Introduction
Lung cancer is associated with one of the highest
mortality rates of all malignant tumors worldwide, resulting in
>1 million mortalities annually (1,2). Non-small
cell lung cancer (NSCLC) is the primary type of lung carcinoma,
accounting for 80–85% of the total number of incidences of lung
cancer (3,4). Despite the emergence of novel drugs and
therapies, the prognosis of lung cancer remains poor, and the
5-year overall survival (OS) rate is ~11% (5,6).
Therefore, additional analysis of the mechanisms resulting in the
occurrence and the development of NSCLC is required to improve the
survival rate of lung cancer.
MicroRNAs (miRNAs) are a class of small single
stranded non-coding RNAs measuring between 20 and 24 nucleotides
(7), which are associated with
post-transcriptional gene regulation (8). miRNAs participate in the regulation of
cellular proliferation, differentiation and apoptosis by inducing
target mRNA degradation or translation inhibition, which affects
cancer occurrence and development. Aberrant miRNA expression is
associated with the development of different types of cancer.
Recent reports (9–11) have suggested that the downregulation
of miRNA (miR)-138, miR-218, miR-34c-3p was found in various types
of cancer, including NSCLC. Therefore, miRNAs serve an important
role in the diagnosis, prediction and treatment of NSCLC and
represent novel research targets in the field of molecular biology,
which is becoming a prominent discipline within the life
sciences.
miR-125a-3p is a member of the miR-125a family, and
in the present study has been demonstrated to exert a tumor
suppressive effect on several types of tumor, such as glioma
(12) and gastric cancer (13). However, there are few studies that
demonstrate the link between miR-125a-3p and lung cancer
specifically (14). Therefore, the
use of miR-125a-3p as a prognostic biomarker for the treatment of
NSCLC remains unknown.
The present study explored the differential
expression of miR-125a-3p within the gene expression omnibus (GEO)
database, and investigated the expression levels of miR-125a-3p in
NSCLC tissues using a quantitative polymerase chain reaction (qPCR)
assay. Additionally, the present study analyzed the association
between miR-125a-3p and patient clinicopathological
characteristics.
Materials and methods
Ethical statement
The present study was approved by the Ethics
Committee of Shanghai 10th People's Hospital, Tongji University
School of Medicine (SHSY-IEC-PAP-15-18; Shanghai, China). All
patients provided written informed consent.
Set-up of server for online survival
calculation
A total of 4 datasets were used for the miR-125a-3p
expression analysis between normal lung and cancer tissues: The
expression levels of miR-125a-3p in a number of cells, reported
previously, (15) were analyzed; 20
pairs of lung cancer samples and adjacent normal lung samples were
compared with GEO datasets, GEO series GSE18692, and 61 lung
squamous carcinoma tissues and 10 normal tissues were investigated,
GEO series GSE16025. The peripheral miRNA blood profiles from
healthy subjects (n=19), lung cancer patients (n=28), and chronic
obstructive pulmonary disease (COPD) samples (n=24) had been
screened for the complete miRNA repertoire; GEO series
GSE24709.
Acquisition of clinical samples
Frozen NSCLC samples (n=118), and paired carcinoma
and adjacent normal lung tissue specimens (n=30) were collected
from a total of 148 patients undergoing surgical resection and
assessed for the expression level of miR-125a-3p. These tissue
samples were collected from Shanghai 10th People's Hospital and
Shanghai Pulmonary Hospital, Tongji University School of Medicine
(Shanghai, China) between January 2008 and December 2012. The
clinical data gathered included the patients' age, gender, smoking
history, lymph-node metastasis, tumor differentiation, histological
grade, tumor node metastasis (TNM) stage, invasion of lung
membrane, vascular invasion, chemotherapy, diameter, OS,
disease-free survival (DFS) rate and miR-125a-3p expression status
of the patients.
RNA extraction
The RNA, including miRNAs, was extracted from the
carcinoma and the adjacent normal lung tissues using TRIzol reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to
protocol of the manufacturer. A Nanodrop 1000 spectrophotometer
(Thermo Fisher Scientific Inc., Wilmington, DE, USA) was used to
assess the RNA concentration and the purity, following the protocol
of the manufacturer. Electrophoresis on 1.5% denaturing agarose
gels was carried out to evaluate the quality of all the RNA
specimens.
RT-qPCR
For the miR-125a-3p qPCR, complementary DNA was
synthesized from 10 ng of the total RNA using the TaqMan Universal
PCR kit (cat no. 4304437; Thermo Fisher Scientific, Inc.) and
individual miR-125a-3p primers. Specific RT primers and TaqMan
probes were used to quantify the expression of hsa-miR-125a-3p (cat
no. 4395310). Samples were normalized to RNU6B (cat no. 4373381) as
indicated. All reactions, including no-template controls and
RT-minus controls, were run in an ABI Prism 7900 HT sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Thermocycling conditions were as follows: Initial
denaturation at 94°C for 10 min, followed by 35 cycles of 94°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec with a final extension
at 72°C for 10 min. Each reaction was independently tested in
duplicate a minimum of 3 times. The quantification cycle (Cq) was
defined as the fractional cycle number at which the fluorescence
passed the fixed threshold. The relative amount of miR-125a-3p to
RNU6B was calculated using the equation 2−ΔΔCq (16).
Statistical analysis
All statistical analyses were performed with IBM
SPSS 19.0 (IBM Corp., Armonk, NY, USA). The results were presented
as the mean ± standard deviation, using at least 3 independent
experiments. A χ2 analysis was carried out to compare
the differences between categorical variables and the Wilcoxon
signed-rank test was performed to compare of differences between
the 2 groups. The Kaplan-Meier method was used to evaluate the
univariate probabilities of OS and DFS, and the comparison of the
survival curves was assessed using a log rank test. A Cox
regression model was used to estimate the univariate and the
multivariate hazard ratios. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-125a-3p using in
silico analysis
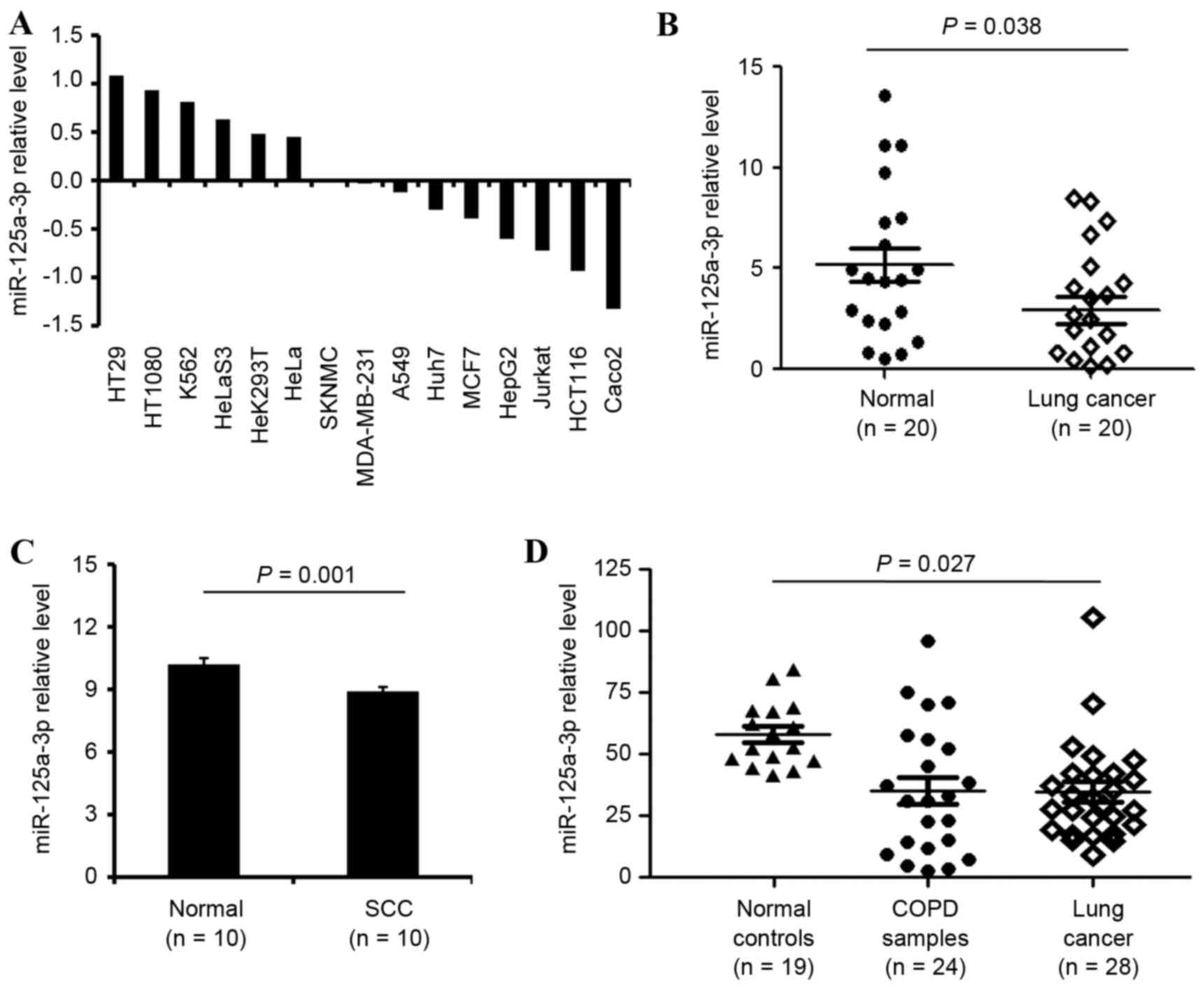
Throughcomparing the expression levels of
miR-125a-3p indifferent cancer cell lines analyzed in a previous
study (15), the present study
demonstrated that miR-125a-3p expression levels werelower in the
lung cancer A549 cells compared withthe other cancer cell lines, as
demonstrated in Fig. 1A. In 20 pairs
of lung cancer and adjacent normal lung samples, the expression
values in the non-tumoral adjacent (n=20) and in the lung cancer
tissues (n=20) were 5.02±0.49 and 3.21±0.58, respectively compared
with the control. This data suggested that the expression of
miR-125a wasdownregulated in lung cancer samples (P=0.038), as
illustrated in Fig. 1B. Additionally,
the lung squamous carcinoma tissues (n=61) and normal tissues
(n=10) were investigated, and these data indicated that miR-125a-3p
expression was lower in lung squamous carcinoma tissues, 8.95±0.16,
in contrast with normal tissues, 10.24±0.31, as demonstrated in
Fig. 1C (P<0.001).
Furthermore, the peripheral miRNA blood profiles
from healthy subjects (n=19), patients with lung cancer (n=28), and
COPD samples (n=24) werescreened for the complete miRNA repertoire,
and their expression values were 61.83±2.05, 32.45±5.75 and
31.06±4.53, respectively. The results revealed that miR-125a-3p was
downregulated in lung cancer (P=0.027), as demonstrated in Fig. 1D.
miRNA-125a-3p expression in NSCLC and
normal lung tissue
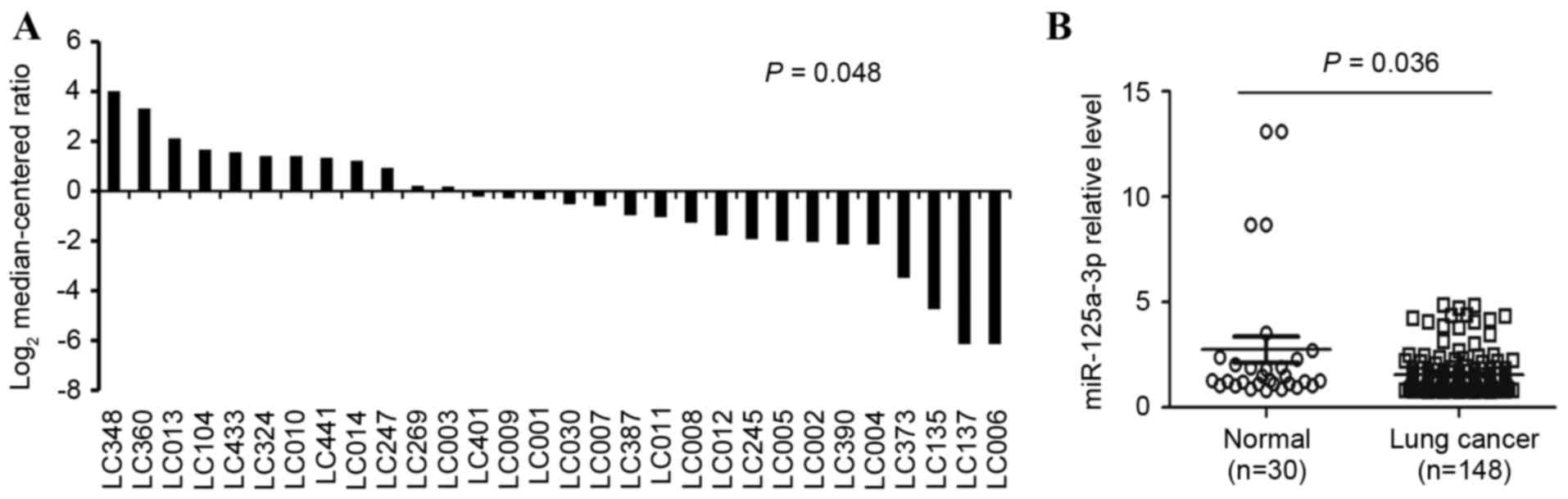
The levelof miR-125a-3p in 30 paired NSCLC samples
were collected and analyzed via RT-qPCR analysis, and it was
identified that miR-125a-3p expression levels were lower in lung
cancer cells, 2.25±0.61, relative to the non-tumor samples, which
exhibited levels of 4.01±0.88 (P=0.048) as demonstrated in Fig. 2A. Additionally, the expression levels
of miR-125a-3p in the 118 lung cancer samples were examined by
RT-qPCR, and are illustrated in Fig.
2B. The expression levels of miR-125a-3p in the adjacent normal
tissues, 4.01±0.88, weremarkedly higher than the NSCLC tumor
biopsies, 1.64±0.37. The difference in the expression levels
between the normal lung tissue and the NSCLC tumor tissue was
statistically significant (P=0.036).
The association between miR-125a-3p
expression and clinical characteristics
Theassociation between miR-125a-3p expression levels
and individual clinical characteristics was investigated. A
univariate analysis demonstrated that the miR-125a-3p expression
level was significantly correlated with lymph-node metastasis
(P=0.004), TNM stage (P=0.031) and tumor diameter (P=0.011), as
demonstrated in Table I in all 148
patients with NSCLC. In contrast, no association was identified
between age, gender, smoking history, tumor differentiation,
histology, invasion of the lung membrane, vascular invasion or
chemotherapy (P>0.05).
 | Table I.Univariate analysis of overall
survival based on patients stratified by clinical
characteristics. |
Table I.
Univariate analysis of overall
survival based on patients stratified by clinical
characteristics.
| Factor | Variable | N | miR-125a-3p
expression (median) | P-value |
|---|
| Age (years) | ≥60 | 87 | 2.78 |
|
|
| <60 | 61 | 3.63 | 0.229 |
| Gender | Male | 92 | 3.86 |
|
|
| Female | 56 | 2.99 | 0.192 |
| Smoking
history | Never | 35 | 2.98 |
|
|
| Ever | 62 | 4.35 | 0.075 |
|
| Unknown | 51 | 3.48 |
|
| Lymph node
metastasis | Negative | 88 | 6.53 |
|
|
| Positive | 53 | 4.42 | 0.004 |
|
| Unknown | 7 | 5.21 |
|
| Tumor
differentiation | Poorly | 52 | 3.69 |
|
|
| Moderately | 90 | 3.47 | 0.409 |
|
| Well | 6 | 5.22 |
|
| Histology | Adenocarcinoma | 50 | 3.85 |
|
|
| Squamous cell
carcinoma | 98 | 5.21 | 0.653 |
| TNM stage | I–II | 88 | 6.89 |
|
|
| III–IV | 60 | 4.11 | 0.031 |
| Invasion of lung
membrane | Negative | 31 | 6.15 |
|
|
| Positive | 109 | 4.23 | 0.063 |
|
| Unknown | 8 | 5.21 |
|
| Vascular
invasion | Negative | 141 | 3.69 |
|
|
| Positive | 3 | 2.52 | 0.809 |
|
| Unknown | 4 | 3.48 |
|
| Diameter (cm) | ≥5 | 36 | 3.22 |
|
|
| <5 | 112 | 6.87 | 0.011 |
Expression of miR-125a-3p was a
prognostic marker for the survival of patients with NSCLC
A univariate survival analysis was performed with a
Kaplan-Meier test to investigate whether several clinical
parameters influenced the prognosis of NSCLC. The clinical
parameters in the univariate analysis stratification included: Age;
gender; smoking history; lymph-node metastasis; tumor
differentiation; histology; TNM stage; invasion of the lung
membrane; vascular invasion; tumor diameter; and miR-125a-3p
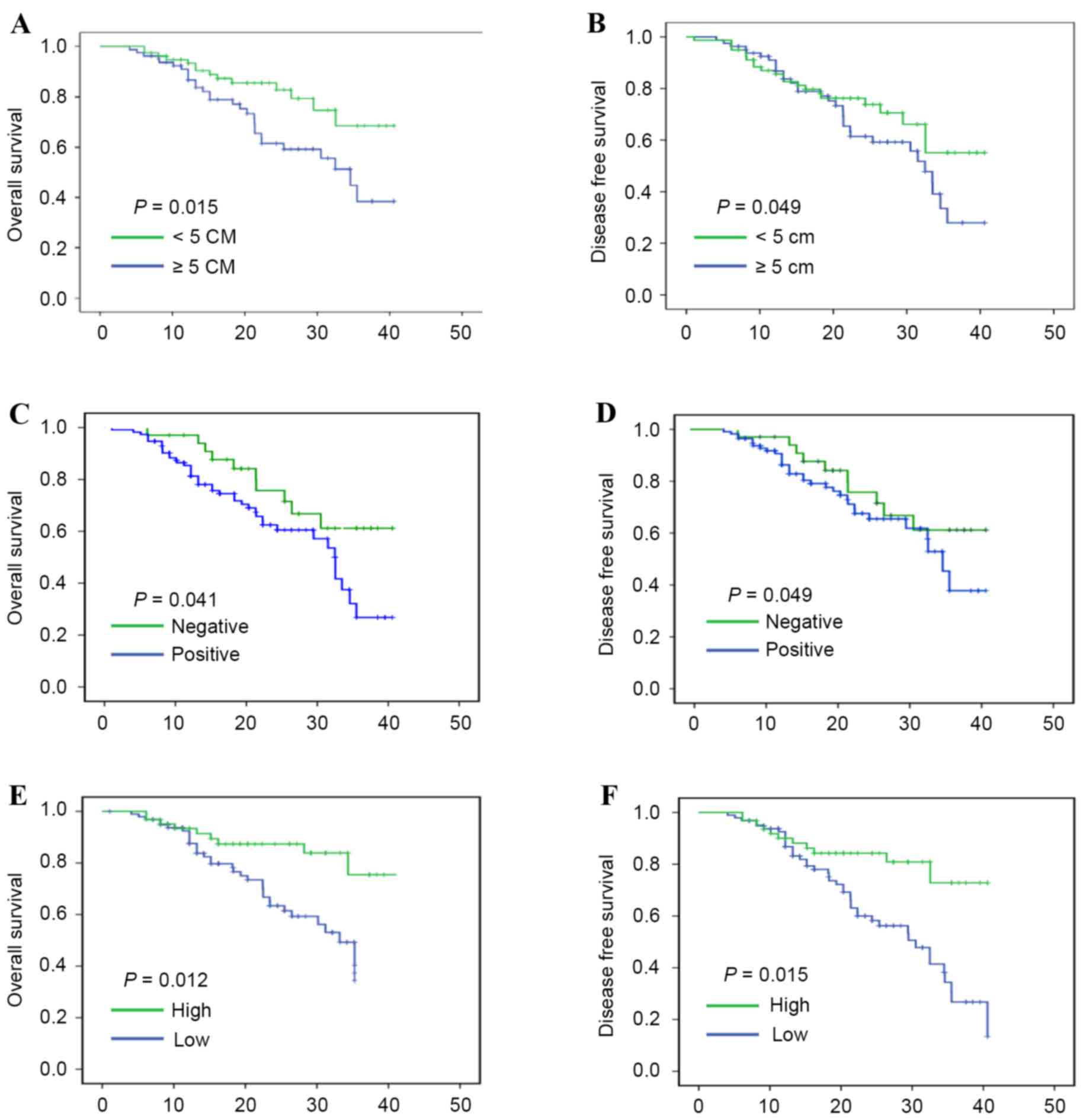
expression. The results indicated that tumor size, TNM stage, and
lymph-node metastasis were correlated with OS and DFS rates in
patients with NSCLC, as illustrated in Fig. 3A and D (P<0.05). Similarly,
miR-125a-3p downregulation was associated with significantly poorer
OS rates (P=0.012, and DFS rates (P=0.015), as demonstrated in
Fig. 3E and F, respectively.
A Cox proportional hazards regression model of
univariate analysis indicated that the prognosis of thepatients
with NSCLC wassignificantly correlated with lymph node metastasis
(P=0.031), TNM stage (P=0.035) and tumor diameter (P=0.005) as
demonstrated in Table II. However,
no correlation was identified between the prognosis and the age,
gender, smoking history, tumor differentiation, histology, invasion
of lung membrane or vascular invasion values of the patients.
Accordingly, the results of the present study demonstrated that the
expression levels of miR-125a-3p were associated with the prognosis
of patients with NSCLC (P=0.012), as illustrated in Table II.
 | Table II.Cox regression model analysis for
overall survival based on various clinical characteristics in
patients with non-small cell lung cancer. |
Table II.
Cox regression model analysis for
overall survival based on various clinical characteristics in
patients with non-small cell lung cancer.
|
|
|
|
| miR-125a-3p
multivariate analysis |
|---|
|
|
|
|
|
|
|---|
| Factor | Hazard ratio | 95% CI
(univariate) | P-value | Hazard ratio | 95% CI
(multivariate) | P-value |
|---|
| Age (≥60 vs.
<60) | 1.274 | 0.698–1.981 | 0.075 |
|
|
|
| Gender (male vs.
female) | 0.811 | 0.533–1.126 | 0.801 |
|
|
|
| Smoking history
(ever vs. never) | 0.991 | 0.423–1.887 | 0.186 |
|
|
|
| Lymph-node
metastasis (positive vs. negative) | 1.737 | 1.186–2.951 | 0.031 | 1.989 | 1.346–3.456 | 0.011 |
| Tumor
differentiation (poorly vs. well) | 0.754 | 0.461–1.234 | 0.261 |
|
|
|
| Histology (adenoma.
vs. squamous) | 0.775 | 0.482–1.247 | 0.294 |
|
|
|
| Tumor node
metastasis stage (III–IV vs. I–II) | 1.683 | 1.116–2.095 | 0.035 | 1.894 | 1.231–2.265 | 0.024 |
| Invasion of lung
membrane (positive vs. negative) | 1.221 | 1.026–1.434 | 0.525 |
|
|
|
| Vascular invasion
(positive vs. negative) | 0.989 | 0.683–1.265 | 0.317 |
|
|
|
| Diameter (≥5 vs.
<5 cm) | 2.382 | 1.305–4.346 | 0.005 | 3.651 | 2.376–4.335 | 0.001 |
| Chemotherapy
(positive vs. negative) | 0.775 | 0.428–1.122 | 0.029 | 0.536 | 0.359–0.721 | 0.004 |
| miR-125a-3p
expression (high vs. low) | 0.671 | 0.423–0.989 | 0.012 |
|
|
|
To confirm whether the expression levels of
miRNA-125a-3p was a prognostic marker in patients with NSCLC, a
multivariate analysis with a Cox proportional hazards regression
model was performed. Through a series of analyses, the ultimate
model of significant predictors of OSrates were defined. These
significant predictors included lymph-node metastasis [P=0.011;
HR=1.989 (1.346–3.456)], TNM stage [P=0.024; HR=1.894
(1.231–2.265)], tumor diameter [P=0.001; HR=3.651 (2.376–4.335)],
as illustrated in Table II. These
data identified that high expression levels of miR-125a-3p were a
predictor of higher OS rates in patients with NSCLC.
Chemotherapy associated with high
expression of miR-125a-3p improves OS and DFS of patients with
NSCLC
As demonstrated in Table
III, there was a statistically significant association between
chemotherapy and OS rates (27.04±3.68 vs. 32.47±2.98, P=0.008) and
DFS rates (26.43±2.96 vs. 28.04±3.36, P=0.035) in patients with
NSCLC. Treatment status as a precondition was used to investigate
the OS and DFS ratesof patients with NSCLC. According to analysis
of the treatment status and expression levels of miR-125a-3p, the
present study observed that chemotherapyand high expression levels
of miR-125a-3p positively prolongedthe OS, 33.78±3.94 vs.
24.32±2.58 (P=0.001), and DFS rates of patients with NSCLC,
30.26±3.79 vs. 22.04±2.86 (P<0.001), comparingpatients with
NSCLC administered non-chemotherapy treatments and low expression
levels of miR-125a-3p, as outlined in Table III.
 | Table III.OS and DFS of patients with non-small
cell lung cancer based on chemotherapy alone vs. chemotherapy and
miR-125a-3p expression. |
Table III.
OS and DFS of patients with non-small
cell lung cancer based on chemotherapy alone vs. chemotherapy and
miR-125a-3p expression.
|
|
| OS |
|
| DFS |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Factor | Median | 95% CI | P-value | Median | 95% CI | P-value |
|---|
| Chemotherapy |
|
|
|
|
|
|
|
Positive | 32.47 | 26.78–34.69 | 0.008 | 28.04 | 26.54–30.18 | 0.035 |
|
Negative | 27.04 | 23.43–29.06 |
| 26.43 | 24.31–28.69 |
|
| Chemotherapy and
expression |
|
|
|
|
|
|
| P &
H | 33.78 | 31.49–35.74 | 0.001 | 30.26 | 26.18–32.54 | <0.001 |
| N &
L | 24.32 | 18.94–26.03 |
| 22.04 | 19.43–24.65 |
|
To confirm the association between miR-125a-3p
expression levels and chemotherapy with OS and DFS rates,
univariate and multivariate survival analyses conducted with
Kaplan-Meier estimates were carried out. The univariate analysis
demonstrated that chemotherapy was associated with prognosis
(P=0.029; HR=0.775 [0.428–1.122]), as illustrated in Table II, and the multivariate survival
analysis indicated that a high expression level of miR-125a-3p with
chemotherapy was associated with prognosis [P=0.004; HR=0.536
(0.359–0.721), also illustrated in Table
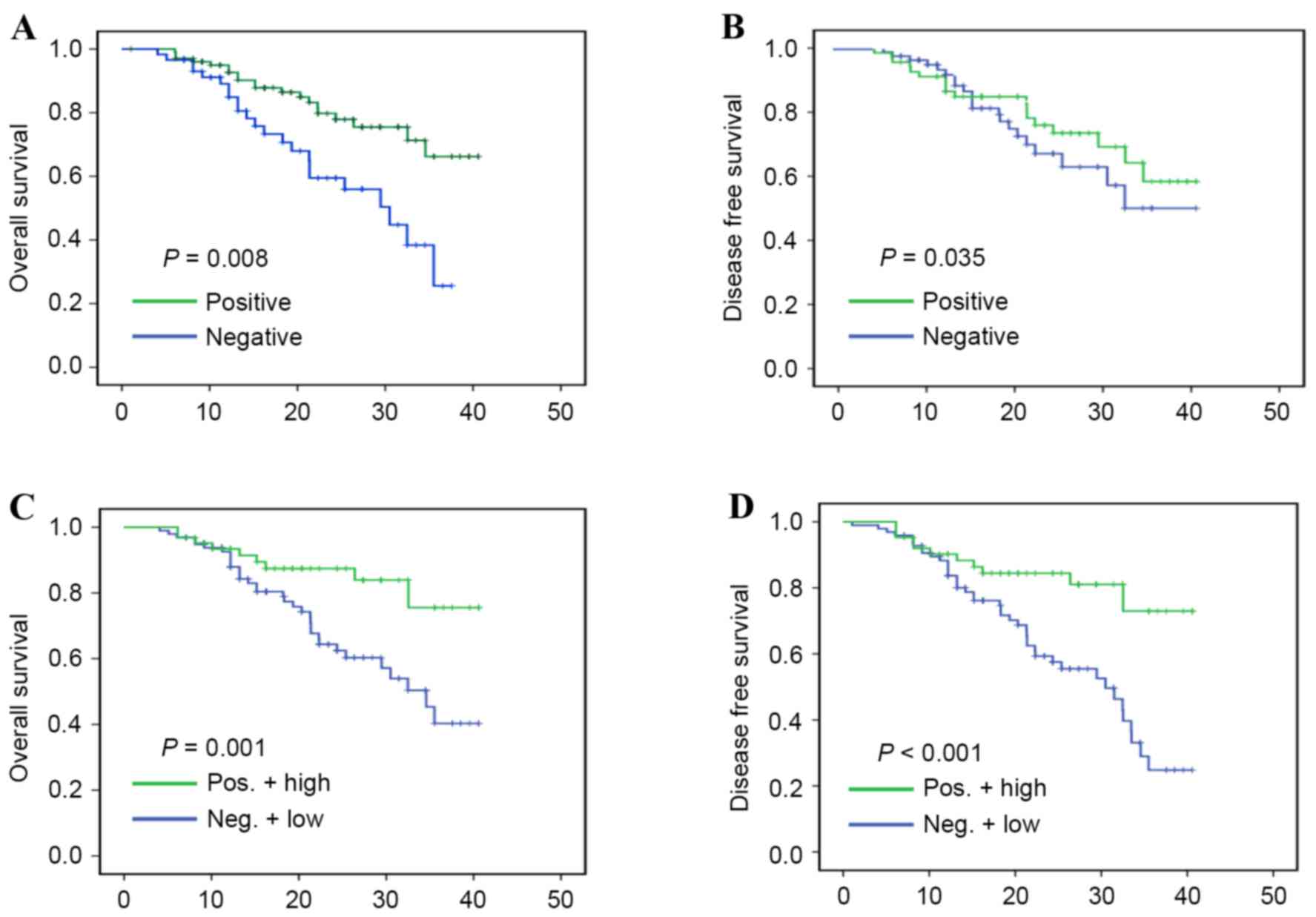
II]. In addition, the results showed that chemotherapy was
positively correlated with OS (P=0.008), and DFS rates (P=0.035),
as demonstrated in Fig. 4A and B,
respectively. Furthermore, a high expression level of miR-125a-3p
in patients with NSCLC who had undergone chemotherapy was also
positively correlated with OS (P=0.001), and DFS rates
(P<0.001), as illustrated in Fig. 4C
and D, respectively, which revealed that patients with NSCLC
with high expression levels of miR-125a-3p may be more likely to
benefit from chemotherapy.
Discussion
The present study revealed that miR-125a-3p
expression levels were lower in the lung cancer A549 cells compared
with the other cancer cell lines, and it is also downregulated in
NSCLC tissues when compared with normal lung tissues. The OS and
DFS rates of patients with low miR-125a-3p expression levels were
higher compared with patients exhibiting high miR-125a-3p
expression levels. The analysis of several clinical factors
suggested that lymph node metastasis and tumor diameter were
associated with poor prognoses in NSCLC. Notably, miR-125a-3p
expression levels as an independent parameter also affected
prognosis and survival. In addition, high expression levels of
miR-125a-3p combined with chemotherapy resulted in high OS rates
compared with the low expression levels of miR-125a-3p with no
chemotherapy. Therefore, miR-125a-3p may act as a biomarker for
chemotherapy in NSCLC.
miR-125a is a type of miRNA that has been
demonstrated to be associated with numerous types of cancer.
According to the 5′ and 3′ ends of pre-miR-125a, the miR-125a
family may be divided into different members, including miR-125a-5p
and miR-125a-3p (17). There are
several reports suggesting that miR-125a-5p is specifically
downregulated in breast and liver cancer, melanoma and bladder and
colon cancer (18–21). In addition, previous studies have
revealed the dysregulated expression of miR-125a-3p in several
types of cancer, for example, miR-125a-3p was downregulated in
malignant glioma (12) and prostate
(22), gastric (13) and pancreatic (23) cancer. Inversely, miR-125a-3p was
significantly upregulated in synovial (24) and rectal carcinoma (25) and multiple myeloma (26). Furthermore, a previous study revealed
that miR-125a-3p is over-expressed in retinoblastoma under hypoxic
conditions (27). miR-125a-3p has
been demonstrated to exhibit a strong association with a regulatory
pathway of lung cancer: Jiang et al (28) demonstrated that miR-125a-3p functioned
as a lung carcinoma suppressor gene that inhibited A549 cell
proliferation and apoptosis, and indicated that the p53 pathway is
not the only pathway involved in inducing lung cancer cell
apoptosis. Previous studies have also suggested miR-125a-3p
participates in the development of lung cancer via the classical
mitogen-activated protein kinase signaling pathway (29) and the Ras homolog gene family, member
A-actomyosin pathway (30). A
previous study indicated that miR-125a-3p suppresses NSCLC cell
proliferation, migration and invasion through the binding of
miR-125a-3p to the 3′-UTR of metastasis-associated gene 1 (MTA1)
(31). Through the aforementioned
analysis, the present study identified that miR-125a-3p suppresses
proliferation, metastasis and apoptosis in lung cancer via numerous
pathways, and participates in the regulation of carcinoma
development. Therefore, the development of novel targets of the
miR-125a-3p gene that improve the treatment of NSCLC requires
attention. miR-125a-3p exhibits a strong association with NSCLC,
and it serves an important role in the molecular therapy of
NSCLC.
The present study revealed a series of molecules
that are closely associated with carcinoma development. These
characteristic molecules are involved in the treatment of cancer;
as such, the role of biomarkers in the clinical diagnosis and
prognosis of patients with cancer is critical. Previous reports
have demonstrated that miRNAs are underlying diagnostic biomarkers
and prognostic factors for lung carcinoma (32,33).
Several miRNAs such as miR-25 (34),
miR-9 (35), miR-498 (36) have been revealed to exhibit clinical
value in the diagnosis of NSCLC. In addition, these novel
biomarkers may possibly promote innovative therapeutic methods
using novel drug compounds.
miRNAs are useful prognostic predictors and
therapeutic targets within clinical chemotherapy. The analysis of
the signal pathways associated with cancer and the predication of
prognosis subsequent to chemotherapy depends on the emergence of
individual biomarkers in patients with NSCLC. Thus, the
identification of unique molecular biomarkers is required to
increase the level of early diagnosis and improve the prognosis of
chemotherapy. At present, efforts are being made to determine
biomarkers with diagnostic and prognostic significance for
NSCLC.
In summary, the results of the present study
revealed that patients who received chemotherapy exhibited higher
OS and DFS rates, compared with untreated ones. additionally,
patients with NSCLC with high expression levels of miR-125a-3p who
were administered chemotherapy demonstrated longer OS and DFS
rates, relative to the untreated patients with low expression
levels of miR-125a-3p. Therefore, miR-125a-3p appears to be
downregulated in NSCLC, which increased OS and DFS rates of
patients receiving chemotherapy. The data of the present study
indicates that miR-125a-3p serves as an independent prognostic
biomarker for chemotherapy within clinical practice. However,
additional studies are required in this field.
The results from the present study also indicated
that the expression levels of miR-125a-3p in normal tissues were
higher than in NSCLC tissues. In addition, analysis suggested that
patients with NSCLC with high expression levels of miR-125a-3p have
an improved prognosis, and may be more likely to respond well to
chemotherapy. In conclusion, the present study demonstrated that
miR-125a-3p is a significant prognostic biomarker for NSCLC, from
which novel therapeutic strategies to combat NSCLC may be
derived.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81372175,
81472501, 81472202 and 81302065), Shanghai Municipal Commission of
Health and Family Planning (grant no. 201440398) and Shanghai
Natural Science Foundation (grant no. 16ZR1428900).
References
|
1
|
Han Y, Jia C, Cong X, Yu F, Cai H, Fang S,
Cai L, Yang H, Sun Y, Li D, et al: Increased expression of TGFβR2
is associated with the clinical outcome of non-small cell lung
cancer patients treated with chemotherapy. PLoS One.
10:e01346822015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng W, Ye Z, Cui R, Perry J,
Dedousi-Huebner V, Huebner A, Wang Y, Li B, Volinia S, Nakanishi H,
et al: MicroRNA-31 predicts the presence of lymph node metastases
and survival in patients with lung adenocarcinoma. Clin Cancer Res.
19:5423–5433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitra R, Edmonds MD, Sun J, Zhao M, Yu H,
Eischen CM and Zhao Z: Reproducible combinatorial regulatory
networks elucidate novel oncogenic microRNAs in non-small cell lung
cancer. RNA. 20:1356–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Wang XF, Ma YS, Xia Q, Zhang F,
Fu D and Wang YC: Quantitative assessment of the influence of TP63
gene polymorphisms and lung cancer risk: Evidence based on 93,751
subjects. PLoS One. 9:e870042014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen S, Yue H, Li Y, Qin J, Li K, Liu Y
and Wang J: Upregulation of miR-136 in human non-small cell lung
cancer cells promotes Erk1/2 activation by targeting PPP2R2A. Tumor
Biol. 35:631–640. 2014. View Article : Google Scholar
|
|
6
|
Fang Y, Fu D and Shen XZ: The potential
role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim
Biophys Acta. 1806:1–6. 2010.PubMed/NCBI
|
|
7
|
Agirre X, Vilas-Zornoza A, Jiménez-Velasco
A, Martin-Subero JI, Cordeu L, Gárate L, José-Eneriz San E,
Abizanda G, Rodríguez-Otero P, Fortes P, et al: Epigenetic
silencing of the tumor suppressor microRNA hsa-miR-124a regulates
CDK6 expression and confers a poor prognosis in acute lymphoblastic
leukemia. Cancer Res. 69:4443–4453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pierson J, Hostager B, Fan R and Vibhakar
R: Regulation of cyclin dependent kinase 6 by microRNA 124 in
medulloblastoma. J Neurooncol. 90:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li JQ, Hu SY, Wang ZY, Lin J, Jian S, Dong
YC, Wu XF, Lan D and Cao LJ: MicroRNA-125-5p targeted CXCL13: A
potential biomarker associated with immune thrombocytopenia. Am J
Transl Res. 15:772–780. 2015.
|
|
10
|
Xie J, Yu F, Li D, Zhu X, Zhang X and Lv
Z: MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in
non-small cell lung cancer by targeting RUNX2. Tumor Biol.
37:1197–1204. 2016. View Article : Google Scholar
|
|
11
|
Zhou YL, Xu YJ and Qiao CW: miR-34c-3p
suppresses the proliferation and invasion of non-small cell lung
cancer (NSCLC) by inhibiting PAC1/MAPK pathway. Int J Clin Exp
Pathol. 8:6312–6322. 2015.PubMed/NCBI
|
|
12
|
Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D
and Chen J: The challenges and the promise of molecular targeted
therapy in malignant gliomas. Neoplasia. 17:239–255. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashiguchi Y, Nishida N, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Mochizuki H, Hase K, Doki Y and Mori
M: Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
14
|
Ruike Y, Ichimura A, Tsuchiya S, Shimizu
K, Kunimoto R, Okuno Y and Tsujimoto G: Global correlation analysis
for micro-RNA and mRNA expression profiles in human cell lines. J
Hum Genet. 53:515–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Zhu X, Li N, Li D, Sha Z, Zheng X
and Wang H: miR-125a-3p targets MTA1 to suppress NSCLC cell
proliferation, migration, and invasion. Acta Biochim Biophys Sin
(Shanghai). 47:496–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raponi M, Dossey L, Jatkoe T, Wu X, Chen
G, Fan H and Beer DG: MicroRNA classifiers for predicting prognosis
of squamous cell lung cancer. Cancer Res. 69:5776–5783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Huang Q, Zhang S, Zhang Q, Chang
J, Qiu X and Wang E: hsa-miR-125a-3p and hsa-miR-125a-5p are
downregulated in non-small cell lung cancer and have inverse
effects on invasion and migration of lung cancer cells. BMC Cancer.
10:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Duan R, Kooy F, Sherman SL, Zhou W
and Jin P: Germline mutation of microRNA-125a is associated with
breast cancer. J Med Genet. 46:358–360. 2008. View Article : Google Scholar
|
|
20
|
Bi Q, Tang S, Xia L, Du R, Fan R, Gao L,
Jin J, Liang S, Chen Z, Xu G, et al: Ectopic expression of miR-125a
inhibits the proliferation and metastasis of hepatocellular
carcinoma by targeting MMP11 and VEGF. PLoS One. 7:e401692012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo
Z, Dong W, Huang J and Lin T: MicroRNA-125b suppresses the
development of bladder cancer by targeting E2F3. Int J Cancer.
128:1758–1769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong Z, Liu N, Lin L, Guo X, Yang D and
Zhang Q: miR-125a-5p inhibits cell proliferation and induces
apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1.
Biomed Pharmacother. 75:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ninio-Many L, Grossman H, Levi M, Zilber
S, Tsarfaty I, Shomron N, Tuvar A, Chuderland D, Stemmer SM,
Ben-Aharon I and Shalgi R: MicroRNA miR-125a-3p modulates molecular
pathway of motility and migration in prostate cancer cells.
Oncoscience. 1:250–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kojima M, Sudo H, Kawauchi J, Takizawa S,
Kondou S, Nobumasa H and Ochiai A: MicroRNA markers for the
diagnosis of pancreatic and biliary-tract cancers. PLoS One.
10:e01182202015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hisaoka M, Matsuyama A, Nagao Y, Luan L,
Kuroda T, Akiyama H, Kondo S and Hashimoto H: Identification of
altered microRNA expression patterns in synovial sarcoma. Genes
Chromosomes Cancer. 50:137–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Della VittoriaScarpati G, Falcetta F,
Carlomagno C, Ubezio P, Marchini S, De Stefano A, Singh VK,
D'Incalci M, De Placido S and Pepe S: A specific miRNA signature
correlates with complete pathological response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. Int J Radiat
Oncol Biol Phys. 83:1113–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chonglei Bi, Chung TH, Huang G, Zhou J,
Yan J, Gregory JA, Fonseca R and Chng WJ: Genome-wide pharmacologic
unmasking identifies tumor suppressive microRNAs in multiple
myeloma. Oncotarget. 6:26508–26518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Jia R, Zhou Y, Song X, Wang J, Qian
G, Ge S and Fan X: Microarray-based analysis: Identification of
hypoxia-regulated microRNAs in retinoblastoma cells. Int J Oncol.
38:1385–1393. 2011.PubMed/NCBI
|
|
29
|
Jiang L, Chang J, Zhang Q, Sun L and Qiu
X: MicroRNA hsa-miR-125a-3p activates p53 and induces apoptosis in
lung cancer cells. Cancer Invest. 31:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou DH, Wang X and Feng Q: EGCG enhances
the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC
A549 cells. Nutr Cancer. 66:636–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang B, Luo W, Sun L, Zhang Q, Jiang L,
Chang J, Qiu X and Wang E: miRNA-125a-3p is a negative regulator of
the RhoA-actomyosin pathway in A549 cells. Int J Oncol.
42:1734–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Zhu X, Li N, Li D, Sha Z, Zheng X
and Wang H: miR-125a-3p targets MTA1 to suppress NSCLC cell
proliferation, migration and invasion. Acta Biochim Biophys Sin
(Shanghai). 47:496–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Zhang Q, Wu L, Jia C, Shi F, Li S,
Peng A, Zhang G, Song X and Wang C: Serum miR-499 as a novel
diagnostic and prognostic biomarker in non-small cell lung cancer.
Oncol Rep. 31:1961–1967. 2014.PubMed/NCBI
|
|
34
|
Zhou DH, Wang X and Feng Q: EGCG enhances
the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC
A549 cells. Nutr Cancer. 66:636–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu FX, Su YL, Zhang H, Kong JY, Yu H and
Qian BY: Prognostic implications for high expression of miR-25 in
lung adenocarcinomas of female non-smokers. Asian Pac J Cancer
Prev. 15:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu T, Liu X, Han L, Shen H, Liu L and Shu
Y: Up-regulation of miR-9 expression as a poor prognostic biomarker
in patients with non-small cell lung cancer. Clin Transl Oncol.
16:469–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang M, Zhang Q, Wang J and Zhai Y:
MicroRNA-498 is downregulated in non-small cell lung cancer and
correlates with tumor progression. J Cancer Res Ther. 11 Suppl
1:S107–S111. 2015. View Article : Google Scholar
|