Introduction
Bladder cancer is one of the most prevalent urologic
malignancy and the fourth common cancer among men worldwide
(1). There are several treatment
choices for bladder cancer patients including radical cystectomy,
radiation therapy and prospective instillation of chemotherapy and
immunotherapy (2,3). Although the treatment of bladder cancer
has been improved in recent years, it frequently recurs due to
metastasis and its prognosis with 5-year overall survival rate are
unsatisfactory (4). Therefore, it is
urgently necessary to elucidate the molecular mechanism of bladder
cancer metastasis to discover novel biomarkers for the further
improvement of bladder cancer treatment.
Among the multiple processes involved in bladder
cancer metastasis, cancer cell invasion is considered a key
process. For cancer cell invasion process, highly invasive cancer
cells form invadopodia, the filamentous actin (F-actin)-based
membrane protrusions to remodel and degrade extracellular matrix
(ECM) and invade surrounding tissues (5). Although invadopodia are considered to
play important roles in the steps of the metastatic cascade, the
details of the invadopodia formation have not been fully understood
yet.
LIM and SH3 protein-1 (LASP-1) is a multi-domain
protein. The LASP-1 protein contains a LIM (Lin-11, IsI1, MEC-3)
protein-protein interaction domain (6,7), two
F-actin-binding nebulin (NEBU) domains and Src homology (SH) 3
domain and it was shown that LASP-1 regulates cytoskeleton dynamics
(8,9).
Several groups reported that overexpression of LASP-1 has been
observed in a variety of malignant tumors including metastatic
breast cancer (10), ovarian cancer
(11) and medulloblastoma (12). Recent clinical studies have
demonstrated that LASP is one of the useful biomarkers for bladder
cancer detection (13,14) and that LASP-1 is involved in bladder
cancer metastasis (15). The above
studies on the LASP-1 function and its high expression in malignant
bladder cancer led us to postulate that LASP-1 is involved in
bladder cancer malignant phenotypes such as cell invasion through
the regulation of actin cytoskeleton.
In the present study, we found the correlation of
LASP-1 expression with clinicopathological characteristics in
bladder cancer and also performed functional analysis to determine
the biological functions of LASP-1 in cancer metastasis.
Materials and methods
Cells, reagents and antibodies
Four established human bladder cancer cell lines
were used. KK-47 was a generous gift of Dr. T. Matsuo (Tohoku
University, Sendai, Japan) (16).
RT-4 and T24 were purchased from American Type Culture Collection.
A human invasive and high-grade bladder cancer cell line, YTS-1 was
a generous gift of Dr Kakizaki H. (Yamagata University, Yamagata,
Japan) (17). Cells were maintained
in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 10% fetal bovine serum (FBS; Capricorn Scientific
GmbH, Ebsdorfergrund, Germany) with 5% CO2 at 37°C. The
monoclonal antibodies to LASP-1 (clone 86C) and
glyceraldehyde-3-phospahte dehydrogenase (GAPDH) (clone 3H12) were
purchased from Abcam (Cambridge, UK) and MBL (Woburn, MA, USA),
respectively.
Analyses of bladder cancer
patients
The cohort consisted of 48 patients with muscle
invasive bladder cancer treated with radical cystectomy at the
Department of Urology, Hirosaki University Graduate School of
Medicine (Hirosaki, Japan). Bladder cancer specimens from patients
were fixed with 10% buffered formalin for 12–24 h.
Paraffin-embedded lymph node samples were cut at 3 µm and subjected
to hematoxylin and eosin staining and immunohistochemistry using a
monoclonal antibody to LASP-1. Anti-mouse immunoglobulin antibody
conjugated with horseradish peroxidase (Agilent Technologies Japan,
Ltd., Tokyo, Japan) was used as a secondary antibody and peroxidase
activity was visualized with Liquid DAB+ Substrate
Chromogen System (Agilent Technologies Japan, Ltd.). Based on the
histochemical staining results, specimens that showed
LASP-1-positive in greater than 50% of cancer cells were judged as
LASP-1-positive. Patient survival was compared to the results of
immunohistochemistry results. The institutional ethics committee of
Hirosaki University Graduate School of Medicine approved this
study.
LASP-1 knockdown (LASPKD)
YTS-1 cells with reduced expression of LASP-1
(LASPKD cells) were generated by shRNA technology as previously
described (18). An shRNA expression
plasmid was constructed using pBAsi-hU6 Neo DNA (Takara Bio, Inc.,
Otsu, Japan). The shRNA sequence for LASP-1 was: GAT CCA
AGGTGAACTGTCTGGATAAGCTGTGAAGCCACAGATGGGCTTATCCAGACAGTTCACCTTTTTTTTA
(The siRNA sequence for LASP-1 was underlined.) (19). A human non-targeting siRNA sequence
(Accell siRNA control; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used to prepare the control cells expressing non-targeting
shRNA. We transfected cells with LASP-1 shRNA expression plasmid
together with pEGFP-C2 vector (BD Biosciences, Palo Alto, CA, USA)
and pTK-Hyg Vector (Clontech Laboratories, Inc., Mountain View, CA,
USA) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). EGFP-positive cells were separated using
BD FACSAria (BD Biosciences, Franklin Lakes, NJ, USA) and then
cultured in RPMI-1640 medium containing 10% FBS and 50 µg/ml
hygromycin B for 2–4 days. Greater than 70% of cultured cells were
positive for EGFP.
Western blotting
Total lysates of cancer cells were prepared by
solubilization in 50 mM Tris-HCl buffer, pH 7.5, containing 1%
Igepal CA-630, 150 mM NaCl and proteinase inhibitors. The lysates
were resolved by SDS-PAGE on an 8–16% gradient gel (Invitrogen;
Thermo Fisher Scientific, Inc.), and transferred to polyvinylidene
fluoride (PVDF) membrane. Western blot analyses were performed
using the specific primary antibodies and a horseradish
peroxidase-conjugated secondary antibody. Signals were visualized
using the ECL PLUS detection system (GE Healthcare Life Sciences,
Little Chalfont, UK).
Immunofluorescence microscopy
Cells seeded on coverslips were fixed in 4%
paraformaldehyde and permeabilized with PBS containing 0.1% saponin
and 1% bovine serum albumin (BSA). Cells were stained with Alexa
Fluor 568-labelled phalloidin (Invitrogen; Thermo Fisher
Scientific, Inc.) together with the monoclonal antibody to LASP-1,
and Alexa Fluor 488-labelled secondary antibody (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for antibody staining. Cell
staining was examined under an Olympus IX-73 fluorescence
microscope (Olympus Corp., Tokyo, Japan).
Matrigel invasion assay
Invasion assay was performed using a conventional
Transwell system (BD Biosciences, San Jose, CA, USA). The bottom
surface of the filter (with 8 µm pore size) of the upper chamber
was coated with 100 µg/ml of fibronectin and the top surface was
covered with 1 mg/ml of Matrigel Matrix (BD Biosciences). The lower
chamber was filled with RPMI-1640 medium supplemented with FBS.
EGFP-positive bladder cancer cells (5×104) were placed
into the upper chamber. After incubation at 37°C for 24 h,
non-migrated cells remaining at the top surface of the filter were
carefully removed with cotton swabs. Migrated cells on the bottom
surface were fixed with 4% paraformaldehyde and counted under a
fluorescence microscope (Olympus IX-73).
Proliferation assay
For cancer cell proliferation assay, cells were
seeded in 96-well plates (1×104 cells per well) and
cultured at 37°C for indicated time. Cell growth was assayed using
Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's specification.
Statistical analysis
We used the statistical program SPSS 12.0 (SPSS,
Inc., Chicago, IL, USA). Statistically significant differences were
determined using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of LASP-1 in bladder cancer
tissues
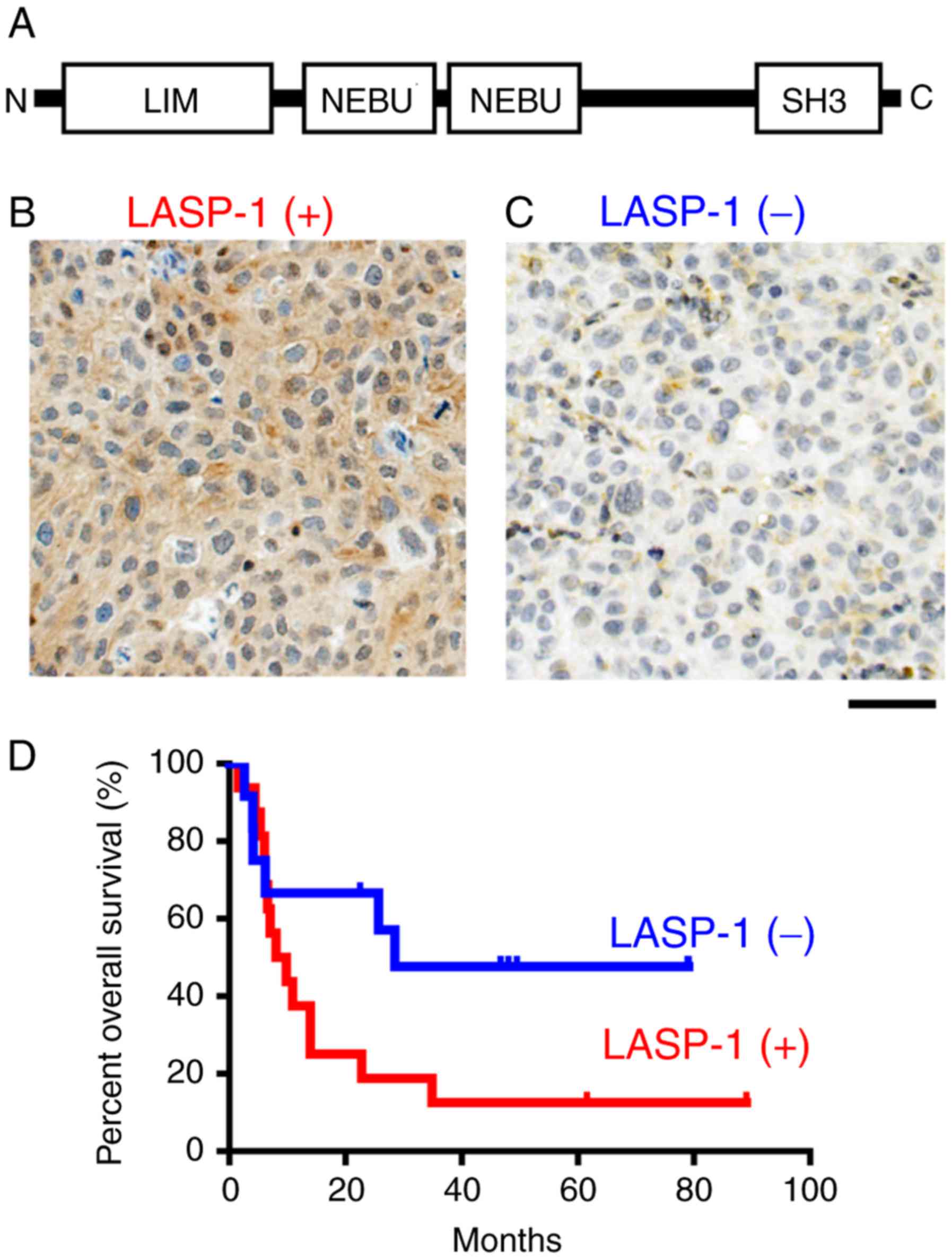
The domain organization of LASP-1 (Fig. 1A) showed that LASP-1 contains the
N-terminal LIM domain, two F-actin binding domain (NEBU domain) and
C-terminal SH3 domain. To evaluate the importance of LASP-1 in
prognosis of bladder cancer patients, we immunohistochemically
examined the expression status of LASP-1 in radical cystectomy
specimens from 48 bladder cancer patients using anti-LASP-1
monoclonal antibody. The typical LASP-1-positive staining pattern
showed LASP-1 in the cytoplasm (Fig.
1B). Based on the staining status, the patients were divided
into two groups, those with LAPS-1-positive specimens (Fig. 1B) and those with C2GnT-negative
specimens (Fig. 1C). A Kaplan-Meier
analysis showed that LASP-1-positive patients (n=33) survived for
shorter time than the LASP-1-negative patients (n=15) (Fig. 1D). The factor that has the strongest
impact on prognosis is recurrence due to metastasis. These results
suggest that bladder cancer highly expressing LASP-1 was malignant
and metastatic.
Increased expression of LASP-1 in
invasive bladder cancer cells
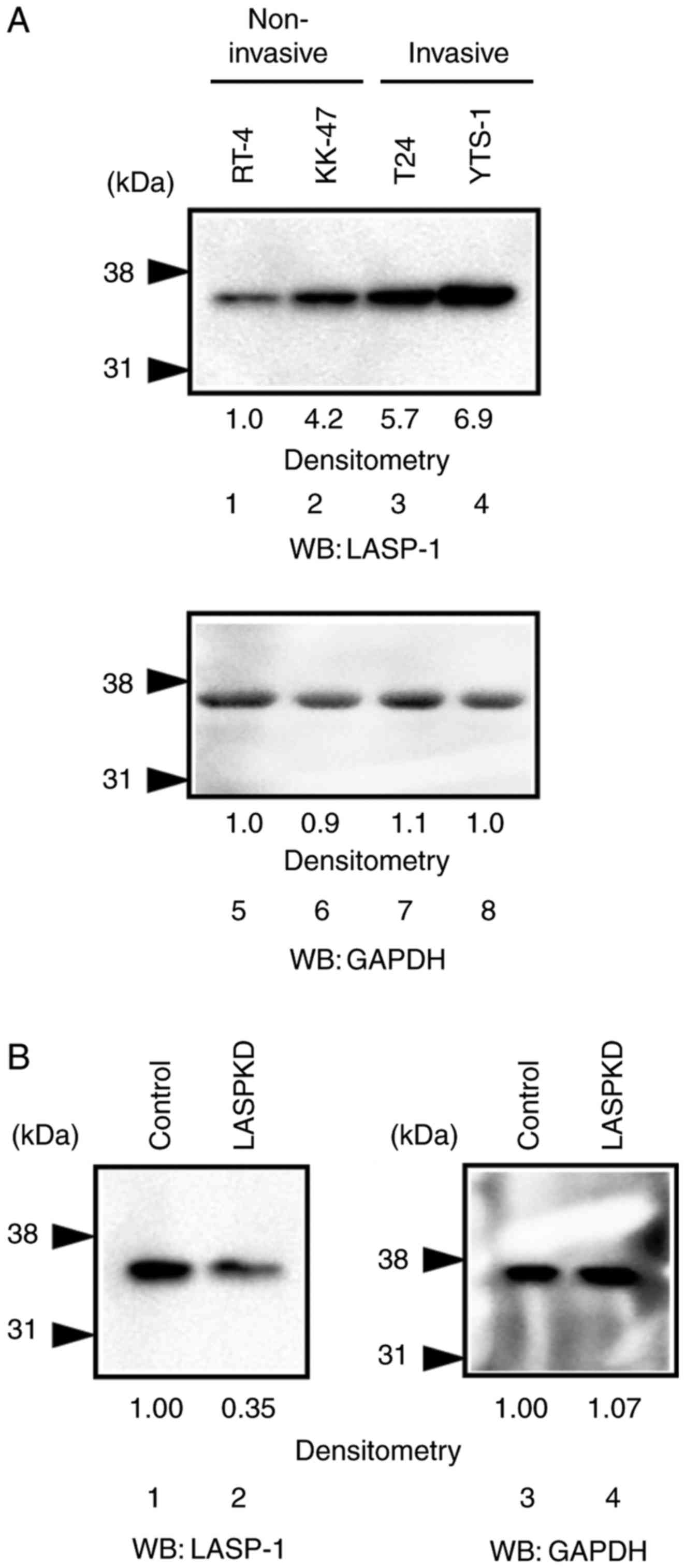
To explore what roles LASP-1 plays in malignant
bladder cancer cells, we first examine the expression level of
LASP-1 in bladder cancer cell lines. The expression level of LASP-1
was increased in invasive bladder cancer cell lines, T24 and YTS-1
(Fig. 2A, lanes 3 and 4), compared
with the non-invasive bladder cancer cell lines, RT-4 and KK-47
(Fig. 2A, lanes 1 and 2). This
suggests that LASP-1 is involved in bladder cancer cell invasion.
To further examine the roles of LASP-1 in cancer cell invasion, we
established LASPKD cells by silencing the expression of LASP-1 in
YTS-1 (an invasive bladder cancer cell line) cells using shRNA
technology. Western blotting showed that LASP-1 expression was
reduced in LASPKD cells (Fig. 2B,
lane 2) compared with control cells (Fig.
2B, lane 1).
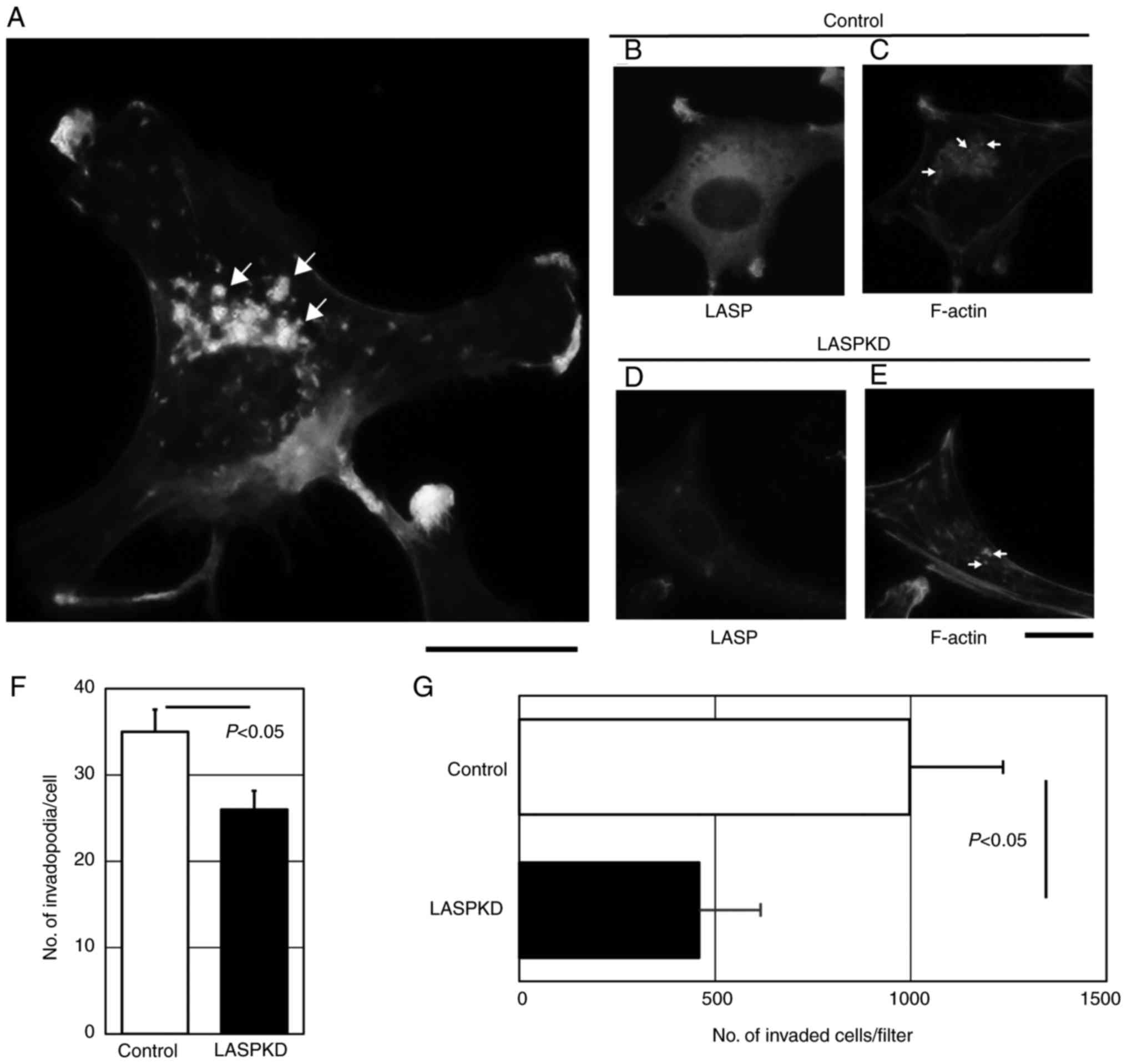
Reduction of invadopodia formation in
LASPKD cells
We previously reported that invadopodia formation is
critical for the invasion capacity of bladder cancer cells
(20–22). We hypothesized that higher expression
of LASP-1 in bladder cancer cells contributes to highly invasive
property by regulating invadopodia formation. Invadopodia are the
membrane protrusions enriched by polymerized filamentous actin
(F-actin). YTS-1 cells which are invasive bladder cancer cells were
permeabilized and stained with Alexa Fluor 568-labeled phalloidin
to visualize F-actin cores of invadopodia. Phalloidin staining of
YTS-1 cells gives lots of F-actin puncta (Fig. 3A). These punctate structures are
typical invadopodia (Several typical ones were denoted by arrows).
To determine the role of LASP-1 in invadopodia formation, we
examined the invadopodia formation in LASPKD cells. YTS-1 cells
expressing human non-targeting siRNA were examined as a control
cell (Control). Control cells exhibited a typical cytosolic
staining of LASP-1 (Fig. 3B) and the
expression of LASP-1 was reduced in LASPKD cells (Fig. 3D). Numerous invadopodia were observed
in control cells (Fig. 3C; several
typical ones were denoted by arrows), which was equivalent to YTS-1
cell (Fig. 3A). Although LASPKD cells
had an ability to form invadopodia, the number of invadopodia was
significantly decreased compared with control cells (denoted by
arrows) (Fig. 3E and F). Invadopodia
play a critical role in cancer cell invasion. We next examined
in vitro invasive capacity of LASPKD cells by using a
conventional invasion assay method in a Boyden chamber. Invasive
capacity of LASPKD cells was significantly lower than that of
control cells (Fig. 3G). These
results indicate that LASPKD cells exhibit less invasive capacity
due to the reduced number of invadopodia, suggesting that LASP-1
play an important role in invasion by promoting invadopodia
formation.
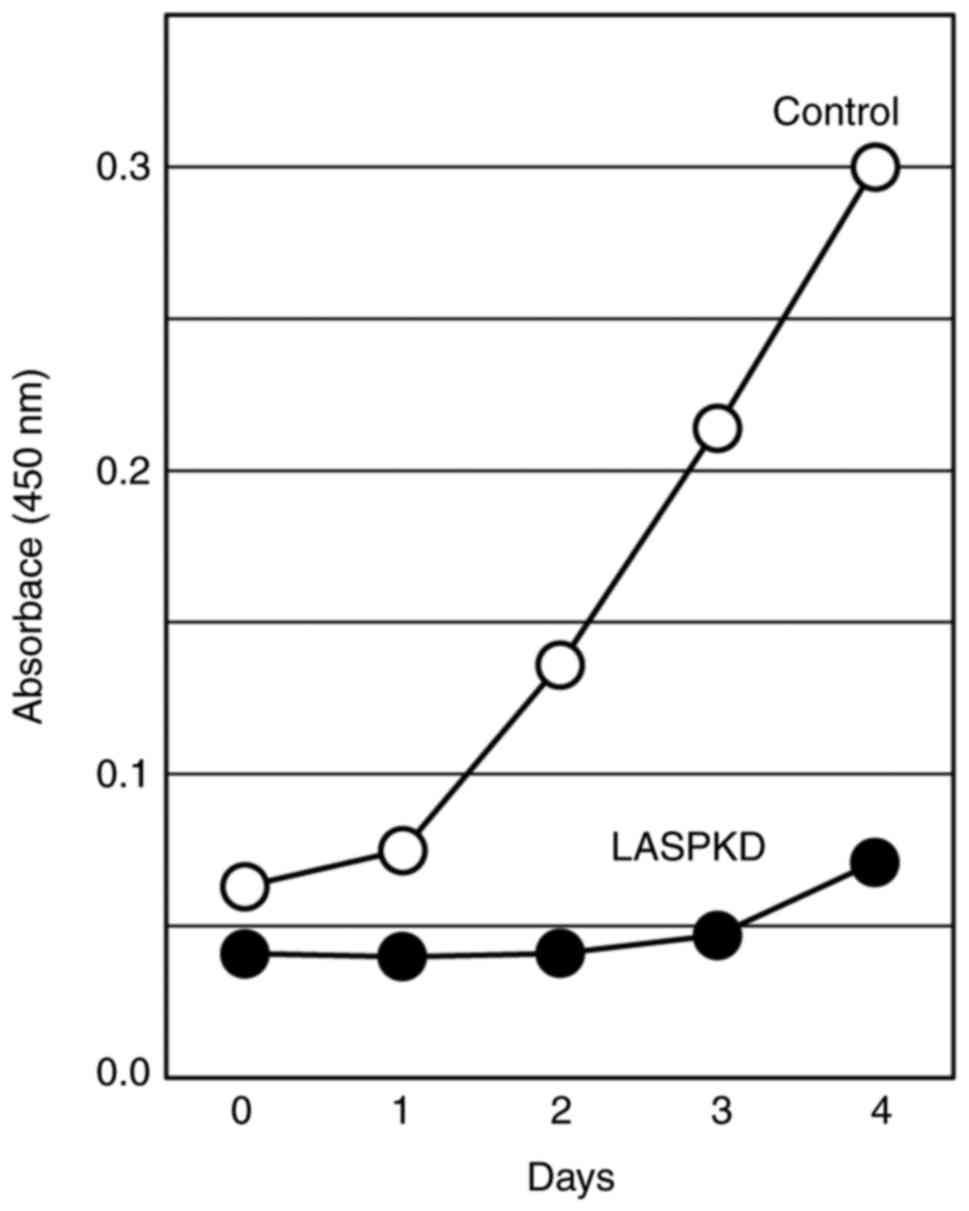
Reduced cell proliferation in LASPKD
cells
Another important biological process in which LASP-1
may be involved is cell proliferation. To examine the impact of
LASP-1 on cellular proliferative ability, we monitored the cell
proliferation in LASPKD cells for four consecutive days. LASPKD
cells exhibited a marked reduction of cell proliferation compared
with control cells (Fig. 4),
suggesting that LASP-1 expression is critical to cell
proliferation.
Discussion
We observed a high expression of LASP-1 in malignant
bladder cancer specimens compared with benign specimens (Fig. 1B). We also showed that higher LASP-1
expression correlated with poor prognosis (Fig. 1C). LASP-1 was originally identified
from a cDNA library of metastatic breast cancer cells (23). In addition, there was a study
reporting that LASP-1 expression was significantly higher in oral
squamous cell carcinoma than that in normal oral tissues (24). Our results taken together with these
previous observations suggest that higher expression of LASP-1 is
involved in the development of metastatic phenotype. This is
supported by the recent observation that LASP-1 can be a promising
urinary marker for detection of bladder cancer (13,14).
One of the malignant phenotypes of cancer which
mediate metastasis is invasion. Higher invasion capacity leads to
metastasis. In order for cancer cells to efficiently invade
surrounding tissues, invadopodia formation is required (25–27). Our
previous studies showed that bladder cancer cells forming
invadopodia exhibited the high capacity of transurothelial invasion
through the bladder muscle layers and extravasation from the blood
vessels, resulting in metastasis (22,28). This
study showed that LASP-1 is necessary for invadopodia formation and
Matrigel invasion (Fig. 3). These
results suggest that LASP-1 plays a critical role in metastasis by
promoting invadopodia formation. This is supported by a recent
study reporting LASP-1 regulates the function of podosomes in
macrophages. Podosomes are the F-actin-based membrane structures
which are homologous with invadopodia (19). Future investigation into the detailed
mechanisms of the involvement of LASP-1 in the formation of
invadopodia and podosomes should be required.
Unrestricted cell proliferation is one of the
hallmarks of malignant cancers. Our result indicates that LASP-1
silencing results in a strong inhibition of cancer cell
proliferation (Fig. 4). This suggests
that LASP-1 plays an essential role in cell proliferation. LASP-1
is involved in cell cycle G2/M phase transition of oral cancer
cells (24). Our result taken
together with this observation strongly suggests that
overexpression of LASP-1 increases cell proliferation in bladder
cancer cells, promoting its malignancy.
In conclusion, our data suggest that LASP-1 plays
the important roles in both invadopodia formation and cell
proliferation, contributing to the promotion of malignant
phenotypes of poor-prognosis bladder cancer. It is noteworthy that
LASP-1 may be a useful biomarker of bladder cancer and a possible
therapeutic target for developing anti-cancer drugs because of its
importance in cancer malignant phenotypes such as high invasiveness
and unrestricted cell proliferation.
Acknowledgements
This study was supported by the grants-in-aid from
the Mizutani Foundation (S.T.) and Japanese Society for the
Promotion of Science (15K06989) (S.T.), (grant nos. 15H02563,
15K15579 and 25220206) (C.O.).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weintraub MD, Li QQ and Agarwal PK:
Advances in intravesical therapy for the treatment of non-muscle
invasive bladder cancer (Review). Mol Clin Oncol. 2:656–660. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Booth CM, Siemens DR, Li G, Peng Y,
Tannock IF, Kong W, Berman DM and Mackillop WJ: Perioperative
chemotherapy for muscle-invasive bladder cancer: A population-based
outcomes study. Cancer. 120:1630–1638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sfakianos JP, Kim PH, Hakimi AA and Herr
HW: The effect of restaging transurethral resection on recurrence
and progression rates in patients with nonmuscle invasive bladder
cancer treated with intravesical bacillus Calmette-Guérin. J Urol.
191:341–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Even-Ram S and Yamada KM: Cell migration
in 3D matrix. Curr Opin Cell Biol. 17:524–532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Way JC and Chalfie M: mec-3, a
homeobox-containing gene that specifies differentiation of the
touch receptor neurons in C. Elegans. Cell. 54:5–16. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karlsson S and Ahrén B: Insulin and
glucagon secretion in swimming mice: Effects of autonomic receptor
antagonism. Metabolism. 39:724–732. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chew CS, Parente JA Jr, Zhou C, Baranco E
and Chen X: Lasp-1 is a regulated phosphoprotein within the cAMP
signaling pathway in the gastric parietal cell. Am J Physiol.
275:C56–C67. 1998.PubMed/NCBI
|
|
9
|
Grunewald TG and Butt E: The LIM and SH3
domain protein family: Structural proteins or signal transducers or
both? Mol Cancer. 7:312008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grunewald TG, Kammerer U, Schulze E,
Schindler D, Honig A, Zimmer M and Butt E: Silencing of LASP-1
influences zyxin localization, inhibits proliferation and reduces
migration in breast cancer cells. Exp Cell Res. 312:974–982. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grunewald TG, Kammerer U, Winkler C,
Schindler D, Sickmann A, Honig A and Butt E: Overexpression of
LASP-1 mediates migration and proliferation of human ovarian cancer
cells and influences zyxin localisation. Br J Cancer. 96:296–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Traenka C, Remke M, Korshunov A, Bender S,
Hielscher T, Northcott PA, Witt H, Ryzhova M, Felsberg J, Benner A,
et al: Role of LIM and SH3 protein 1 (LASP1) in the metastatic
dissemination of medulloblastoma. Cancer Res. 70:8003–8014. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ardelt P, Grunemay N, Strehl A, Jilg C,
Miernik A, Kneitz B and Butt E: LASP-1, a novel urinary marker for
detection of bladder cancer. Urol Oncol. 31:1591–1598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Payton S: Bladder cancer: LASP-1-a
promising urine marker for detection of bladder cancer. Nat Rev
Urol. 9:2402012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Chen Y, Wang X, Zhang H, Zhang Y,
Gao Y, Weng M, Wang L, Liang H, Li M, et al: LASP-1 induces
proliferation, metastasis and cell cycle arrest at the G2/M phase
in gallbladder cancer by down-regulating S100P via the PI3K/AKT
pathway. Cancer Lett. 372:239–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota Y, Nakada T, Yanai H, Kakizaki H,
Sasagawa I and Watanabe M: Electropermeabilization in bladder
cancer chemotherapy. Cancer Chemother Pharmacol. 39:67–70. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hisazumi H, Uchibayashi T, Katoh M,
Kobayashi T, Nakajima K, Naitoh K, Misaki T and Kuroda K:
Anticancer drug sensitivity in vitro in the bladder cancer cell
line, KK-47 and prophylactic use of carbazilquinone and urokinase
in bladder cancer. Urol Res. 9:231–235. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka
N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T,
et al: A novel strategy for evasion of NK cell immunity by tumours
expressing core2 O-glycans. EMBO J. 30:3173–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stölting M, Wiesner C, van Vliet V, Butt
E, Pavenstädt H, Linder S and Kremerskothen J: Lasp-1 regulates
podosome function. PLoS One. 7:e353402012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sutoh M, Hashimoto Y, Yoneyama T, Yamamoto
H, Hatakeyama S, Koie T, Okamoto A, Yamaya K, Saitoh H, Funyu T, et
al: Invadopodia formation by bladder tumor cells. Oncol Res.
19:85–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto H, Sutoh M, Hatakeyama S,
Hashimoto Y, Yoneyama T, Koie T, Saitoh H, Yamaya K, Funyu T,
Nakamura T, et al: Requirement for FBP17 in invadopodia formation
by invasive bladder tumor cells. J Urol. 185:1930–1938. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoneyama Sutoh M, Hatakeyama S, Habuchi T,
Inoue T, Nakamura T, Funyu T, Wiche G, Ohyama C and Tsuboi S:
Vimentin intermediate filament and plectin provide a scaffold for
invadopodia, facilitating cancer cell invasion and extravasation
for metastasis. Eur J Cell Biol. 93:157–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomasetto C, Moog-Lutz C, Regnier CH,
Schreiber V, Basset P and Rio MC: Lasp-1 (MLN 50) defines a new LIM
protein subfamily characterized by the association of LIM and SH3
domains. FEBS Lett. 373:245–249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimizu F, Shiiba M, Ogawara K, Kimura R,
Minakawa Y, Baba T, Yokota S, Nakashima D, Higo M, Kasamatsu A, et
al: Overexpression of LIM and SH3 Protein 1 leading to accelerated
G2/M phase transition contributes to enhanced tumourigenesis in
oral cancer. PLoS One. 8:e831872013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caldieri G, Ayala I, Attanasio F and
Buccione R: Cell and molecular biology of invadopodia. Int Rev Cell
Mol Biol. 275:1–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linder S, Wiesner C and Himmel M:
Degrading devices: Invadosomes in proteolytic cell invasion. Annu
Rev Cell Dev Biol. 27:185–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: Characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imanishi K, Yoneyama MS, Hatakeyama S,
Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Nakamura T, Ohyama
C and Tsuboi S: Invadopodia are essential in transurothelial
invasion during the muscle invasion of bladder cancer cells. Mol
Med Rep. 9:2159–2165. 2014.PubMed/NCBI
|