Introduction
Breast cancer is a major disease threatening women's
health. According to statistics, breast cancer accounts for
one-third in all new-onset tumors in female (1). The occurrence and development of breast
cancer are multi-gene and multi-step chain processes, which is
promoted by gene abnormality; besides, abnormal epigenetic
regulation also plays an important role in the occurrence process
of breast cancer (2). In recent
years, studies have found that long non-coding RNA (lncRNA) plays
an important role in the occurrence and development of breast
cancer through the epigenetic regulation mechanism (3).
PTEN (phosphatase and tensin homolog deleted on
chromosome ten) has been reported to inhibit progression and
development of various cancers, and it was established as a tumor
suppressor (4). For instance, PTEN
can participate in suppressing the proliferation of cancer cells
via negatively regulating the PI3K/AKT pathway (5,6), which
suggests that PTEN is important in malignant transformation of
cancer cells. However, little is known about the detailed mechanism
how PTEN is changed during carcinogenesis. In recent years,
non-coding RNA (ncRNA) has drawn increasing attention in the field
of cancer. As reported, PTENp1 (PTEN pseudogene 1) could regulate
the expression of the ancestral gene PTEN, thus exerted an effect
on the process of carcinogenesis (7).
However, the function and mechanism of PTEN pseudogene 1 (PTENP1)
in breast cancer cells have not been completely clarified. Breast
cancer cell lines expressing lncRNA PTENP1 stably were established
in this study, so as to investigate the role of PTENP1 in the
proliferation and migration of breast cancer cells and its
mechanism.
Materials and methods
Materials
Breast cancer cell line MCF7 and cell line 293T used
for lentivirus packaging were purchased from Institute of
Biochemistry and Cell Biology, Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences. TRIzol reagent was purchased
from Invitrogen (Carlsbad, CA, USA), trypsin and fetal bovine serum
DMEM were purchased from Gibco (Grand Island, NY, USA), dimethyl
sulfoxide was from Sigma (St. Louis, MO, USA), CCK-8 kit was from
Dojindo (Kumamoto, Japan), RNA extraction kit and TRIzol were
obtained from Takara (Otsu, Japan), and the rabbit anti-human
p-p44/42MAPK, p-p38 MAPK, p-AKT, cyclin A2, CDK2 and mouse
anti-human GAPDH monoclonal antibodies were purchased from Cell
Signaling Technology (Beverly, MA, USA).
Cell culture
MCF7 cells were cultured in DMEM medium containing
10% fetal bovine serum and the medium was placed in an incubator
containing 5% CO2 at 37°C. Cells were digested by 0.25% trypsin,
followed by passage; and cells in logarithmic phase were used for
the experiment.
Construction of lncRNA PTENP1
lentiviral expression vector
According to DNA sequence of PTENP1 in National
Center for Biotechnology Information (NCBI), the target gene
PTENP1-3′UTR was amplified with DNA in human skin tissue as the
template. Gene primer sequences were as follows: sense strand:
5′-AGTCACCTGTTAAGAAAATGAGAAGACAAA-3′, antisense strand:
5′-CTGTCCCTTATCAGATACATGACTTTCAA-3′. The recombinants expressing
PTENP1 were constructed by LV003-GFP vector, followed by sequencing
and identification.
Lentivirus packaging
Four kinds of plasmids (pspax2, pMD2G,
pLVX-IRES-ZsGreen1 and pLV003-PTENP1) in lentiviral packaging
system were prepared; ZsGreen1 expression cassette on the plasmid
could express GFP; 293T cells were transfected with the plasmids
containing the target gene and the four kinds of plasmids in
lentiviral packaging system for virus packaging, and the virus
supernatant was collected; the supernatant was centrifuged in a 40
ml ultra-speed centrifuge tube at 80,000 × g for 2 h at 4°C; the in
a 40 ml ultracentrifuge tube. The virus precipitation was
resuspended using iced PBS, dissolved at 4°C overnight and stored
at −80°C.
Construction of breast cancer cell
line MCF7 expressing lncRNA PTENP1 stably
When the cell fusion of MCF7 reached 60–70%,
GFP-PTENP1 and LV003-GFP were added on the cell surface at the
multiplicity of infection (MOI)=10, respectively. After 8 h, the
normal DMEM medium containing 10% fetal calf serum was used. The
experimental group (MCF7 cells transfected with LV003-GFP-PTENP1)
and control group (MCF7 cells transfected with LV003-GFP) were set
up. Puromycin (1 µl/ml) was added for screening after green
fluorescence was observed under the fluorescence microscope,
fluorescence photos were taken after 3 days, and the expression of
PTENP1 was detected by real-time fluorescent quantitative PCR.
Detection of proliferation capacity of
breast cancer cells via CCK-8
Cells taken from the two groups were inoculated onto
the 96-well plates (2×103/well) with 5 repetition wells
in each group. CCK-8 solution was added with 10 µl/well at 12, 24,
48 and 72 h, respectively, and the cells were incubated in an
incubator for 2 h. A450 was measured with the microplate reader,
and the growth curve was drawn according to A450s.
Detection of clone formation capacity
of breast cancer cells via plate clone assay
Cells taken from experimental group and control
group were digested to prepare the single-cell suspension, and the
cell density was adjusted to 2×103/ml, and 100 µl cell
suspension was taken and inoculated onto the 6-well plate, and then
2 ml medium was added. The cell suspension was cultured in an
incubator for 1–2 weeks and was seeded when the clone formation was
visible, followed by Giemsa staining; and the number of clones was
counted.
Detection of migration rate of breast
cancer cells via scratch assay
The above-mentioned cells were inoculated onto the
6-well plate, and when the cell fusion reached 100%, a straight
line was drawn in the middle of each well with 1-ml spearhead, and
the photos of cells were taken after 24 h; the area change before
and after the scratch was calculated. Cell migration rate (%) =
(area in scratch - area after 24 h/area in scratch) × 100%.
Detection of protein expression via
western blot method
Cells in logarithmic phase were taken from
experimental group and control group; the total protein of each
group was extracted and quantified according to the procedures in
protein extraction kit. The protein was transferred to
polyvinylidene chloride membrane by wet transfer method after
acrylamide gel electrophoresis. The membrane was sealed by skimmed
milk for 1 h. And then primary rabbit monoclonal p-AKT antibody
(dilution, 1:500; cat. no. ab81283); rabbit polyclonal t-AKT
antibody (dilution, 1:500; cat. no. ab38449); rabbit monoclonal
p-P44/42MAPK antibody (dilution, 1:500; cat. no. ab53277); rabbit
monoclonal t-P44/42 MAPK antibody (dilution, 1:500; cat. no.
ab50011); rabbit polyclonal p-p38 MAPK antibody (dilution, 1:500;
cat. no. ab47363); rabbit polyclonal t-p38 MAPK antibody (dilution,
1:500; cat. no. ab197348); rabbit monoclonal cyclinA2 antibody
(dilution, 1:500; cat. no. ab32386); rabbit monoclonal CDK2
antibody (dilution, 1:500; cat. no. ab32147); rabbit polyclonal
GAPDH antibody (dilution, 1:500; cat. no. ab37168) were added. All
the antibodies were all purchased from Abcam (Cambridge, MA, USA).
Then the protein was incubated overnight at 4°C. After the protein
was washed with TBST three times, secondary goat anti-rabbit (HRP)
IgG antibody (dilution, 1:2,000; cat. no. ab6721) was added for
incubation for 1 h, followed by development via enhanced
chemiluminescence (ECL).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data analysis. The t-test was used for the comparison
of protein expression levels and absorbance values between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Influence of lncRNA PTENP1 on
proliferation capability of breast cancer MCF7 cells
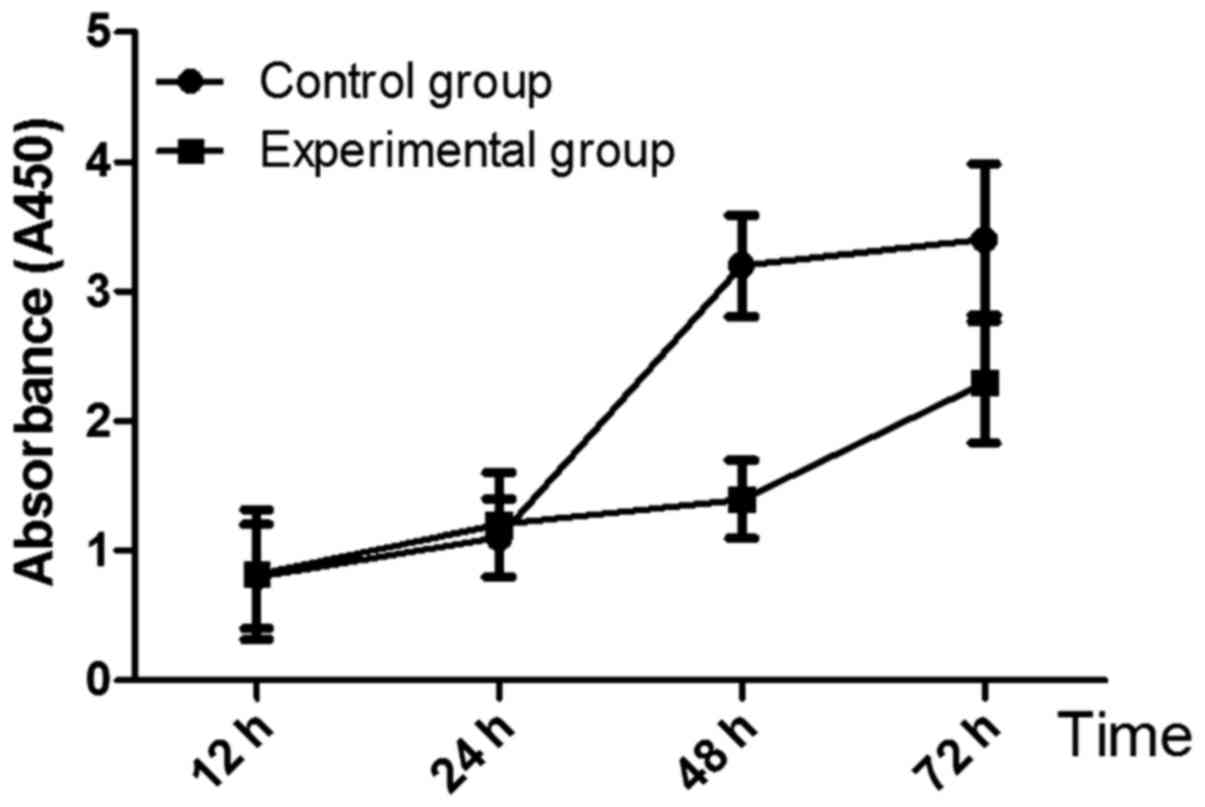
The growth curve of breast cancer MCF7 cells is
shown in Fig. 1. A450 of cells in
experimental group at 48 h and 72 h was 1.4±0.3 and 2.3±0.47,
respectively, which were significantly lower than those in control
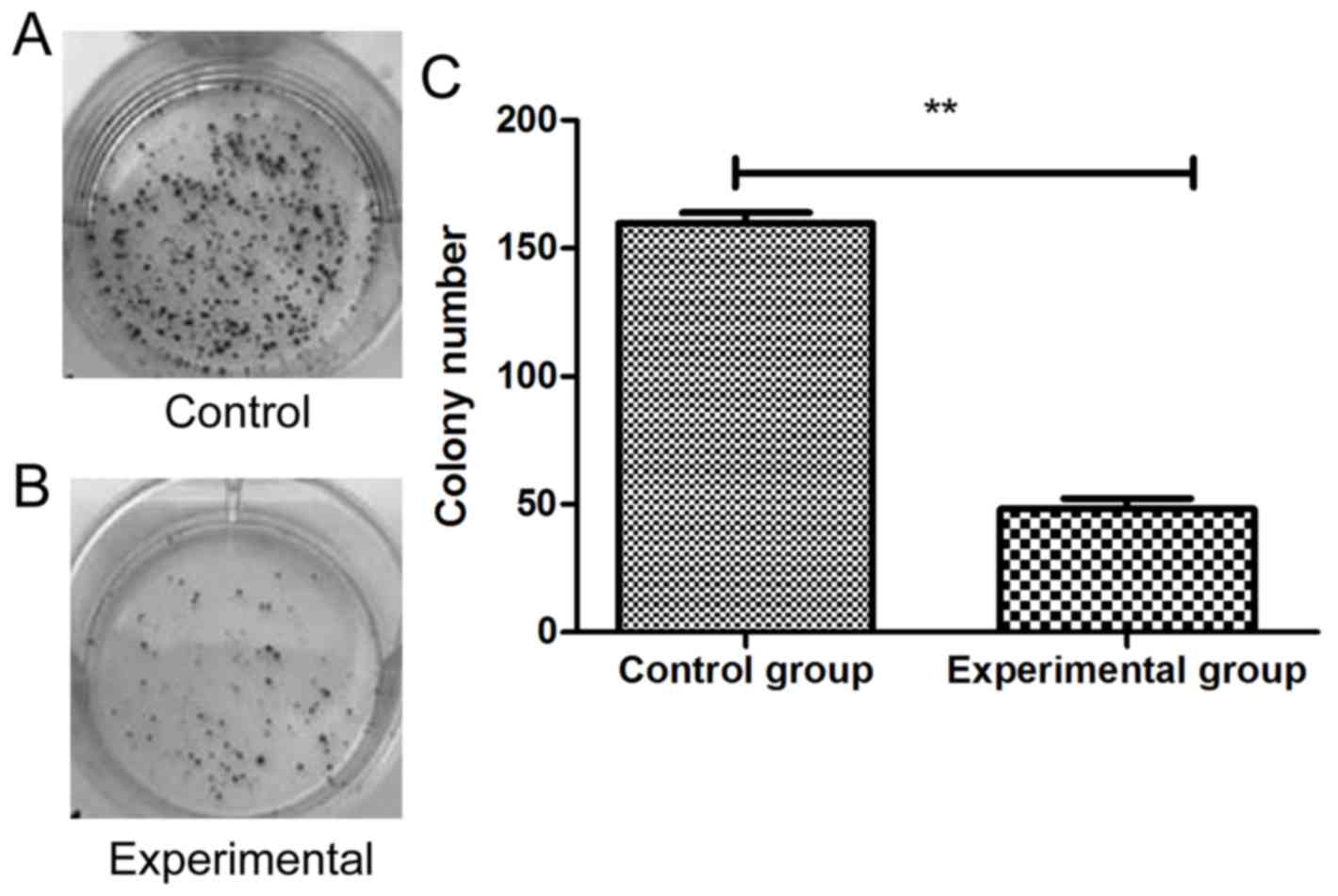
group (3.2±0.39, 3.4±0.58) (P<0.05). Colony-forming assay is
shown in Fig. 2, and the results
showed that the number of cell clones in experimental group was
48±13, which was significantly lower than that in control group
(159±16) (P<0.01).
Influence of lncRNA PTENP1 on
migration capacity of breast cancer MCF7 cells
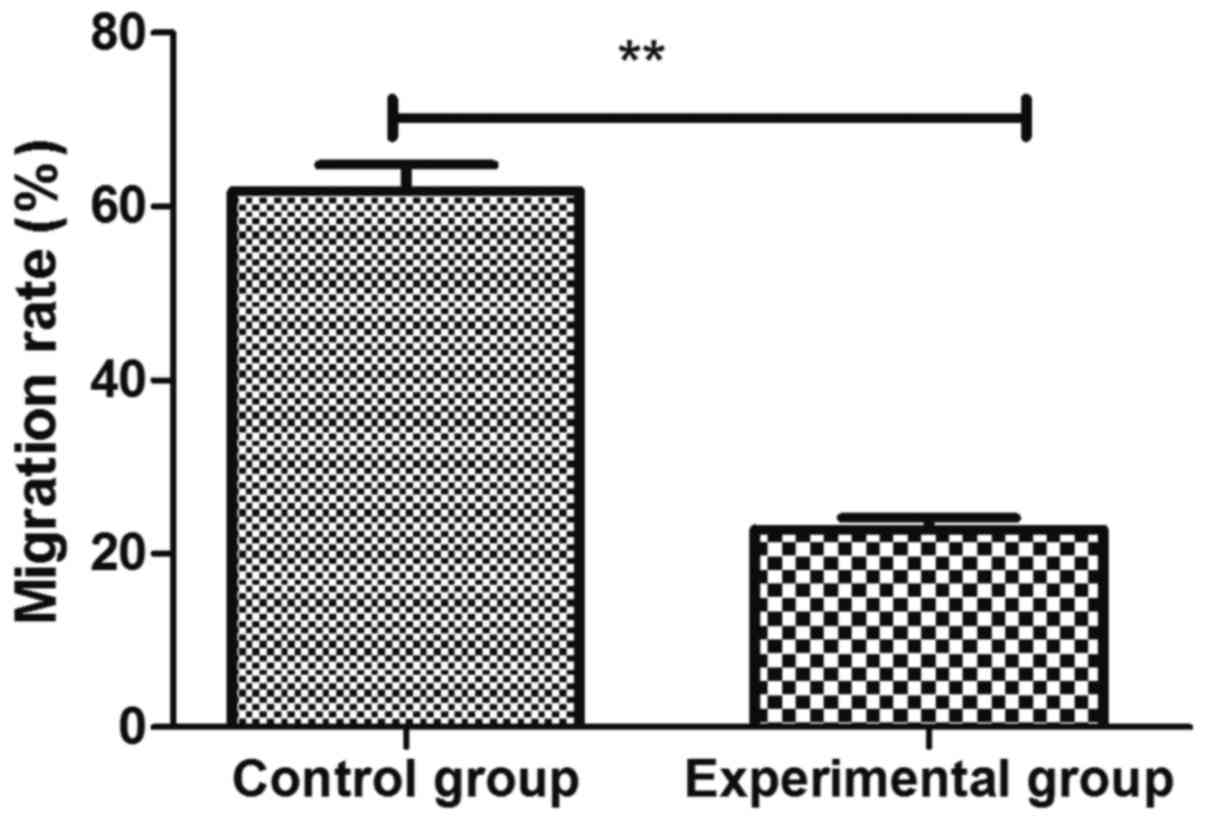
Scratch assay showed that the cell migration rate in
experimental group was 22.8±3.3%, which was significantly lower
than that in control group 61.8±5.2% (p<0.01) (Fig. 3).
Influence of lncRNA PTENP1 on cyclin
of breast cancer MCF7 cells
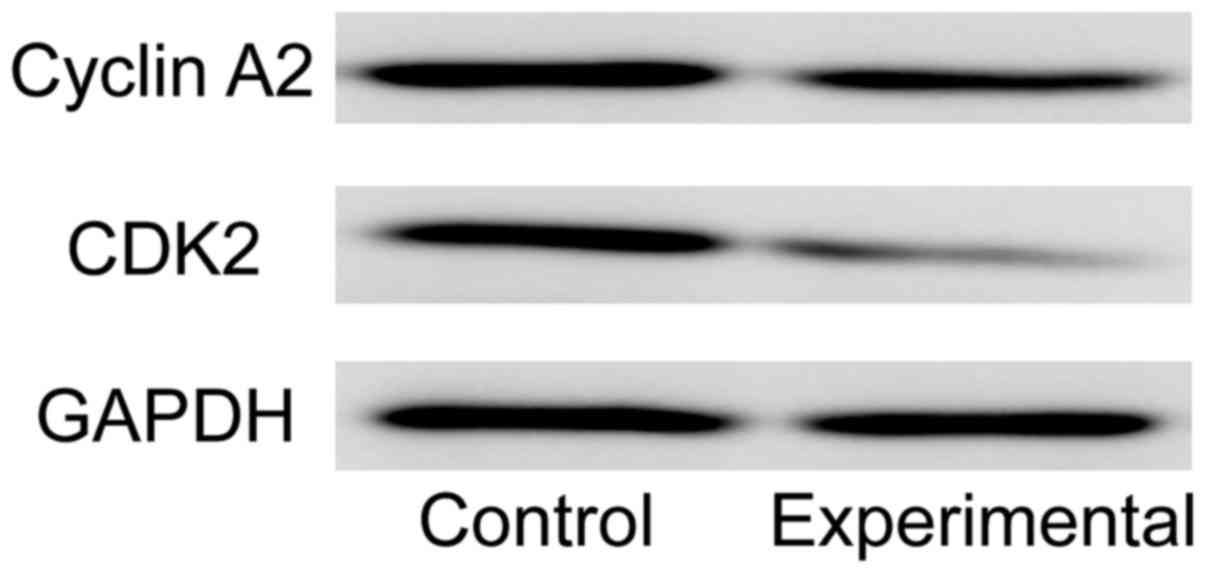
Cyclin A2 and CDK2 are the important proteins that
promote cells to transform from S phase to G2 phase. Western blot
detection showed that the expression levels of cyclin A2 and CDK2
in experimental group were significantly lower than those in
control group (Fig. 4).
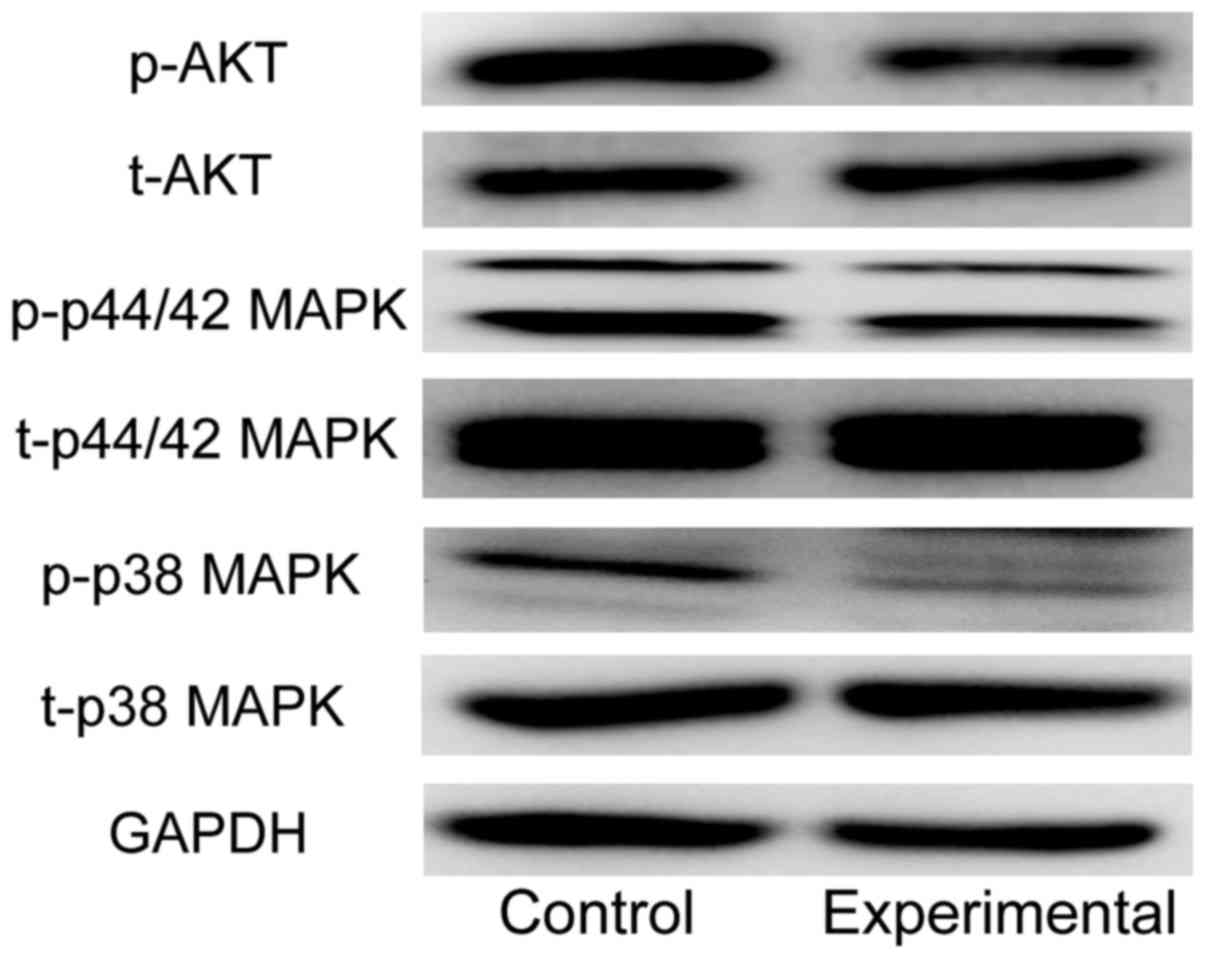
Influence of lncRNA PTENP1 on AKT and
MAPK signaling pathways
Western blot detection showed that the expression of
p-AKT protein, an important factor in AKT signaling pathway, in
experimental group was significantly decreased compared with that
in control group. In addition, the protein expression levels of
p-p38 MAPK and p-p44/42 MAPK, important factors in MAPK signaling
pathway, in experimental group were significantly decreased
compared with those in control group (Fig. 5).
Discussion
lncRNA is a transcription RNA with the length of
more than 200 nucleotides. lncRNA can not be translated into
protein because of lack of a promoter. More and more studies have
confirmed that lncRNA regulates the proliferation, apoptosis,
differentiation and migration of tumor cells through its
participation in regulating chromatin modification, transcriptional
activation, intranuclear interference and silencing of X chromosome
(8). Studies have shown that lncRNA
also plays an important regulatory role in the occurrence and
development of breast cancer. In ERα-positive breast cancer, lncRNA
H19 can improve the chemotherapy resistance of breast cancer by
downregulating BIK, a pro-apoptotic gene (9). In breast cancer cells, long non-coding
RNA UCA1 can enhance the chemotherapy resistance of tamoxifen,
which may be realized through the regulation of miR-18a-HIF1α
feedback loop (10). In this study,
breast cancer cell MCF7 expressing PTENP1 was established. CCK-8
proliferation assay and plate clone assay showed that PTENP1 could
inhibit the proliferation and growth of breast cancer cells,
scratch assay showed that PTENP1 could inhibit the migration of
breast cancer cells.
Via directly inhibiting phosphoinositide 3-kinase
(PI3K) signaling pathway and negatively regulating miR-21, PTEN
could affect a series of cellular processes (11,12). As
showed in our study, PTENp1 inhibited cell proliferation and
migration via AKT signaling pathway as well as cell cycle related
proteins such as cyclin A2 and CDK2. This discovery revealed that
PTENp1, a kind of pseudogene transcript that blocks miRNA activity
in breast cancer, could post-transcriptionally regulate many
potential biological roles.
MAPK is a serine/threonine protein kinase in cells,
which plays an important role in breast cancer. Abnormal changes of
MAPK signaling pathway play an important role in the growth,
differentiation and other physiological activities of breast
cancer. To investigate the role of PTENP1 in MAPK signaling pathway
in breast cancer cells, the protein expression levels of p-p44/42
MAPK and p-p38 MAPK in MCF7 expressing PTENP1 were detected, and it
was found that PTENP1 could downregulate the phosphorylation of
Erk1/2 (P44/42 MAPK) and p38 MAPK protein, important factors in
MAPK signaling pathway in breast cancer cells, indicating that
PTENP1 can also regulate the proliferation and migration of breast
cancer cells via regulating MAPK signaling pathway, in addition to
inhibiting the activity of AKT signaling pathway in breast cancer
cells.
In recent years, some scholars have proposed the
competitive endogenous RNA (ceRNA) hypothesis; in other words,
there are common microRNA binding sites in lncRNA and mRNA in
corresponding functional network, which weakens the inhibiting
effect of microRNA on homologous mRNA via microRNA adsorption
(13–15). Studies show that lncRNA PTENP1 can
also regulate the expression of PTEN through the method in ceRNA
hypothesis, thus affecting the progression of tumor cells (16–18).
Whether the mechanism of ceRNA is involved in breast cancer cells
needs further study.
In conclusion, lncRNA PTENP1 can inhibit the
proliferation and migration of breast cancer cells, which may be
realized through downregulating cyclin A2 and CDK2, the important
proteins in cell cycle, and AKT and MAPK signaling pathways. lncRNA
PTENP1 plays an important role in the occurrence and development of
breast cancer, and the in-depth study on it can reveal not only the
related mechanism of PTEN, its cancer suppressor gene, but also the
role and mechanism of lncRNA in breast cancer. In addition, the
in-depth study on lncRNA can provide new ideas for the prevention
and treatment of breast cancer.
References
|
1
|
Shi Y, Yang F, Sun Z, Zhang W, Gu J and
Guan X: Differential microRNA expression is associated with
androgen receptor expression in breast cancer. Mol Med Rep.
15:29–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marzese DM, Gago FE, Vargas-Roig LM and
Roqué M: Methylation profile of human breast cancer: A possible
biomarker for the detection of circulating tumor cells. J Clin
Oncol. 27 Suppl 15:111122009.
|
|
3
|
Yu X and Li Z: Long non-coding RNA HOTAIR:
A novel oncogene (Review). Mol Med Rep. 12:5611–5618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez-Tenorio G, Alkhori L, Olsson B,
Waltersson MA, Nordenskjöld B, Rutqvist LE, Skoog L and Stål O:
PIK3CA mutations and PTEN loss correlate with similar prognostic
factors and are not mutually exclusive in breast cancer. Clin
Cancer Res. 13:3577–3584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karreth FA, Tay Y, Perna D, Ala U, Tan SM,
Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al:
In vivo identification of tumor- suppressive PTEN ceRNAs in an
oncogenic BRAF-induced mouse model of melanoma. Cell. 147:382–395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang
E, Shao L, Li A, Yang N, Han X, et al: LncRNA H19 confers
chemoresistance in ERα-positive breast cancer through epigenetic
silencing of the pro-apoptotic gene BIK. Oncotarget. 7:81452–81462.
2016.PubMed/NCBI
|
|
10
|
Li X, Wu Y, Liu A and Tang X: Long
non-coding RNA UCA1 enhances tamoxifen resistance in breast cancer
cells through a miR-18a-HIF1α feedback regulatory loop. Tumour
Biol. 37:14733–14743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Simpson L, Takahashi M, Miliaresis
C, Myers MP, Tonks N and Parsons R: The PTEN/MMAC1 tumor suppressor
induces cell death that is rescued by the AKT/protein kinase B
oncogene. Cancer Res. 58:5667–5672. 1998.PubMed/NCBI
|
|
12
|
Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH,
Kim JG and Kim CH: Ganglioside GM3 modulates tumor suppressor
PTEN-mediated cell cycle progression - transcriptional induction of
p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway.
Glycobiology. 16:573–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu G, Yao W, Gumireddy K, Li A, Wang J,
Xiao W, Chen K, Xiao H, Li H, Tang K, et al: Pseudogene PTENP1
functions as a competing endogenous RNA to suppress clear-cell
renal cell carcinoma progression. Mol Cancer Ther. 13:3086–3097.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alimonti A, Carracedo A, Clohessy JG,
Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ,
Brogi E, et al: Subtle variations in Pten dose determine cancer
susceptibility. Nat Genet. 42:454–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnsson P, Ackley A, Vidarsdottir L, Lui
WO, Corcoran M, Grandér D and Morris KV: A pseudogene
long-noncoding-RNA network regulates PTEN transcription and
translation in human cells. Nat Struct Mol Biol. 20:440–446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC,
Hwang SM and Hu YC: Suppression of hepatocellular carcinoma by
baculovirus-mediated expression of long non-coding RNA PTENP1 and
MicroRNA regulation. Biomaterials. 44:71–81. 2015. View Article : Google Scholar : PubMed/NCBI
|