.700 | (18) |
|
| >18 (17) |
| MS (8) |
| 4 | 4 |
|
|
| Chilosi et
al, 2014 | <18 (11) | 38 | SS (33) | Pyrosequencing and
VE1 immunoreactivity | 17 | 16 | 0.343 | (17) |
|
| >18 (27) |
| MS (5) |
| 1 | 4 |
|
|
| Haroche et
al, 2012 | <18 (N/A) | 29 | SS (N/A) | Pyrosequencing | – | – |
| (22) |
|
| >18 (N/A) |
| MS (N/A) |
| – | – |
|
|
| Sahm et al,
2012 | <18 (49) | 89 | SS (85) | Direct sequencing
and VE1 immunoreactivity | 31 | 54 | 0.154 | (23) |
|
| >18 (40) |
| MS (4) |
| 3 | 1 |
|
|
| Wei et al,
2013 | <18 (36) | 52 | SS (43) | Direct
sequencing | 25 | 18 | 0.684 | (15) |
|
| >18 (16) |
| MS (7) |
| 3 | 4 |
|
|
| Berres et
al, 2014 | <18 (97) | 100 | SS (45) | Qiagen
BRAFV600E qPCR mutation assay and | 27 | 18 | 0.532 | (24) |
|
| >18 (3) |
| MS (55) | Sanger
sequencing | 37 | 18 |
|
|
| Go et al,
2014 | <18 (19) | 27 | SS (N/A) | Direst Sanger
sequencing, Peptide nucleic acid | 6 | 21 |
| (19) |
|
| >18 (8) |
| MS (N/A) | clamp qPCR
(PNAcqPCR) assay and Anyplex™ qPCR assay |
|
|
|
|
BRAFV600E sequencing
Research Ethics Committee (REC) approval at
Hammersmith Hospital (London, UK) was obtained for the present
study (REC reference no. 60/Q0406/107). In total, 33 adult LCH
samples, representing 30 patients were available to screen for the
BRAFV600E mutation. DNA was extracted from 21 archival
paraffin embedded LCH biopsies and 12 fresh biopsies enriched for
CD1a positive cells using an AllPrep DNA/RNA FFPE kit and AllPrep
DNA/RNA Mini kit from Qiagen, Inc. (Valencia, CA, USA),
respectively. The extractions were performed according to the
manufacturer's protocol. CD1a-positive cell selection was performed
using MACS CD1a MicroBeads kit purchased from Miltenyi Biotec, Inc.
(Cambridge, MA, USA). Cells were selcted using magnetic beads
coated with a human anti-mouse IgG monoclonal antibody and a
magnetic particle concentrator according to the manufacturer's
instructions. DNA quality was assessed using a NanoDrop (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Amplification and
sequencing of exon 15 of the BRAF gene was performed by Source
BioScience (Nottingham, UK). Source BioScience designed the primer
pairs and performed region-specific PCR optimization, amplification
and sequencing. The oligonucleotide sequences were as follows: PCR
forward primer (5′AAC ACA TTT CAA GCC CCA AA); PCR reverse primer
(5′AGC ATC TCA GGG CCA AAA AT); forward sequencing primer (5′TCA
TAA TGC TTG CTC TGA TAG GA); reverse sequencing primer (5′GGC CAA
AAA TTT AAT CAG TGG A). PCR reaction mixes contained 25 ng DNA (or
a no template control), 0.6 µM of each primer and Roche
High-Fidelity master mix at a 1X final concentration with the
following conditions: Initial denaturation at 94°C for 279 sec;
followed by 35 cycles of 94°C for 20 sec, 55°C for 15 sec and 65°C
for 30 sec; followed by a final elongation step at 65°C for 60 sec.
PCR products were cleaned up using Zymo ZR-96 clean and
concentrator kit (Zymo Research Corp, Irvine, CA, USA). Products
were sequenced as standard using an ABI 3730 machine (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using Prism
statistics software (version 4; GraphPad Software, Inc., La Jolla,
CA, USA). Analysis was performed using χ2 and Fisher's
exact tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
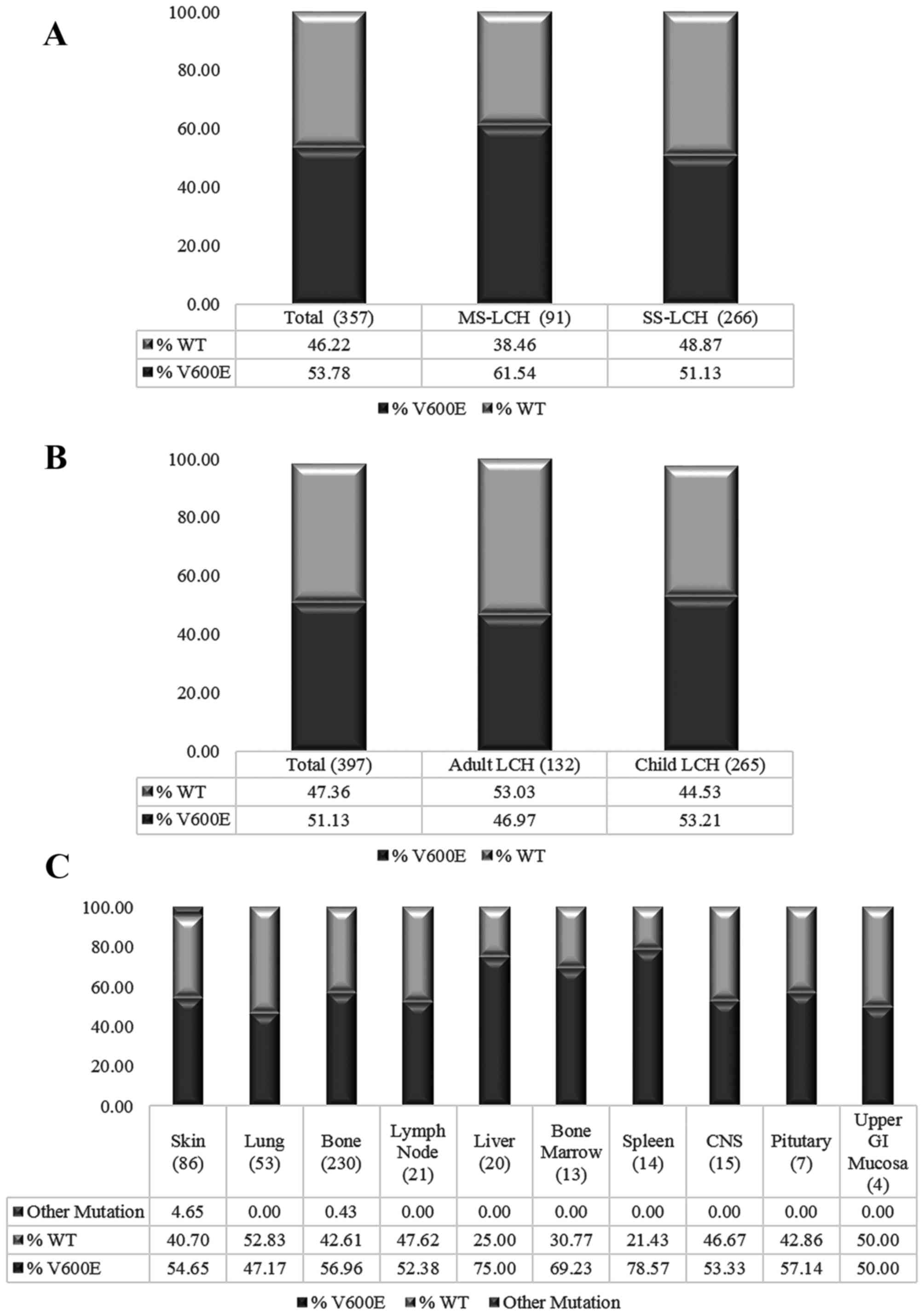
A meta-analysis of existing LCH BRAFV600E
studies was performed to invesitigate the heterogeneity of LCH at
the genetic level. Fig. 1A reveals no
difference in the prevalence of BRAFV600E mutations
between adult and paediatric LCH. Similarly, when the incidence of
the BRAFV600E mutation in various clinical
classifications of LCH was evaluated, no significant differences
were identified between multi-system (MS)-LCH and focal or single
system (SS) LCH (Fig. 1B). In
addition, investigation of the mutation in varying sites revealed
no consistent pattern (Fig. 1C).
The results of BRAFV600E sequencing in
adult LCH cases are presented in Table
III. Of the 29 patients analysed, 11 patients exhibited a
BRAFV600E mutation, which corresponds to 38% of patients
with LCH being BRAFV600E-positive for the mutation. There were 3
patients for whom multiple samples were analysed. Lesional gum and
bone samples from patient 16 exhibited differential status with
respect to the BRAFV600E mutation. However, follow up
with PCR revealed the two samples to be
BRAFV600E-positive (data not shown), suggesting a low
level of mutated cells in the gum sample below the sensitivity
threshold of sequencing. It is of note that PCR analysis did not
identify V600E mutations in any other biopsies identified as
wild-type by sequencing. A total of 3 lung samples were available
for patient 28, all of which were BRAFV600E-positive.
There were two lymph node samples available for patient 20, of
which one was BRAFV600E-positive. It is possible that
one of these lymph node samples was obtained from a node not
involved in the lesion; however, the clinical history of these
samples is not available.
 | Table III.BRAFV600E mutation
screening in adult LCH cases using Sanger Sequencing. |
Table III.
BRAFV600E mutation
screening in adult LCH cases using Sanger Sequencing.
| Patient no. | Type | Tissue type | Clinical
status | BRAF status |
|---|
| 1 | Cells | N/A | LCH | WT |
| 2 | Cells | N/A | LCH | WT |
| 3 | Cells | Skin | MS | WT |
| 4 | Cells | N/A | LCH | WT |
| 5 | Cells | N/A | LCH | WT |
| 6 | Cells | N/A | LCH | V600E |
| 7 | Cells | BALF | SS | WT |
| 8 | Cells | BALF | MS | WT |
| 9 | Cells | Skin | SS | V600E |
| 10 | Cells | BALF | SS | WT |
| 11 | Cells | Skin | LCH | V600E |
| 12 | Cells | BALF | SS | WT |
| 13 | FFPE | Skin | MS-HR | WT |
| 14 | FFPE | Skin | MS-HR | WT |
| 15 | FFPE | Skin | MS-HR | WT |
| 16a | FFPE | Gum | MS | WT |
| 16b | FFPE | Bone |
| V600E |
| 17 | FFPE | Skin | SS | WT |
| 18 | FFPE | Skin | SS | WT |
| 19 | FFPE | Skin | SS | WT |
| 20a | FFPE | LN | LCH | WT |
| 20b | FFPE | LN |
| V600E |
| 21 | FFPE | LN | LCH | V600E |
| 22 | FFPE | N/A | LCH | WT |
| 23 | FFPE | Liver | LCH | WT |
| 24 | FFPE | N/A | LCH | WT |
| 25 | FFPE | Skin | LCH | V600E |
| 26 | FFPE | Skin | MS | V600E |
| 27 | FFPE | Skin | SS | V600E |
| 28a | FFPE | Lung |
| V600E |
| 28b | FFPE | Lung | LCH | V600E |
| 28c | FFPE | Lung |
| V600E |
| 29 | FFPE | Thyroid | LCH | V600E |
Discussion
The BRAFV600E mutant was present in the
adult population in the current study at a slightly lower frequency
than that indicated by the meta-analysis (38 vs. 47%,
respectively), albeit with no discernible pattern linking the
mutation to lesional site (skin or bone) or disease severity (SS or
MS-LCH). While the meta-analysis revealed a higher prevalence of
mutations in high-risk organs, BRAFV600E status by
itself does not necessarily identify high-risk disease. The present
findings are broadly comparable with those reported by Berres et
al (25).
Consistency in BRAFV600E mutation status
in more than one lesion from the same individual (with the
exception of one lymph node biopsy) is consistent with a clonal
origin of the disease (4,5). Moreover, the fact that dendritic cells
derive from circulating myeloid precursors, and that lesional
Langerhans cells have an immature phenotype, suggests that LCH can
be considered to be a haematological tumour (14,19).
Haematopoietic forms of cancer typically exhibit
arrested cell development at a discrete stage of an ordered
developmental pathway, frequently associated with distinct patterns
of mutation. In addition, the increased prevalence of
BRAFV600E mutations in higher risk organs including the
liver and spleen (Fig. 1C) was
concordant with results from Héritier et al (26) suggesting that the expression of this
mutation in at-risk organs increases the aggressiveness of LCH,
particularly in younger patients. Clinically, LCH is currently
treated as a haematological disease in paediatric cases.
BRAF mutations in haematological malignancy are
relatively rare (27,28). The BRAFV600E mutation has a
high prevalence in in hairy cell leukaemia and has been suggested
to be the disease-defining event in this disorder (29). It is, however, rare in other B-cell or
associated lymphoproliferative disorders (28) and is notably absent from chronic and
acute myeloid neoplasms (30,31).
BRAF mutation-targeting therapy, including the BRAF
inhibitors vemurafenib and dabrafenib, have demonstrated evidence
of therapeutic activity in several BRAFV600E-mutated
cancer types, including hairy cell leukaemia (29,32–34).
However, the results of the current study suggest that, prior to
administering BRAF therapy, clinicians must be aware that the
mutation characterizes a subset of LCH and administration of such
therapies should be predicted upon genotyping. Furthermore, the
resistance-profile of BRAF inhibitors in melanoma (35) must be considered to ensure LCH is
treated and eliminated, rather than driving drug resistance and
limiting future clinical options.
The present study has demonstrated that
BRAFV600E mutations are present within a sub-population
of LCH patients. The haematological tumour profile exhibited by LCH
suggests that certain treatments that are currently undertaken in
paediatric LCH cases and for other haematopoietic types of cancer
may aid the treatment of LCH. An investigation of the effective
treatments for hairy cell leukaemia may offer more therapeutic
options for this disease.
Acknowledgements
The current study was sponsored by the Cotswold
Trust.
References
|
1
|
Chu T, D'Angio GJ, Favara BE, Ladisch S,
Nesbit M and Pritchard J: Histiocytosis syndromes in children.
Lancet. 2:41–42. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu T and Jaffe R: The normal Langerhans
cell and the LCH cell. Br J Cancer Suppl. 23:S4–S10.
1994.PubMed/NCBI
|
|
3
|
Egeler RM, Favara BE, van Meurs M, Laman
JD and Claassen E: Differential In situ cytokine profiles of
Langerhans-like cells and T cells in Langerhans cell histiocytosis:
Abundant expression of cytokines relevant to disease and treatment.
Blood. 94:4195–4201. 1999.PubMed/NCBI
|
|
4
|
Willman CL, Busque L, Griffith BB, Favara
BE, McClain KL, Duncan MH and Gilliland DG: Langerhans'-cell
histiocytosis (histiocytosis X)-a clonal proliferative disease. N
Engl J Med. 331:154–160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu RC, Chu C, Buluwela L and Chu AC:
Clonal proliferation of Langerhans cells in Langerhans cell
histiocytosis. Lancet. 343:767–768. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salotti JA, Nanduri V, Pearce MS, Parker
L, Lynn R and Windebank KP: Incidence and clinical features of
Langerhans cell histiocytosis in the UK and Ireland. Arch Dis
Child. 94:376–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aricò M, Girschikofsky M, Généreau T,
Klersy C, McClain K, Grois N, Emile JF, Lukina E, De Juli E and
Danesino C: Langerhans cell histiocytosis in adults. Report from
the International Registry of the Histiocyte society. Eur J Cancer.
39:2341–2348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitz L and Favara BE: Nosology and
pathology of Langerhans cell histiocytosis. Hematol Oncol Clin
North Am. 12:221–246. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alston RD, Tatevossian RG, McNally RJ,
Kelsey A, Birch JM and Eden TO: Incidence and survival of childhood
Langerhans cell histiocytosis in Northwest England from 1954 to
1998. Pediatr Blood Cancer. 48:555–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
A multicentre retrospective survey of
Langerhans' cell histiocytosis: 348 cases observed between 1983 and
1993. The French Langerhans' Cell Histiocytosis Study Group. Arch
Dis Child. 75:17–24. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Histiocytosis association (2014) LCH in
children.
|
|
12
|
Howarth DM, Gilchrist GS, Mullan BP,
Wiseman GA, Edmonson JH and Schomberg PJ: Langerhans cell
histiocytosis: Diagnosis, natural history, management, and outcome.
Cancer. 85:2278–2290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gadner H, Grois N, Pötschger U, Minkov M,
Aricò M, Braier J, Broadbent V, Donadieu J, Henter JI, McCarter R,
et al: Improved outcome in multisystem Langerhans cell
histiocytosis is associated with therapy intensification. Blood.
111:2556–2562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen CE, Li L, Peters TL, Leung HC, Yu A,
Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, et al:
Cell-specific gene expression in Langerhans cell histiocytosis
lesions reveals a distinct profile compared with epidermal
Langerhans cells. J Immunol. 184:4557–4567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahm F, Capper D, Preusser M, Meyer J,
Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M,
Kulozik A, et al: BRAFV600E mutant protein is expressed in cells of
variable maturation in Langerhans cell histiocytosis. Blood.
120:e28–e34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoecklein NH, Hosch SB, Bezler M, Stern
F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel
WT, et al: Direct genetic analysis of single disseminated cancer
cells for prediction of outcome and therapy selection in esophageal
cancer. Cancer Cell. 13:441–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badalian-Very G, Vergilio JA, Degar BA,
MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH,
Stevenson KE, Kehoe SM, et al: Recurrent BRAF mutations in
Langerhans cell histiocytosis. Blood. 116:1919–1923. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh T, Smith A, Sarde A, Lu HC, Mian S,
Trouillet C, Mufti G, Emile JF, Fraternali F, Donadieu J and
Geissmann F: B-RAF mutant alleles associated with Langerhans cell
histiocytosis, a granulomatous pediatric disease. PLoS One.
7:e338912012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Go H, Jeon YK, Huh J, Choi SJ, Choi YD,
Cha HJ, Kim HJ, Park G, Min S and Kim JE: Frequent detection of
BRAF (V600E) mutations in histiocytic and dendritic cell neoplasms.
Histopathology. 65:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bates SV, Lakshmanan A, Green AL, Terry J,
Badalian-Very G, Rollins BJ, Fleck P, Aslam M and Degar BA: BRAF
V600E-Positive multisite Langerhans cell histiocytosis in a preterm
neonate. AJP Rep. 3:63–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yousem SA, Dacic S, Nikiforov YE and
Nikiforova M: Pulmonary Langerhans cell histiocytosis: Profiling of
multifocal tumors using next-generation sequencing identifies
concordant occurrence of BRAF V600E mutations. Chest.
143:1679–1684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chilosi M, Facchetti F, Caliò A, Zamò A,
Brunelli M, Martignoni G, Rossi A, Montagna L, Piccoli P, Dubini A,
et al: Oncogene-induced senescence distinguishes indolent from
aggressive forms of pulmonary and non-pulmonary Langerhans cell
histiocytosis. Leuk Lymphoma. 55:2620–2626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haroche J, Charlotte F, Arnaud L, von
Deimling A, Hélias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay
D, Lesot A, Mokhtari K, et al: High prevalence of BRAF V600E
mutations in Erdheim-Chester disease but not in other
non-Langerhans cell histiocytoses. Blood. 120:2700–2703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei R, Wang Z, Li X, Shu Y and Fu B:
Frequent BRAFV600E mutation has no effect on tumor invasiveness in
patients with Langerhans cell histiocytosis. Biomed Rep. 1:365–368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berres ML, Lim KP, Peters T, Price J,
Takizawa H, Salmon H, Idoyaga J, Ruzo A, Lupo PJ, Hicks MJ, et al:
BRAF-V600E expression in precursor versus differentiated dendritic
cells defines clinically distinct LCH risk groups. J Exp Med.
211:669–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Héritier S, Emile JF, Barkaoui MA, Thomas
C, Fraitag S, Boudjemaa S, Renaud F, Moreau A, Peuchmaur M,
Chassagne-Clément C, et al: BRAF mutation correlates with high-risk
Langerhans cell histiocytosis and increased resistance to
first-line therapy. J Clin Oncol. 34:3023–3030. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiacci E, Trifonov V, Schiavoni G, Holmes
A, Kern W, Martelli MP, Pucciarini A, Bigerna B, Pacini R, Wells
VA, et al: BRAF mutations in hairy-cell leukemia. N Engl J Med.
364:2305–2315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davidsson J, Lilljebjörn H, Panagopoulos
I, Fioretos T and Johansson B: BRAF mutations are very rare in B-
and T-cell pediatric acute lymphoblastic leukemias. Leukemia.
22:1619–1621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maevis V, Mey U, Schmidt-Wolf G and
Schmidt-Wolf IG: Hairy cell leukemia: Short review, today's
recommendations and outlook. Blood Cancer J. 4:e1842014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tadmor T, Tiacci E, Falini B and Polliack
A: The BRAF-V600E mutation in hematological malignancies: A new
player in hairy cell leukemia and Langerhans cell histiocytosis.
Leuk Lymphoma. 53:2339–2340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trifa AP, Popp RA, Cucuianu A, Coadă CA,
Urian LG, Militaru MS, Bănescu C, Dima D, Farcaş MF, Crişan TO, et
al: Absence of BRAF V600E mutation in a cohort of 402 patients with
various chronic and acute myeloid neoplasms. Leuk Lymphoma.
53:2496–2497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dietrich S, Hüllein J, Hundemer M, Lehners
N, Jethwa A, Capper D, Acker T, Garvalov BK, Andrulis M, Blume C,
et al: Continued response off treatment after BRAF inhibition in
refractory hairy cell leukemia. J Clin Oncol. 31:e300–e303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kainthla R, Kim KB and Falchook GS:
Dabrafenib for treatment of BRAF-mutant melanoma. Pharmgenomics
Pers Med. 7:21–29. 2013.PubMed/NCBI
|
|
35
|
Sullivan RJ and Flaherty KT: Resistance to
BRAF-targeted therapy in melanoma. Eur J Cancer. 49:1297–1304.
2013. View Article : Google Scholar : PubMed/NCBI
|