Introduction
Cancer is characterized by uncontrolled growth and
spreading of abnormal cells. It is one of the most life-threatening
and challenging diseases of modern time, caused by a number of
internal factors, including immune condition and inherited
mutation, and external factors, including radiation, tobacco and
chemical exposure (1). Liver, breast,
colorectal, lung, cervical and nasopharyngeal cancer are the most
prevalent cancer types in the modern age. The present study focused
on liver and breast cancer due to their high occurrence rates. In
2006, hepatocellular carcinoma (HCC) was identified to account for
70–85% of total primary liver cancer types (2). HCC is primarily caused by chronic viral
hepatitis B or C infection, iron overload, aflotoxin exposure,
obesity, alcohol-associated cirrhosis and possibly non-alcoholic
fatty liver disease (3,4). Breast cancer continues to be the leading
cause of cancer-associated mortality among women (5), and the occurrence rate has increased in
China and other Asian countries (6,7). Breast
cancer can be categorized into two main types, ductal carcinoma and
lobular carcinoma. Ductal carcinoma begins in the ducts that
transport milk from the breast to the nipple, whereas lobular
carcinoma starts in the lobules that produce milk. Atypical
hyperplasia of the breast, family history, early menarche, late
menopause and Li-Fraumeni syndrome are some of the known risk
factors for breast cancer (8–10).
To date, the common treatments for cancers are
surgical removal of the tumour tissue, chemotherapy and ionizing
radiation. Apart from destroying adjacent normal cells (11), these treatments also cause severe side
effects (12–14). Therefore, current studies have focused
on the search for alternative medicines from plant-based sources.
Alternative medicine not only improves the efficacy of conventional
medicines, it reduces the side effects of chemotherapy (15) and strengthens the immune system to
fight against cancer (16).
Among the medicinal plants, one of the commonly
found plants in tropical countries is Strobilanthes crispa
Blume. It is traditionally known as daun pecah beling in Jakarta
and enyoh kilo, kecibeling or kejibeling in Java (17). In Malaysia, it is known as pecahkaca
or jinbatu (17). This bush-like
plant is scattered throughout the regions of Madagascar to Malay
Archipelago (18). S. crispa
has high mineral and phenolic content, and exhibits high
antioxidant activity (19,20). In addition, this plant contains
alkaloids, tannins, polyphenols, water-soluble vitamins, caffeine,
catechins (19,20) and bioactive compounds, including
β-sitosterol, and stigmasterol (21).
A previous study demonstrated that S. crispa leaves possess
anti-diabetic, diuretic and blood pressure lowering properties
(17). Furthermore, a number of
studies have recorded the potency of S. crispa extracts in
inhibiting cancer cell growth (22–25).
Thus, the aim of the present study was to
investigate the cytotoxic and anti-proliferative effects of S.
crispa extracts on HepG-2 and MDA-MB-231 cancer cell lines.
Furthermore, one of the aims was to determine the effects of the
extracts on cell cycle and caspase-8 activation.
Materials and methods
Preparation of plant extracts
The botanical identity of S. crispa was
determined and authenticated by a taxonomist from the Forest
Research Institute Malaysia (Kuala Lumpur, Malaysia; sample no. PID
040114–04). Fresh plant materials (leaves and stems) were collected
and dried in an oven at 40°C until a constant weight was obtained.
Dried leaves and stems were separated, crushed into fine pieces and
then ground with a mill grinder into powder form. A total of four
organic solvent extracts of the leaves and stems were prepared
individually. The organic solvents used were hexane, chloroform,
ethyl acetate and methanol (all Friendemann Schmidt, Parkwood, WA,
USA). The extraction was performed by mixing 500 ml of solvent into
100 g of powdered leaves or stems, and then macerating in the dark
at room temperature for 3 days. The suspensions were filtered using
Whatman paper (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and then evaporated using a rotary evaporator (Büchi R-2154; Büchi
Labortechnik AG, Flawil, Switzerland) to obtain desired crude
extracts. The extracts obtained were as follows: Leaf hexane (LH),
leaf chloroform (LC), leaf ethyl acetate (LEA), leaf methanol (LM),
stem hexane (SH), stem chloroform (SC), stem ethyl acetate (SEA)
and stem methanol (SM).
The dried plant materials (10 g) were mixed with 500
ml distilled water and kept at 60°C for 3 h. The resulting
suspensions were then filtered and followed by freeze drying. The
extracts obtained were designated as leaf water (LW) and stem water
(SW).
Cell culture
Hepatocellular carcinoma HepG-2, breast cancer
MDA-MB-231 and normal rat kidney NRK-52E cell lines were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific,
Inc.), and maintained at 37°C in a humidified atmosphere of 5%
CO2.
MTT assay
HepG-2, MDA-MB-231 and NRK-52E cells were seeded at
a density of 1×105 cells/ml into a 96-well plate and treated with
varying concentrations of S. crispa extracts (12.5, 25, 50,
100 and 200 µg/ml) including LH, LC, LEA, LM, LW, SH, SC, SEA, SM
and SW for 72 h at 37°C. Subsequently, 10 µl MTT solution (Bio
Basic, Inc., Amherst, NY, USA) was added into each well and cells
were incubated for 4 h at 37°C. Subsequently, the solution in each
well was removed and 100 µl of dimethyl sulphoxide (Friendemann
Schmidt) was added. The coloured solution was measured using a
Dynex Opsys MR microplate reader (Dynex Technologies Inc.,
Chantilly, VA, USA) at 570 nm wavelength. Cell viability (%) was
calculated according to the following formula: Optical density (OD)
of samples/OD of control ×100, where the control represents
untreated cells. The control cells were incubated with DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin (all Gibco; Thermo Fisher Scientific, Inc.) for 72 h
at 37°C. The half maximal inhibitory concentration
(IC50), which is defined as the concentration required
to inhibit cell viability by 50%, was determined through the
construction of a dose-response curve.
HepG-2 and MDA-MB-231 cells were also treated with
5-flurouracil (5-Fu; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), the positive control of the experiment, at the
concentrations of 12.5, 25, 50, 100 and 200 µg/ml for 72 h at 37°C.
IC50 values were determined and the cytotoxicity of
S. crispa extracts were compared to the effects of the known
anti-cancer drug, 5-Fu.
The selectivity index (SI) in the present study was
calculated from the IC50 of the extracts in normal rat
kidney cells (NRK-52E) vs. cancer cells. The extract was considered
to have high selectivity for cancer cells if the SI was >3
(26).
Observation of morphological
changes
HepG-2 and MDA-MB-231 cells were seeded at a density
of 1×106 cells/well into 6-well plates and treated with the SH at
their respective IC50 concentrations for 72 h at 37°C.
Untreated and treated cells were then observed under an inverted
microscope (Nikon Eclipse TS100; Nikon Corporation, Tokyo, Japan)
and images were captured with the attached camera.
Determination of cell doubling
time
HepG-2 and MDA-MB-231 cells were seeded into a
24-well plate at the density of 1×104 cells/ml. Subsequently, the
cells were treated with SH and incubated at 37°C for 72 h. Two
different concentrations were used: The pre-determined
IC50; and 2x the IC50. On the third day, the
treated cells were trypsinised and stained with trypan blue (Gibco;
Thermo Fisher Scientific, Inc.). Live cells were counted under an
inverted microscope (Nikon Eclipse TS100) using a hemocytometer
(Paul Marienfeld GmbH & Co. KG, Lunda-Königshofen, Germany).
The cell doubling time was calculated by dividing the total
duration (h) by the total number of generations. The number of
generations was calculated using the following formula: 3.32
(logNN-logN1), where NN is the
number of cells counted and N1 is the number of cells
seeded (27).
Cell cycle analysis
HepG-2 and MDA-MB-231 cells were seeded into 6-well
plates at a density of 1×106 cells/well and treated with SH at
IC50 for 72 h at 37°C. The cells were fixed with 70%
ethanol subsequently. Then the cells were washed twice with cold
phosphate buffer saline (PBS) and resuspended in 1 ml of PBS at a
concentration of 1×106 cells/ml. Subsequently, 5 µl of 10 mg/ml
RNase (Sigma-Aldrich; Merck KGaA) was added to the cell suspension
and incubated at 37°C for 1 h. Subsequently, 10 µl propidium iodide
(1 mg/ml; Sigma-Aldrich; Merck KGaA) was added and the suspension
was incubated in the dark for 30 min at 37°C. Finally, the samples
were analysed using flow cytometry (FACScan; BD Biosciences,
Franklin Lakes, NJ, USA) and CELLQuest™ software (BD Biosciences),
version 3.0. Flow cytometric analysis of the samples stained with
propidium iodide was run by collecting 25,000 events per
sample.
Detection of caspase-8
The caspase-8 assay kit was purchased from
Calbiochem (Merck KGaA). The caspase activity in SH-treated cells
was measured according to the manufacturer's protocol. Briefly,
cells were seeded at a density of 1×106 cells/ml into 6-well
plates. Cells were trypsinised following treatment with the SH at
IC50 for 72 h at 37°C. The cells were then transferred
into an Eppendorf tube. Subsequently, 1 µl staining reagent was
added to the cell suspension and incubated at 37°C for 30 min.
Cells were washed with PBS and transferred into a black well plate.
Fluorescence intensity at excitation 485 nm and emission 535 nm was
then recorded.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance with Tukey's honest significant difference
post-hoc test with GraphPad Instat software (version 3.0; GraphPad
Software, Inc., La Jolla, CA, USA) or Student's t-tests with
Microsoft Excel (version 2013; Microsoft Corporation, Redmond, WA,
USA). All data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxic effect of S. crispa
extracts
The effect of S. crispa on the growth
activities of HepG-2 and MDA-MB-231 cells was investigated using
MTT assays (Fig. 1). In total, 5
extracts (LH, LC, LEA, SH and SC) were demonstrated to induce cell
death in the two cell lines in a concentration-dependent manner
(P<0.05) while SEA displayed moderate cytotoxicity. However,
methanolic and water extracts of S. crispa (LM, LW, SM and
SW) revealed no evident cytotoxic activity against the cell lines.
In fact, the extracts promoted the growth of cancer cells, when
compared with the untreated control (Fig.
1). However, the differences were not statistically
significant.
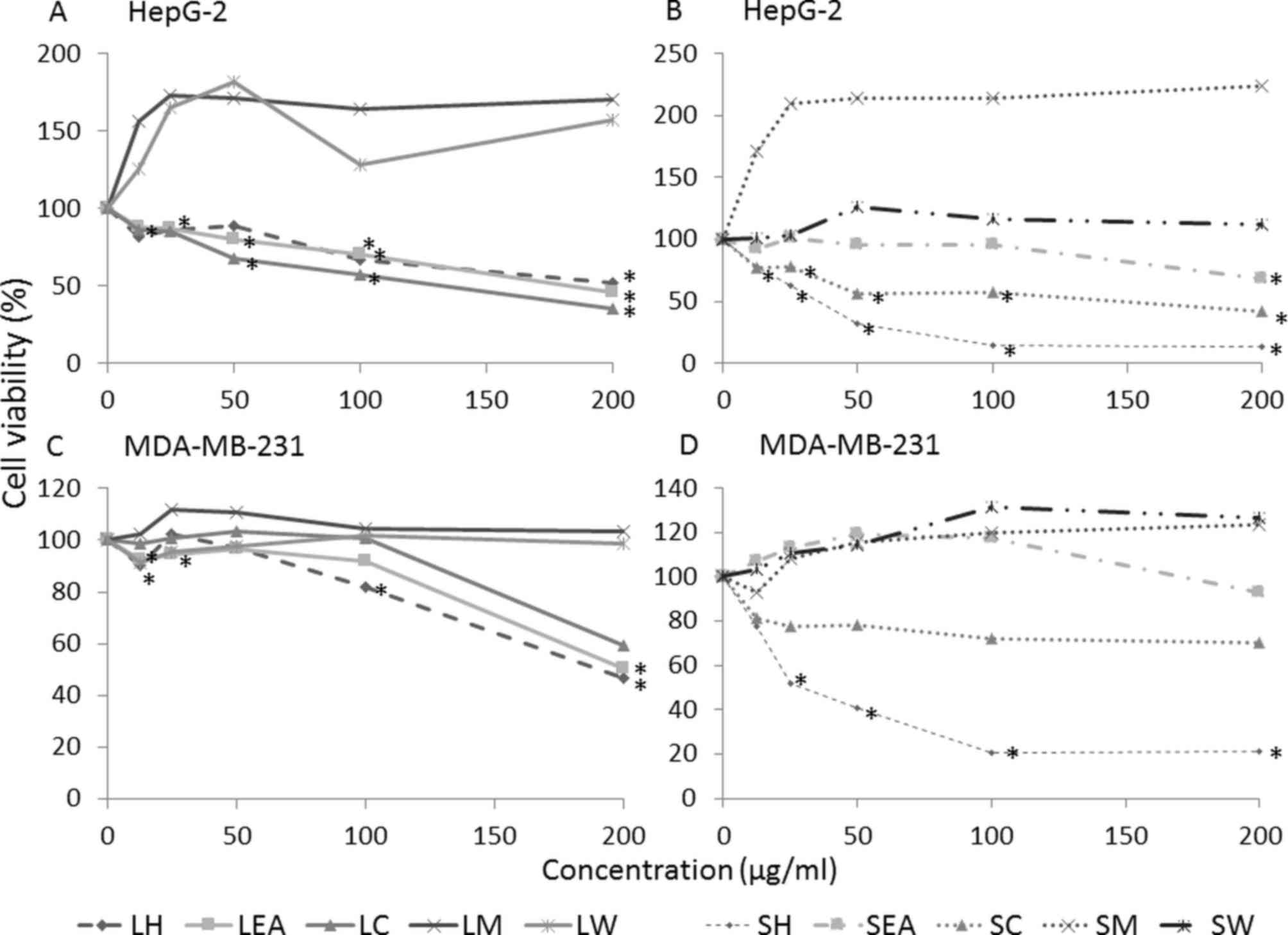 | Figure 1.Dose-response curve of
Strobilanthes crispa extracts on HepG-2 liver cancer and
MDA-MB-231 breast cancer cell lines. Cells were treated with
various concentrations (12.5, 25, 50, 100 and 200 µg/ml) of S.
crispa extracts for 72 h and percentage of cell viability was
determined using an MTT assay. HepG-2 cells treated with extracts
derived from the (A) leaves and (B) stems of Strobilanthes
crispa. MDA-MB-231 cells treated with extracts derived from the
(C) leaves and (D) stems of Strobilanthes crispa. Data are
presented as the mean following 3 independent experiments.
*P<0.05, compared with the untreated control (representing 100%
of cell viability). LH, leaf hexane; LEA, leaf ethyl acetate; LC,
leaf chloroform; LM, leaf methanol; LW, leaf water; SH, stem
hexane; SEA, stem ethyl acetate; SC, stem chloroform; SM, stem
methanol; SW, stem water. |
Based on the dose-response curve generated,
IC50 of each extracts was determined and summarized in
Table I. Among the extracts tested,
SH exhibited the highest potency with the lowest IC50
values in mediating HepG-2 and MDA-MB-231 cell death. The
IC50 value obtained from HepG-2 cells (38.8 µg/ml) was
comparable to the commercially available anti-cancer drug, 5-Fu
(IC50, 37.3 µg/ml). The IC50 determined in
MDA-MB-231 cells, 42.5 µg/ml was 1.4 times lower compared with the
5-Fu (IC50, 60 µg/ml).
 | Table I.IC50 and SI values of extracts of
Strobilanthes crispa on HepG-2 and MDA-MB-231 cells. |
Table I.
IC50 and SI values of extracts of
Strobilanthes crispa on HepG-2 and MDA-MB-231 cells.
|
| IC50,
µg/ml (SI) |
|---|
|
|
|
|---|
|
Extract/treatment | HepG-2 | MDA-MB-231 |
|---|
| Leaf |
|
|
|
Hexane | N/A | 192.50±3.54
(0.44) |
|
Chloroform | 175.70±35.40
(1.05) | N/A |
| Ethyl
acetate | 176.70±15.30
(0.94) | N/A |
|
Methanol | N/A | N/A |
|
Water | N/A | N/A |
| Stem |
|
|
|
Hexane | 38.80±8.50
(0.28) | 42.50±39.67
(0.26) |
|
Chloroform | 173.30±5.80
(>1.15) | N/A |
| Ethyl
acetate | N/A | N/A |
|
Methanol | N/A | N/A |
|
Water | N/A | N/A |
|
5-Fluorouracil | 37.30±6.75
(0.26) | 60.00±14.14
(0.16) |
SI calculated based on predetermined IC50
values on NRK-52E cells demonstrated that none of the extracts,
apart from SC, exhibited high selectivity towards cancer cells
because the SI values were <3 (Table
I). Furthermore, the anti-cancer drug, 5-Fu exhibited poor
selectivity. The SI value of SC was markedly higher compared with
the other extracts (>1.15).
Effect of SH on cell morphological
changes
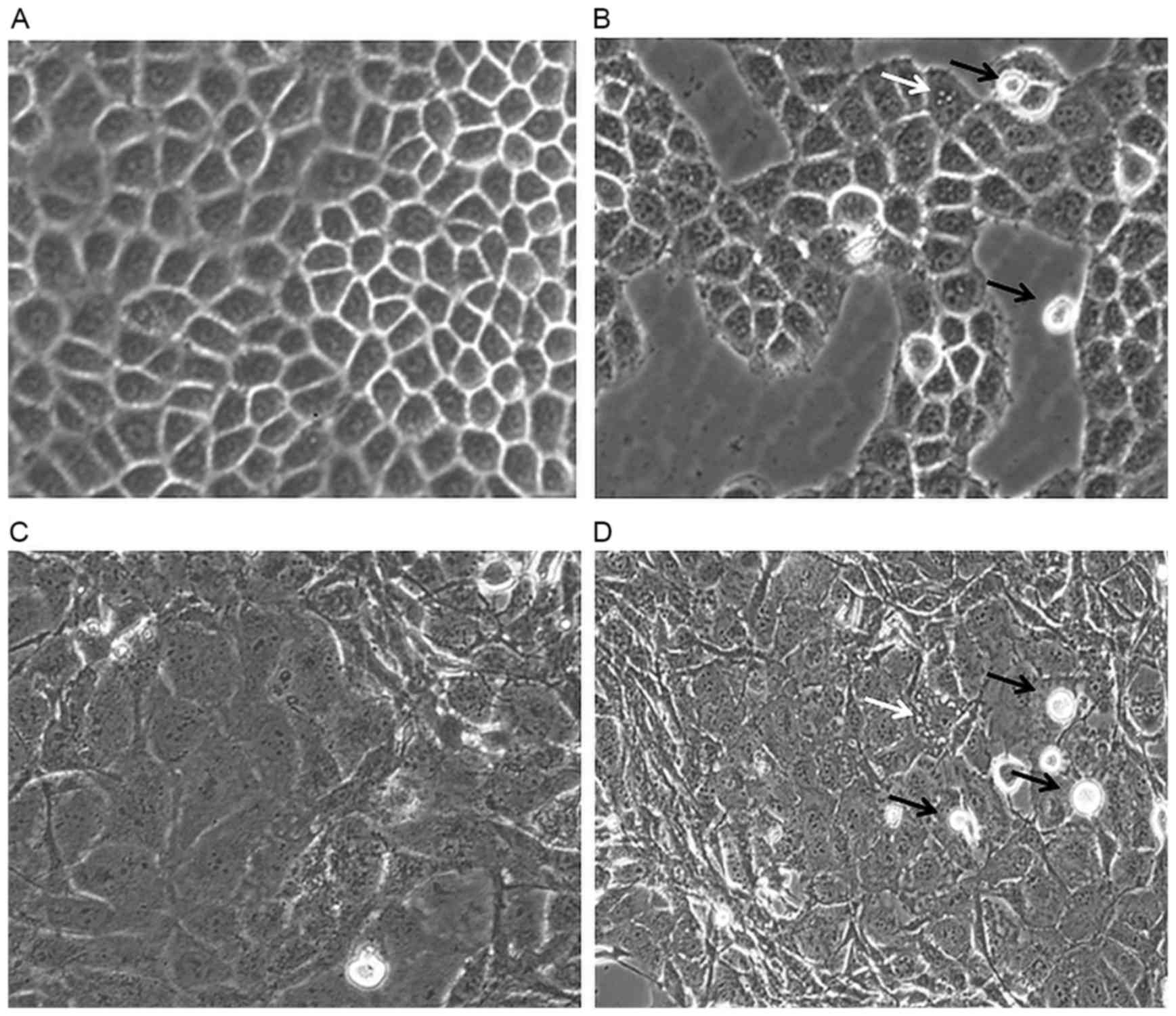
HepG-2 and MDA-MB-231 cells were observed under an
inverted microscope for morphological changes and the results are
presented in Fig. 2. Fig. 2A demonstrates confluent HepG-2 cells
that are flattened, grossly polygonal in shape and arranged in
monolayer. In the SH-treated HepG-2 cells, morphological changes,
including the formation of vacuoles within the cell were observed.
Furthermore, floating dead cells and a reduction in cell number
were also noted (Fig. 2B).
SH also induced MDA-MB-231 cell death as more
floating cells were observed in the treated compared with the
control cells. Untreated MDA-MB-231 cells remained normal, healthy
and confluent throughout the treatment period (Fig. 2C). However, vacuolation of cells was
noted in the treated cells (Fig.
2D).
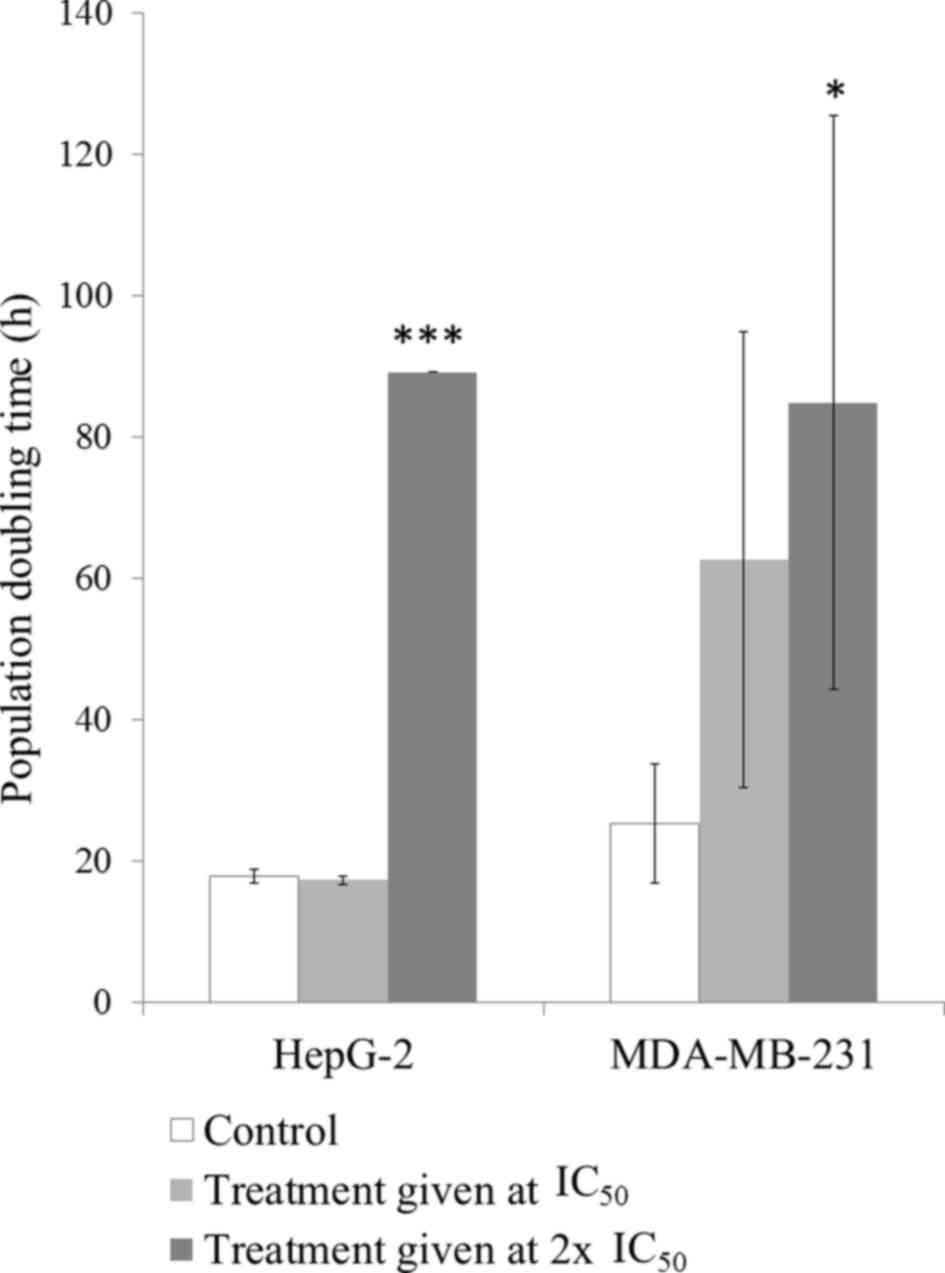
Effect of SH on cell doubling
time
Fig. 3 demonstrates
the effect of SH on HepG-2 and MDA-MB-231 cell proliferation. When
HepG-2 cells were exposed to SH at the IC50, no
significant differences in the cell population doubling time were
observed. However, cells treated with double the IC50 of
SH exhibited a significant increase in the cell doubling time
compared with the untreated control group (P<0.001). However,
MDA-MB-231 cells responded to SH in a dose-dependent manner. SH
treatment at the IC50 caused a 2.5-fold delay in cell
proliferation while twice the IC50 of SH delayed cell
doubling by 3.4-fold. The delayed cell doubling in cells treated
with the higher dose was statistically significant when compared
with the untreated control cells (P<0.05).
Effect of SH on cell cycle
profile
Treatment with SH caused HepG-2 cell cycle arrest at
the G0/G1 phase. This in turn caused a
decrease in percentage of cells that entered the S and
G2/M phases. The percentage of cells in sub-G phase in
SH-treated group was ~50% compared with that of the control cells
(Table II).
 | Table II.Effect of S. crispa stem
hexane extract on cell cycle progression of HepG-2 cells. |
Table II.
Effect of S. crispa stem
hexane extract on cell cycle progression of HepG-2 cells.
|
| Percentage of
cells |
|
|---|
|
|
|
|
|---|
| Cell cycle
phase | Control | Stem hexane | P-value |
|---|
| Sub-G | 0.79±0.01 | 0.41±0.01 | 0.017 |
|
G0/G1 | 60.68±1.22 | 71.16±0.68 | 0.009 |
| S | 2.22±0.05 | 1.80±0.01 | 0.008 |
|
G2/M | 35.37±0.71 | 26.13±0.25 | 0.003 |
Table III shows the
distribution of MDA-MB-231 cells in different cell cycle phases
following treatment with SH. Increased sub-G and S phases were
noted following the treatment; however, no significant differences
were identified.
 | Table III.Effect of S. crispa stem
hexane extract on cell cycle progression of MDA-MB-231 cells. |
Table III.
Effect of S. crispa stem
hexane extract on cell cycle progression of MDA-MB-231 cells.
|
| Percentage of
cells |
|
|---|
|
|
|
|
|---|
| Cell cycle
phase | Control | Stem hexane | P-value |
|---|
| Sub-G | 0.56±0.59 | 5.04±3.44 | 0.181 |
|
G0/G1 | 70.25±9.68 | 57.86±10.23 | 0.270 |
| S | 0.94±0.86 | 8.57±8.02 | 0.293 |
| G2/M | 28.31±8.29 | 28.98±13.75 | 0.956 |
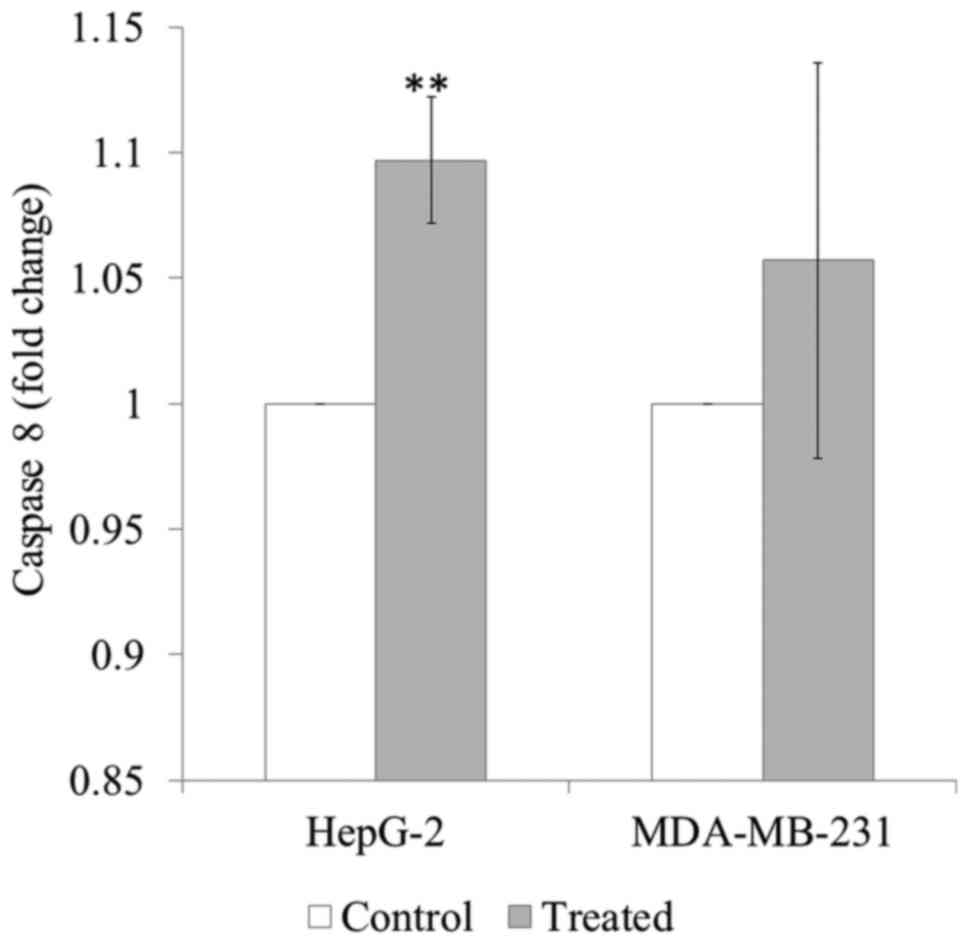
Effect of SH on caspase-8
activation
SH treatment significantly induced caspase-8
activation in HepG-2 cells (P<0.01). SH treatment appeared to
induce caspase-8 activation in MDA-MB-231 cells, although this was
not significant (Fig. 4).
Discussion
Cancer remains one of the leading causes of
mortality worldwide. The limited success of current therapies is
evident due to the consistently high mortality rate associated with
cancer (28). Therefore, the
identification of novel potential anti-cancer therapeutic agents is
warranted to provide more treatments options. Natural products
serve as a valuable source that offer cytotoxic properties, and
have gained significant recognition for their role in the
management of cancer. The present study evaluated the anticancer
effects of S. crispa extracts on HepG-2 liver cancer and
MDA-MB-231 breast cancer cells.
An MTT assay was used in the present study to screen
the plant extracts for their anti-cancer properties. As
demonstrated by the MTT assay LH, LEA, LC, SH and SC reduced cell
viability in a concentration-dependent manner in both cell lines.
Previous studies have revealed that S. crispa extracts are
able to kill liver and breast cancer cells (22–24,29). For
example, the chloroform extract of S. crispa was
demonstrated to possess cytotoxic effects against HepG-2 cells
(22,23), while the methanolic extract exhibited
strong cytotoxicity on MDA-MB-231 and HepG-2 cells (22). Furthermore, the ethanolic extract
(29) and a sub-fraction of the
dichloromethane extract of S. crispa (24) demonstrated the ability to consistently
kill breast cancer cells. Notably, the IC50 values
obtained in the present study were higher compared with that in the
previous studies mentioned. For instance, Asmah et al
(22) identified the IC50
value of the S. crispa chloroform extract in HepG-2 cells to
be 28 µg/ml, while the present study reported an IC50
value of 175.7 µg/ml. The methanolic extracts demonstrated no
cytotoxic effect on HepG-2 and MDA-MB-231 cells in the present
study, but Asmah et al (22)
reported that the extract inhibited the growth of the cells with
IC50 values of 29.3 and 27.2 µg/ml, respectively
(22). These contradicting
observations may be due to the differences in the geographical
areas from which the plants were collected. In fact, a study by
Chinkwo et al (30) reported
that the bioactivity of extracts from the medicinal plant
Sutherlandia frutescens that was collected from different
areas varied so much that only plants grown in a specific province
exhibited a chemotherapeutic effect. Differences in environmental
factors, including soil composition and temperature may influence
the production of the bioactive compounds in the plants (31). In addition, the maturity of plants may
influence the amount of bioactive compounds produced; generally
older plants accumulate a greater amount of content (19).
Among the extracts tested, the hexane extract from
the stem of S. crispa demonstrated the most potent cytotoxic
effect with the lowest IC50 values in the current study.
This suggests that the anti-cancer compounds are extractable by
hexane, a hydrophobic solvent. Previous studies have revealed that
hexane is useful for the extraction of stigmasterol (32–34) and
β-sitosterol (33–36). Stigmasterol and β-sitosterol extracted
from S. crispa have been demonstrated to possess anti-cancer
effects on liver, breast, and colon carcinoma (22). Other anti-cancer compounds that may be
extracted include alkaloids and tannins (20), which have been documented to exhibit
anti-cancer properties (37–40). In order to identify the compounds
responsible for the anti-cancer effects, further studies on the
isolation and purification of the bioactive compounds from S.
crispa are required.
SI, an indicator of the safety of drug use, was
determined for the compounds investigated in the present study. A
compound is considered to have high selectivity towards cancer
cells if the SI value is >3 (26).
In the present study, all SI values determined were <3, except
for SC, indicating the effect of the extracts was not specific to
the cancer cells as they were also killing the normal cells.
Furthermore, the anti-cancer drug, 5-Fu also demonstrated poor
selectivity for tumouricidal activity (41,42). The
non-selectiveness of compounds may impose severe side effects on
patients with cancer, as they are capable of damaging healthy cells
(43). In the current study, SC
possessed a relatively high SI value, indicating that it may cause
less side effects compared with the other extracts evaluated. In
order to improve the selectivity of a compound towards cancer
cells, drug modification can be performed (44). Strategies to improve the affinity
towards target cells and at the same time lower the affinity
towards off-target molecules should be considered (45). Previously, a number of antiepileptic
drugs, including levetiracetam, carbamazepine and felbamate, have
undergone drug modification to improve their tolerability and
efficacy (46). Thus, drug
modification to increase the selectively of SH to cancer cells may
be an ideal option to further improve its anticancer potency and
limit the side effects.
Treatment with SH was observed to delay the doubling
time of HepG-2 and MDA-MB-231 cells in the current study. The cell
cycle analysis demonstrated that HepG-2 cells were arrested at the
G0/G1 phase, indicating that SH inhibited
liver cancer cell growth through cell cycle modulation. However, no
significant changes in the cell cycle phases were reported in
MDA-MB-231 cells. Thus, the reduction in MDA-MB-231 cell
proliferation was independent of cell cycle progression and may be
attributed to other mechanisms.
Cell apoptosis, an important event in controlling
the programmed cell death, may serve an important role in the
SH-mediated cytotoxicity observed in the current study. This is
evident through the presence of cytoplasmic vacuolation in the
HepG-2 and MDA-MB-231 cells, which is a typical feature of
apoptosis (47,48). Cell apoptosis is an autonomous
dismantled process, which removes individual cell components while
avoiding inflammation that usually occurs during necrosis. Thus,
apoptosis is only limited to single cells and does not affect
normal adjacent cells (49). In
cancer studies, induction of apoptosis and inhibition of cellular
proliferation are considered as imperative properties in
chemoprevention and chemotherapy (50–52). To
further confirm the apoptotic activity of SH, caspase activation
was determined in the present study.
Caspases can be activated through the intrinsic or
extrinsic signalling pathway. Mitochondrial integrity is disrupted
by cellular stress in the intrinsic pathway while stimulation of
death receptors in the plasma membrane is initiated in the
extrinsic pathway (53). In response
to cellular stress, the initiator caspases (caspase-2, −8, −9 and
−10) cleave and activate the effector caspases (caspase-3, −6 and
−7); in turn the effector caspases execute the apoptotic process
(54). The present study demonstrated
that SH significantly activated caspase-8 in HepG-2 but not
MDA-MB-231 cells.
Taken together, the results of the present study
demonstrated that SH induced cytotoxicity and reduced cell
proliferation in HepG-2 cells through cell cycle modulation and
caspase-8 activation. However, the anti-cancer effect of SH was
independent of cell cycle and caspase-8 activation in MDA-MB-231
cells. This suggests that SH exhibited cytotoxic effects in
MDA-MB-231 cells via other mechanisms. One of the possible
mechanisms could be due to the down-regulation of c-myc gene
expression (23). C-myc oncogene has
been verified to account for the growth and progression of breast
cancer in hormone-dependent and -independent breast cancer cell
lines (55). Other possible
mechanisms that may be involved include the p53-dependent
signalling pathway (56), tumour
necrosis factor-related apoptosis-inducing ligand mechanism
(57) and p73-dependent signalling
pathway (58). Hence, further studies
investigating these mechanisms are warranted to confirm the
mechanism of action of SH on the MDA-MB-231 cell line.
In conclusion, S. crispa extracts were
revealed to exert strong anti-cancer effects on liver and breast
carcinoma cells. Among the extracts tested, the SH extract
demonstrated the most potent activity and could be further
developed as a potential anti-cancer therapeutic drug in the
future.
Acknowledgements
The present study was supported by the International
Medical University, Kuala Lumpur, Malaysia [grant no. BMSc I01/09
(03) 2011].
References
|
1
|
Sunilson AJ, Rejitha G, Anandarajagopal K,
Das A, Muthappan M and Promwichit P: Cytotoxic effect of Cayratia
carnosa leaves on human breast cancer cell lines. Int J Cancer Res.
5:115–122. 2009. View Article : Google Scholar
|
|
2
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Epidemiology of
hepatocellular carcinoma in USA. Hepatol Res. 37 Suppl 2:S88–S94.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Röcken C and Carl-McGrath S: Pathology and
pathogenesis of hepatocellular carcinoma. Dig Dis. 19:269–278.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sezgin C: Arsenic trioxide has additive
cytotoxic effects on MCF-7 breast cancer cell line with taxanes.
Turkish J Med Sci. 32:439–444. 2002.
|
|
6
|
Park S, Bae J, Nam BH and Yoo KY:
Aetiology of cancer in Asia. Asian Pac J Cancer Prev. 9:371–380.
2008.PubMed/NCBI
|
|
7
|
Ziegler RG, Anderson WF and Gail MH:
Increasing breast cancer incidence in China: The numbers add up. J
Natl Cancer Inst. 100:1339–1341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelsey JL and Bernstein L: Epidemiology
and prevention of breast cancer. Annu Rev Public Health. 17:47–67.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelsey JL, Gammon MD and John EM:
Reproductive factors and breast cancer. Epidemiol Rev. 15:36–47.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hulka BS and Stark AT: Breast cancer:
Cause and prevention. Lancet. 346:883–887. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu
K, Luan J, Duan H, Lu Z, Wang F, et al: Suppression of growth,
migration and invasion of highly-metastatic human breast cancer
cells by berbamine and its molecular mechanisms of action. Mol
Cancer. 8:812009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beer TM and Bubalo JS: Complications of
chemotherapy for prostate cancer. Semin Urol Oncol. 19:222–230.
2001.PubMed/NCBI
|
|
13
|
Leonard RC, Williams S, Tulpule A, Levine
AM and Oliveros S: Improving the therapeutic index of anthracycline
chemotherapy: Focus on liposomal doxorubicin (Myocet). Breast.
18:218–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wonders KY and Reigle BS: Trastuzumab and
doxorubicin-related cardiotoxicity and the cardioprotective role of
exercise. Integr Cancer Ther. 8:17–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldstein MS: Complementary and
Alternative Medicine. J Psychosoc Oncol. 21:1–212. 2003. View Article : Google Scholar
|
|
16
|
Chan LL, George S, Ahmad I, Gosangari SL,
Abbasi A, Cunningham BT and Watkin KL: Cytotoxicity effects of
Amoora rohituka and chittagonga on breast and pancreatic cancer
cells. Evid Based Complement Alternat Med. 2011:8606052011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sunarto PA: Materia Medica Indonesia1st.
Penerbitan Directorat Jenderal Pengawasan Obat dan Makanan.
Jakarta: pp. 95–99. 1977
|
|
18
|
Burkill I, Birtwistle W, Foxworthy F,
Scrivenor J and Watson J: A dictionary of the economic products of
the Malay Peninsula1st. Published on behalf of the governments of
the Straits settlements and Federated Malay states by the Crown
agents for the colonies. London: pp. 2086–2087. 1935
|
|
19
|
Bakar Abu MF, Teh AH, Rahmat A, Othman F,
Hashim N and Fakurazi S: Antiproliferative properties and
antioxidant activity of various types of Strobilanthes crispus tea.
Int J Cancer Res. 2:152–158. 2006. View Article : Google Scholar
|
|
20
|
Ismail M, Manickam E, Danial AM, Rahmat A
and Yahaya A: Chemical composition and antioxidant activity of
Strobilanthes crispus leaf extract. J Nutr Biochem. 11:536–542.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asmah R, Susi E, Patimah I, Yap Taufiq YH
and Fadzelly Mohd AB: Chemical constituents, antioxidant activity
and cytotoxic effects of essential oil from Strobilanthes crispus
and Lawsonia inermis. J Biol Sci. 6:1005–1010. 2006. View Article : Google Scholar
|
|
22
|
Asmah R, Susi E, Abdah MA, Patimah I, Yap
Taufiq YH and Fadzelly Mohd AB: Anticarcinogenic properties of
Strobilanthes crispus extracts and its compounds in vitro. Int J
Cancer Res. 2:47–49. 2006. View Article : Google Scholar
|
|
23
|
Susi E, Asmah R, Patimah I and Taufiq-Yap
YH: Comparing of the cytotoxicity properties and mechanism of
Lawsonia inermis and Strobilanthes crispus extract against several
cancer cell lines. J Med Sci. 7:1098–1102. 2007. View Article : Google Scholar
|
|
24
|
Yaacob NS, Hamzah N, Kamal Nik Mohamed NN,
Abidin Zainal SA, Lai CS, Navaratnam V and Norazmi MN: Anticancer
activity of a sub-fraction of dichloromethane extract of
Strobilanthes crispus on human breast and prostate cancer cells in
vitro. BMC Complement Altern Med. 10:422010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koh RY, Sim YC, Toh HJ, Liam LK, Ong RS,
Yew MY, Tiong YL, Ling AP, Chye SM and Ng KY: Cytotoxic and
apoptogenic effects of Strobilanthes crispa Blume extracts on
nasopharyngeal cancer cells. Mol Med Rep. 12:6293–6299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahavorasirikul W, Viyanant V,
Chaijaroenkul W, Itharat A and Na-Bangchang K: Cytotoxic activity
of Thai medicinal plants against human cholangiocarcinoma,
laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern
Med. 10:552010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schoene NW and Kamara KS: Population
doubling time, phosphatase activity, and hydrogen peroxide
generation in Jurkat cells. Free Radic Biol Med. 27:364–369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chong HZ, Rahmat A, Yeap SK, Akim Md A,
Alitheen NB, Othman F and Gwendoline-Ee CL: In vitro cytotoxicity
of Strobilanthes crispus ethanol extract on hormone dependent human
breast adenocarcinoma MCF-7 cell. BMC Complement Altern Med.
12:352012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinkwo KA: Sutherlandia frutescens
extracts can induce apoptosis in cultured carcinoma cells. J
Ethnopharmacol. 98:163–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wink M: Functions in plants secondary
metabolites and their exploration in biotechnology. Sheffield
Academic Press; United Kingdom: pp. 188–2731. 1999
|
|
32
|
Ahmad A, Alkarkhi AFM, Hena S and Khim LH:
Extraction, separation and identification of chemical ingredients
of Elephantopus Scaber L. using factorial design of experiment. Int
J Chem. 1:2009. View Article : Google Scholar
|
|
33
|
Ahmed Y, Rahman S, Akhtar P, Islam F,
Rahman M and Yaakob Z: Isolation of steroids from n-hexane extract
of the leaves of Saurauia roxburghii. Int Food Res J. 20:2939–2943.
2013.
|
|
34
|
Saludes JP, Garson MJ, Franzblau SG and
Aguinaldo AM: Antitubercular constituents from the hexane fraction
of Morinda citrifolia Linn. (Rubiaceae). Phytother Res. 16:683–685.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Panal S, Urip H, Pandapotan M and Tonel B:
Isolation of β-sitosterol from n-hexane extract of Picria
fel-terrae Lour. leave and study of its antidiabetic effect in
alloxan induced diabetic mice. Int J PharmTech Res. 6:137–141.
2014.
|
|
36
|
Saiin C, Rattanajak R, Kamchonwongpaisan
S, Ingkaninan K, Sukontason K, Baramee A and Sirithunyalug B:
Isolation and in vitro antimalarial activity of hexane extract from
Thai Picrasma javanica B1 stembark. Southeast Asian J Trop Med
Public Health. 34 Suppl 2:S51–S55. 2003.
|
|
37
|
Kabashima H, Miura N, Shimizu M, Shinoda
W, Wang X, Wang Z, Takahashi S, Harada T, Maruyama H, Tashiro S, et
al: Preventive impact of alkaloids with anti-cancer effect
extracted from natural herb and the derivatives. WebmedCentral Prev
Med. 1:WMC005192010.
|
|
38
|
Lu JJ, Bao JL, Chen XP, Huang M and Wang
YT: Alkaloids isolated from natural herbs as the anticancer agents.
Evid Based Complement Alternat Med. 2012:4850422012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marzouk MS, Moharram FA, Mohamed MA,
Gamal-Eldeen AM and Aboutabl EA: Anticancer and antioxidant tannins
from Pimenta dioica leaves. Z Naturforsch C. 62:526–536. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Melo Gomes J, de Sousa Araújo TA, de
Almeida e Castro Thijan Nobre V, de Vasconcelos Cabral Lyra D, do
Desterro Rodrigues M, do Nascimento Carneiro S, de Amorim
Cavalcanti EL and de Albuquerque UP: Antiproliferative activity,
antioxidant capacity and tannin content in plants of semi-arid
northeastern Brazil. Molecules. 15:8534–8542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khanna A, Walker GR, Livingstone AS,
Arheart KL, Rocha-Lima C and Koniaris LG: Is adjuvant 5-FU-based
chemoradiotherapy for resectable pancreatic adenocarcinoma
beneficial? A meta-analysis of an unanswered question. J
Gastrointest Surg. 10:689–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kurt E, Kurt M, Kanat O, Cetintas SK,
Aygun S, Palazoglu T, Ozkan L, Evrensel T, Kaya E and Manavoglu O:
Phase II study of induction chemotherapy with gemcitabine plus
5-fluorouracil followed by gemcitabine-based concurrent
chemoradiotherapy for unresectable locally advanced pancreatic
cancer. Tumori. 92:481–486. 2006.PubMed/NCBI
|
|
43
|
Widakowich C, de Castro G Jr, de Azambuja
E, Dinh P and Awada A: Review: Side effects of approved molecular
targeted therapies in solid cancers. Oncologist. 12:1443–1455.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huggins DJ, Sherman W and Tidor B:
Rational approaches to improving selectivity in drug design. J Med
Chem. 55:1424–1444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawasaki Y and Freire E: Finding a better
path to drug selectivity. Drug Discov Today. 16:985–990. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Landmark CJ and Johannessen SI:
Modifications of antiepileptic drugs for improved tolerability and
efficacy. Perspect Medicin Chem. 2:21–39. 2008.PubMed/NCBI
|
|
47
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Syam S, Abdul AB, Sukari MA, Mohan S,
Abdelwahab SI and Wah TS: The growth suppressing effects of
girinimbine on HepG2 involve induction of apoptosis and cell cycle
arrest. Molecules. 16:7155–7170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cullen SP and Martin SJ: Caspase
activation pathways: Some recent progress. Cell Death Differ.
16:935–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shiu RP, Watson PH and Dubik D: c-myc
oncogene expression in estrogen-dependent and -independent breast
cancer. Clin Chem. 39:353–355. 1993.PubMed/NCBI
|
|
56
|
Shen Y and White E: p53-dependent
apoptosis pathways. Adv Cancer Res. 82:55–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ramadan S, Terrinoni A, Catani MV, Sayan
AE, Knight RA, Mueller M, Krammer PH, Melino G and Candi E: p73
induces apoptosis by different mechanisms. Biochem Biophys Res
Commun. 331:713–717. 2005. View Article : Google Scholar : PubMed/NCBI
|