Introduction
Colorectal carcinoma (CRC) is one of the most
prevalent types of cancer in humans globally (1,2). In the
United States, a report revealed that CRC incidence rates were
lowest among Asian/Pacific residents, and highest in Alaska
natives. CRC-induced mortality decreased by 34% in individuals aged
≥50 between 2000 and 2014, but increased by 13% among those aged
<50 (3). Patients with CRC present
with multiple symptoms that are commonly associated with the
disease, including abdominal pain, fatigue (4), obstruction, perforation and bleeding
(5). The medical literature
associated with emergencies in patients with CRC is dominated by
reports of obstruction, perforation, overt bleeding (6), and systemic inflammatory response
subsequent to curative resection of CRC (7). To the best of our knowledge, systemic
inflammatory response syndrome (SIRS) has not been previously
reported as the primary indication of CRC or other diseases. SIRS
is the result of occult infection or sterile inflammation (8), and is characterized by multi-organ
failure and increased mortality (9,10). A
prompt diagnosis of underlying disease is necessary for early
intervention and appropriate treatment. SIRS is associated with
poor prognosis in patients with CRC (11,12). The
present study described a patient with CRC that presented with SIRS
as the early clinical indication without a history of chronic
diseases.
Case report
A 68-year-old woman presented to the Emergency
Department of Changsha Central Hospital (Changsha, China) with
worsening symptoms of abdominal pain late in the evening of June 1,
2015. These symptoms were accompanied by an urge to defecate 4
times/day, severe abdominal distention and vomiting, and a
low-grade fever with a body temperature not exceeding 37.9°C
(normal range, 36–37°C). Abdominal radiographs were performed to
ascertain the cause of the symptoms (Fig.
1A). The patient was diagnosed with acute gastroenteritis and
admitted to the hospital for the administration of 80 mg
phloroglucinol intramuscularly. However, the symptoms were found to
be exacerbated even after treatment. Bloody diarrhea was reported
four times a day, and the urine volume decreased to a maximum of
100 ml in a 24 h period (normal range, 1,000–2,000 ml in a 24 h
period). The patient was consequently referred to the Department of
Gastroenterology of the Second Xiangya Hospital (Central South
University, Changsha, China) for further diagnosis and treatment on
June 2, 2015.
The patient had a history of long-term consistent
abdominal pain that was relieved following defecation. The patient
had also suffered from asthma for >10 years. There was no
previous history of weight loss, cigarette smoking or renal
disease. A physical examination revealed shock, with limb
clamminess, a body temperature of 37°C, blood pressure of 92/50
mmHg (normal range, 90–120/60–80 mmHg), tachycardia (145 beats/min;
normal range, 60–100 beats/min) and tachypnea (22 breaths/min;
normal range, 12–20 breaths/min). There were diminished breath
sounds at each lung base. Upon abdominal examination, peritonitis
with a firm consistency was found, with rebound tenderness and
hypoactive bowel sounds at a frequency of 3–4 times/min. Laboratory
tests revealed a white blood cell count (WBC) of
1.526×1010/l (normal range, 3.5–9.5×109/l)
with 92.8% neutrophils (normal range, 40–75%), a serum hemoglobin
level of 135 g/l (normal range, 130–175 g/l), a platelet count of
1.62×1011/l (normal range, 1.25–3.50×1011/l),
an elevated erythrocyte sedimentation rate (ESR) that ranged from
20–118 mm/h (normal range, 0–20 mm/h), a procalcitonin (PCT) level
of 25.06 g/l (normal range, <0.05 ng/ml) and a C-reactive
protein level of 351 mg/l (normal range, 0–8 mg/l). Hepato-renal
function tests demonstrated increased levels of blood urea nitrogen
(BUN) at 46.69 mmol/l (normal range, 2.9–7.14 mmol/l), creatinine
at 367.2 µmol/l (normal range, 40–133 µmol/l) and alanine
aminotransferase (ALT) at 107.9 µ/l (normal range, 9–50 µ/l), and a
raised aspartate aminotransferase (AST) level of 136.9 U/l (normal
range, 15–40 U/l). The BNP level reached 8,392 pg/ml (normal range,
0–900 pg/ml). Arterial blood gas and electrolyte related laboratory
data showed acidosis with a pH of 7.281 (normal range, 7.35–7.45),
partial pressure (Pa)CO2 of 55.6 mmHg (normal range,
32–45 mmHg), PaO2 of 55.6 mmHg (normal range, 83–108
mmHg), a bicarbonate level of 13.5 mmol/l (normal range, 21–28
mmol/l) and electrolyte disturbance with a low blood calcium level
of 1.41 mmol/l (normal range, 2.03–2.54 mmol/l). Coagulation
function tests showed increased fibrinogen degradation products at
44.89 µg/ml (normal range, 0–5 µg/ml), an extended prothrombin time
of 17.9 sec (normal range, 11–13 sec), a prolonged activated
partial thromboplastin time of 66.1 sec (normal range, 25–33 sec)
and a fibrinogen concentration of 70.6 mg/dl (normal range, 200–400
mg/dl). The concentration of carcinoembryonic antigen (CEA) was
87.720 ng/ml (normal range, <5 ng/ml). No positive results were
found for serum antinuclear antibodies, extractable nuclear antigen
or any other immunological biomarkers.
An electrocardiogram (ECG) indicated
supraventricular tachycardia. Chest X-rays showed exudative lesions
at each lung base. An abdominal X-ray disclosed the existence of
incomplete intestinal obstruction (Fig.
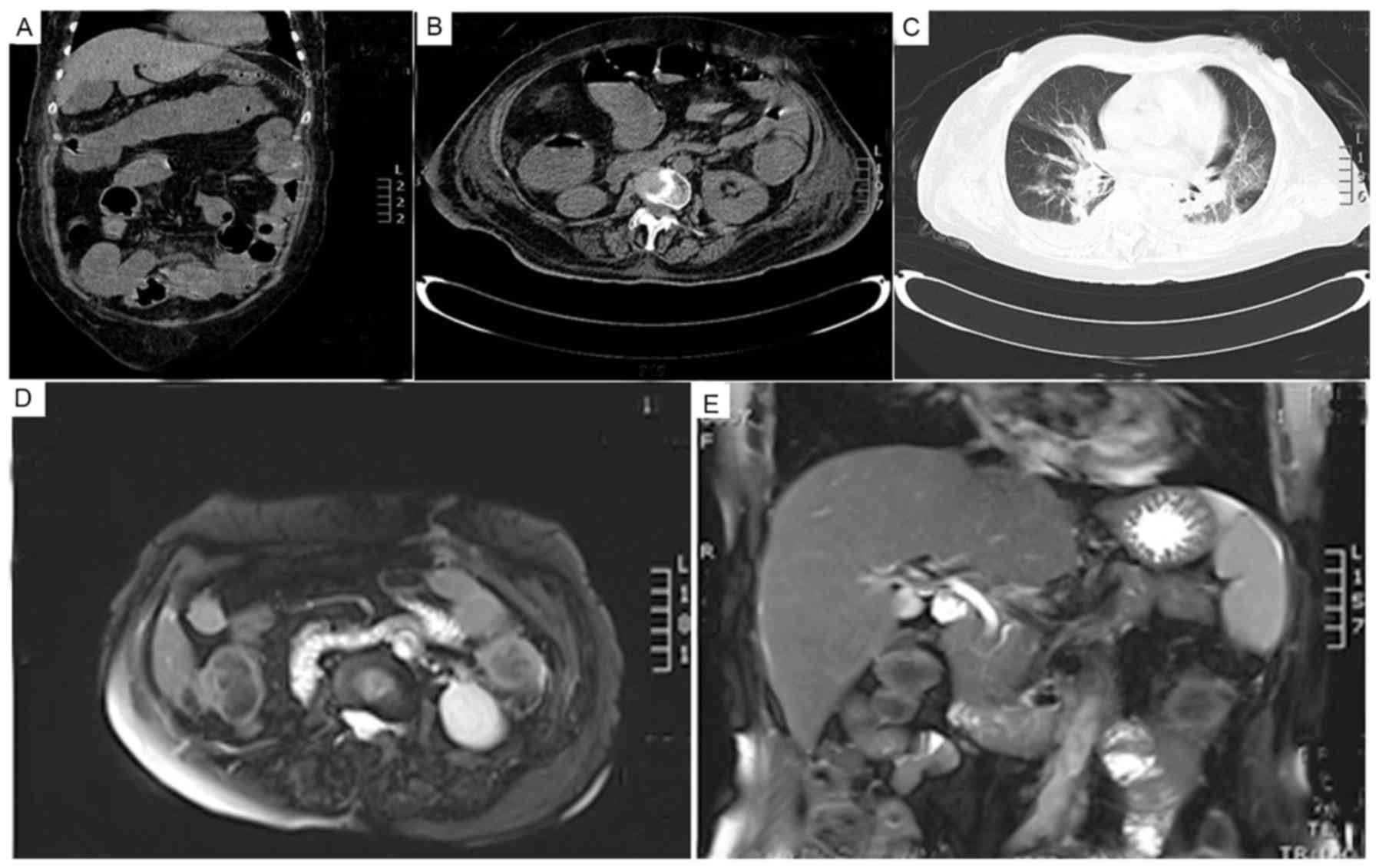
1B). Scans of the thorax and abdomen were made prior to and
following treatment of SIRS (Fig. 2).
As shown in Fig. 2A-C, lung infection
and intestinal obstruction with dilatation were observed on
computed tomography scans prior to treatment.
According to a consensus conference in 1992, the
defining characteristic of SIRS is the presence of any two
diagnostic criteria among the following: Abnormal temperature
(>38°C or <36°C), heart rate (>90 beats/min), respiratory
rate (>20 breaths/min) and WBC count (>12×109/l or
<4×109/l) (13–15). In the present case, the heart rate was
145 beats/min and the respiratory rate was 22 breaths/min. In
consideration of acute multi-organ function disturbances such as
kidney, heart and lung dysfunction, the patient was diagnosed with
SIRS. The patient was immediately administered 1 g meropenem
intravenously every 8 h along with nutrition support therapy. After
almost 1 month of treatment, the symptoms were observed to improve.
The WBC count improved to 8.15×109/l with 64%
neutrophils, PCT decreased to 0.08 g/l and hepato-renal function
tests showed normal levels of BUN, ALT and AST. Furthermore, a
sinus rhythm was also detected upon ECG. Most importantly, the
patient exhibited no trace of infection or multi-organ
disturbance.
However, unexpectedly, the hemoglobin level was
reduced to 95 g/l (normal range, 130–175 g/l), and the stool occult
blood test remained positive. A secondary abdominal X-ray revealed
trapped gas located mainly in the lower right quadrant of the
abdomen (Fig. 1C).
Considering the presence of anemia, bloody stool and
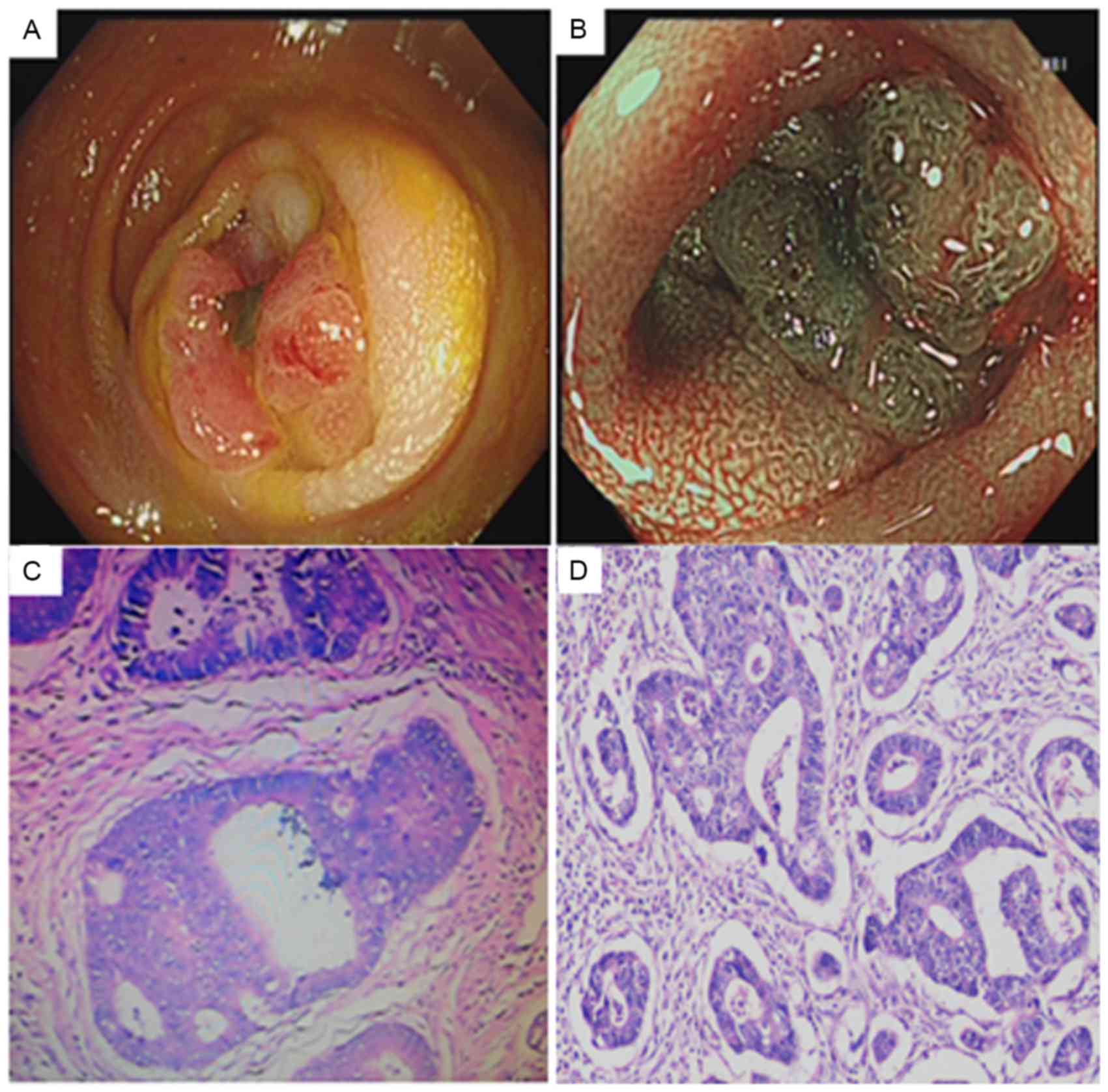
intestinal dilatation, a colonoscopy was performed to aid the
diagnosis. A laterally spreading tumor with uneven nodules was
detected in the descending colon. Narrow band imaging staining
disclosed the presence of type IIIA and type VA pits based on pit
structure and superficial microvessel features, and identified
using Kudo's pit pattern classification (16), which were indicative of a carcinogenic
lesion. This lesion was thus endoscopically diagnosed as CRC
(Fig. 3A and B). Biopsy of the tumor
areas showed atypical tissues suggestive of adenocarcinoma, as
shown in Fig. 3C. Thus, the patient
was advised to undergo surgical removal of 12 cm of the colon
descendens. The final histopathological diagnosis was of a
moderately differentiated colorectal adenocarcinoma without
metastasis (Fig. 3D).
Subsequent to the surgery, the patient refused to
undergo chemotherapy. The patient's quality of life was mostly
normal, with no evidence of any complications. During the 1-month
follow-up after surgery, no recurrence or metastasis of the CRC
were observed upon magnetic resonance imaging (Fig. 2D and E). As of April 1, 2016, 8 months
post-surgery, no fever, abdominal pain or other symptoms were
present, and the blood tests, including blood cell levels, the
serum levels of BUN, ALT, AST and CEA, and the ESR have been
normal. A telephone follow-up will be performed every 6 months
until the time of patient mortality, and a telephone follow-up on
25th June, 2017 showed the patient has been feeling well so far
with no signs of recurrence and metastasis were tracked following
CT examination.
Written informed patient consent was obtained for
the publication of the present study.
Discussion
Recent studies have reported that systemic
inflammatory response is a predictor of prognosis in patients with
CRC (7,11,17,18).
Certain published studies have also reported the association
between emergency presentation and poor cancer-specific survival
times following curative resection (6). However, systemic inflammation has not
been previously reported as the primary manifestation. The
association between SIRS and clinical symptoms of CRC is thus rare
and this rarity could render this symptom as a warning sign for its
diagnosis.
SIRS is a rapidly progressive syndrome that causes
significant morbidity and has a high rate of mortality if not
treated promptly, and it is usually secondary to other clinical
manifestations, including carcinoma and severe infection (19). Due to SIRS patients presenting as an
emergency case at first admission, the primary cause tends to be
ignored, leading to a missed diagnosis of the primary disease
(20).
Potential mechanisms involved in the occurrence of
SIRS in CRC patients may be associated with the fact that the gut
is a habitat pool for 100 trillion microbiota, a number of which
have been reported to be associated with the development of CRC
(21–23). Gut microbiota can influence the normal
development and function of the mucosal immune system, such as
modification of T-cell repertoires and T-helper cell cytokine
profiles (24–26). Intestinal epithelial cells maintain a
moderate immune response against, or tolerance for, non-pathogenic
bacteria via Toll-like receptors (TLRs), which recognize particular
molecular motifs of the pathogens (27). Activation of TLRs may lead to the
activation of nuclear factor-κB and mitogen-activated protein
kinase pathways, which lead to the development of pro-inflammatory
cytokines, such as tumor necrosis factor-α and interleukin-6
(28). Consequently, a cascade
reaction is triggered by inflammatory cytokines, which can lead to
the development of SIRS with multi-organ dysfunction involving the
lungs, heart, kidney and liver.
SIRS is characterized by acute systemic
inflammation. However, SIRS could not explain the long-term
abdominal pain in the present case. Furthermore, the characteristic
symptoms of bloody stool, anemia and persistent incomplete
intestinal obstruction warrant more attention. In the current case,
a diagnosis of CRC in the early stages of the disease had
significant implications on the treatment choices and prognosis of
the patient.
Thus, the present study describes an emergency case
of SIRS as a primary symptom of CRC, which highlights the
importance of analyzing obscure manifestations carefully upon
emergency presentation in order to obtain a satisfactory
outcome.
Acknowledgements
The authors would like to thank Miss Lei Shi from
the Department of Pathology, Second Xiangya Hospital of Central
South University, for providing pathological support.
Glossary
Abbreviations
Abbreviations:
|
SIRS
|
systemic inflammatory response
syndrome
|
|
CRC
|
colorectal carcinoma
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müller F, Tuinman MA, Janse M, Almansa J,
Sprangers MAG, Smink A, Ranchor AV, Fleer J and Hagedoorn M:
Clinically distinct trajectories of fatigue and their longitudinal
relationship with the disturbance of personal goals following a
cancer diagnosis. Br J Health Psychol. Jun 21–2017.(Epub ahead of
print). View Article : Google Scholar
|
|
5
|
Baer C, Menon R, Bastawrous S and
Bastawrous A: Emergency presentations of colorectal cancer. Surg
Clin North Am. 97:529–545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McArdle CS and Hole DJ: Emergency
presentation of colorectal cancer is associated with poor 5-year
survival. Br J Surg. 91:605–609. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoque R, Farooq A and Mehal WZ: Sterile
inflammation in the liver and pancreas. J Gastroenterol Hepatol. 28
Suppl 1:S61–S67. 2013. View Article : Google Scholar
|
|
9
|
Beal AL and Cerra FB: Multiple organ
failure syndrome in the 1990s. Systemic inflammatory response and
organ dysfunction. JAMA. 271:226–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scott HF, Deakyne SJ, Woods JM and Bajaj
L: The prevalence and diagnostic utility of systemic inflammatory
response syndrome vital signs in a pediatric emergency department.
Acad Emerg Med. 22:381–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carruthers R, Tho LM, Brown J, Kakumanu S,
McCartney E and McDonald AC: Systemic inflammatory response is a
predictor of outcome in patients undergoing preoperative
chemoradiation for locally advanced rectal cancer. Colorectal Dis.
14:e701–e707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McSorley ST, Black DH, Horgan PG and
McMillan DC: The relationship between tumour stage, systemic
inflammation, body composition and survival in patients with
colorectal cancer. Clin Nutr. May 19–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM consensus conference committee.
American college of chest physicians/society of critical care
medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Landis RC: 20 Years on: Is it time to
redefine the systemic inflammatory response to cardiothoracic
surgery? J Extra Corpor Technol. 47:5–9. 2015.PubMed/NCBI
|
|
15
|
Castillo-Lara RA, Soria-Ruiz M and
Martínez-Carrera O: Definitions for sepsis and organ failure. JAMA.
270:9391993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujita T: Preoperative but not
postoperative systemic inflammatory response correlates with
survival in colorectal cancer (Br J Surg 2007; 94: 1028–1032). Br J
Surg. 94:1439–1440. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crozier JE, Leitch EF, McKee RF, Anderson
JH, Horgan PG and McMillan DC: Relationship between emergency
presentation, systemic inflammatory response, and cancer-specific
survival in patients undergoing potentially curative surgery for
colon cancer. Am J Surg. 197:544–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vincent JL, Opal SM, Marshall JC and
Tracey KJ: Sepsis definitions: Time for change. Lancet.
381:774–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Comstedt P, Storgaard M and Lassen AT: The
systemic inflammatory response syndrome (SIRS) in acutely
hospitalised medical patients: A cohort study. Scand J Trauma
Resusc Emerg Med. 17:672009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cuevas-Ramos G, Petit CR, Marcq I, Boury
M, Oswald E and Nougayrède JP: Escherichia coli induces DNA damage
in vivo and triggers genomic instability in mammalian cells. Proc
Natl Acad Sci USA. 107:11537–11542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Becker S, Oelschlaeger TA, Wullaert A,
Vlantis K, Pasparakis M, Wehkamp J, Stange EF and Gersemann M:
Bacteria regulate intestinal epithelial cell differentiation
factors both in vitro and in vivo. PLoS One. 8:e556202013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta A, Madani R and Mukhtar H:
Streptococcus bovis endocarditis, a silent sign for colonic tumour.
Colorectal Dis. 12:164–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazmanian SK, Liu CH, Tzianabos AO and
Kasper DL: An immunomodulatory molecule of symbiotic bacteria
directs maturation of the host immune system. Cell. 122:107–118.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rakoff-Nahoum S, Paglino J,
Eslami-Varzaneh F, Edberg S and Medzhitov R: Recognition of
commensal microflora by toll-like receptors is required for
intestinal homeostasis. Cell. 118:229–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gagnière J, Raisch J, Veziant J, Barnich
N, Bonnet R, Buc E, Bringer MA, Pezet D and Bonnet M: Gut
microbiota imbalance and colorectal cancer. World J Gastroenterol.
22:501–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cario E: Bacterial interactions with cells
of the intestinal mucosa: Toll-like receptors and NOD2. Gut.
54:1182–1193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|