Introduction
Aggressive fibromatosis, also known as desmoid
tumor, is a semi-malignant soft tissue neoplasm of clonal
myofibroblastic origin that arises from the musculoaponeurotic
structures, fascial planes and ligaments throughout the body. The
incidence is estimated to be 3–4 cases/million people/year in
Europe and the USA, accounting for ~3% of all soft-tissue tumors
analyzed by biopsy (1,2). Aggressive fibromatosis can occur
sporadically or be associated with familial adenomatous polyposis
in Gardner syndrome. Among all cases of sporadic aggressive
fibromatosis, >70% are associated with β-catenin mutations;
however, the clinical implication of this finding has not been
determined completely (3–5). Although aggressive fibromatosis does not
have the ability to metastasize, it is characterized by locally
aggressive growth with destructive infiltration of the surrounding
tissues and high rates of local recurrence despite surgical
resection, leading to significant functional impairments and
morbidity.
Numerous analyses have been conducted to assess the
prognostic factors that affect recurrence-free survival (RFS) in
patients with aggressive fibromatosis (1,2,6–16). Among
these factors, anatomical site, tumor size and patient age are
considered to be the most significant for RFS (10,11).
Notably, the prognostic significance of negative surgical margins
on RFS remains a subject of debate, and inconsistent results have
been presented in published studies investigating the clinical
significance of surgical margins in aggressive fibromatosis,
questioning the impact of curative surgical resection in
general.
Prior to 1999, limb-sparing surgical resection with
clear margins was considered the therapy of choice in the vast
majority of cases, reflecting the standard approach for the
treatment of soft tissue sarcomas. In 1998 and 1999, the
Massachusetts General Hospital and the M.D. Anderson Cancer Center
(MDACC) reported improved RFS rates for patients with negative
margins, following analyses of 92 and 168 patients, respectively
(6,7).
Shortly afterwards, Merchant et al (8) analyzed the outcomes of a series of 105
surgically treated patients with primary disease at the Memorial
Sloan-Kettering Cancer Center (MSKCC), and were unable to detect
any significant effect of positive margins on RFS. In 2003, Gronchi
et al (10) from the Instituto
Nazionale Tumori (INT) in Milan reported similar observations in
203 patients. A more recent analysis from the MDACC in 2007 was
unable to reproduce the results from 1999, and margin positivity
could no longer be substantiated as a significant prognostic factor
(17). Thereafter, the MSKCC
published its actualized data analyzing 495 patients, revealing no
statistical association between surgical margin status and RFS
(11); however, in the specific
subgroup analysis of tumors measuring <5 cm, R1 margins were
found to have an increased risk of local recurrence compared with
R0. In 2011, a European multicenter-based study including 426
patients was unable to determine any significant differences in RFS
when comparing patients with R0 and R1 margins (1).
The aforementioned findings have subsequently
subverted the role of surgical resection as an initial treatment
step, and have prompted the present review of our institutional
experience. The aim of the current study was to identify the
prognostic indicators of RFS in patients with primary aggressive
fibromatosis who underwent surgical resection. The analysis focused
particularly on the effect of surgical margins on disease
outcome.
Patient and methods
Patients
Between June 2000 and July 2014, 114 patients with
aggressive fibromatosis were treated surgically at BG-University
Hospital Bergmannsheil (Bochum, Germany). Of the 114 patients, 82
presented with primary disease in our institution, while 32
patients were subsequently referred to our center following
incomplete resection or the diagnosis of recurrence ≥3 months after
definitive surgery on the primary tumor performed at other
institutions. From this group of 114 patients, 17 patients were
excluded due to the unavailability of data regarding the surgical
margins of the initial surgical procedure. Furthermore, 7 patients
were lost to follow-up. Thus, the current analyses were restricted
to 90 participants with full information available on the surgical
margins at the initial procedure. The clinicopathological
characteristics of the patients are summarized in Tables I and II. Patient follow-up information was
obtained from our database and from patient correspondence. The
study was approved by the local ethics committee and all patients
provided their written informed consent.
 | Table I.Results of univariate analyses to
determine factors predictive of recurrence-free survival in 90
patients with aggressive fibromatosis. |
Table I.
Results of univariate analyses to
determine factors predictive of recurrence-free survival in 90
patients with aggressive fibromatosis.
|
|
|
| Estimated RFS rate, %
(95% CI) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Total no. of
patients | No. of patients with
recurrences | 1-year | 2-year | 5-year | P-value
(log-rank)a |
|---|
| Patient age,
years |
|
|
|
|
| 0.794 |
|
<50 | 61 | 31 | 78.5 (65.9–86.9) | 70.2 (56.9–80.1) | 54.5 (40.4–66.6) |
|
| ≥50 | 29 | 14 | 86.2 (67.3–94.6) | 57.7 (37.6–73.4) | 46.6 (27.6–63.6) |
|
| Gender |
|
|
|
|
| 0.315 |
| Male | 37 | 22 | 83.5 (67.0–92.2) | 72.2 (54.4–84.0) | 59.2 (40.7–73.7) |
|
|
Female | 53 | 23 | 79.2 (65.7–87.9) | 62.3 (47.8–73.8) | 47.8 (33.6–60.7) |
|
| Tumor site |
|
|
|
|
| 0.387 |
|
Extremity | 51 | 21 | 78.4 (64.4–87.4) | 58.5 (43.7–70.6) | 40.0 (25.9–53.8) | 0.074b |
| Abdominal
cavity | 14 | 10 | 85.7 (53.9–96.2) | 78.6 (47.2–92.5) | 78.6 (47.2–92.5) | 0.147c |
|
Head/neck | 7 | 3 | 57.1 (17.2–83.7) | 57.1 (17.2–83.7) | 57.1 (17.2–83.7) | 0.530d |
| Truncal
wall | 18 | 11 | 94.1 (65.0–99.1) | 82.4 (54.7–93.9) | 63.5 (35.9–81.8) | 0.241e |
| Tumor size, cm |
|
|
|
|
| 0.799 |
|
<5 | 26 | 15 | 84.3 (63.3–93.8) | 63.5 (41.5–79.1) | 59.0 (37.1–75.6) |
|
| ≥5 | 64 | 30 | 79.7 (67.6–87.7) | 67.2 (54.2–77.2) | 50.3 (37.2–62.0) |
|
| Previous history of
trauma at disease site |
|
|
|
|
| 0.296 |
| Yes | 15 | 5 | 80.0 (50.0–93.1) | 53.3 (26.3–74.4) | 45.7 (20.1–68.3) |
|
| No | 75 | 40 | 81.2 (70.3–88.4) | 68.9 (57.0–78.1) | 53.3 (40.8–64.3) |
|
 | Table II.Univariate analyses of recurrence-free
survival with respect to treatment characteristics. |
Table II.
Univariate analyses of recurrence-free
survival with respect to treatment characteristics.
|
|
|
| Estimated RFS rate, %
(95% CI) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Total no. of
patients | No. of patients
with recurrences | 1-year | 2-year | 5-year | P-value
(log-rank)a |
|---|
| Margin status after
primary resection |
|
|
|
|
|
|
| R0 | 50 | 34 | 87.9
(75.0–94.4) | 75.5
(60.8–85.3) | 68.8
(53.5–79.9) |
|
|
R1/2 | 40 | 11 | 72.5
(55.9–83.7) | 55.0
(38.5–68.8) | 34.1
(19.9–48.9) | 0.001b |
| R1 | 28 | 7 | 71.4
(50.9–84.6) | 46.4
(27.6–63.3) | 28.6
(13.5–45.6) |
<0.001c |
| R2 | 12 | 4 | 75.0
(40.8–91.2) | 75.0
(40.8–91.2) | 47.6
(18.2–72.4) | 0.341d |
| Distance of closest
negative surgical margin at resection of the primary tumor (R0
group), mm |
|
|
|
|
|
|
| ≤1 | 26 | 16 | 84.4
(63.7–93.9) | 76.2
(54.4–88.6) | 62.9
(40.5–78.8) | 0.301e |
|
>1 | 24 | 18 | 91.7
(70.6–97.8) | 75.0
(52.6–87.9) | 75.0
(52.6–87.9) |
|
| ≤5 | 44 | 31 | 88.5
(74.6–95.1) | 79.1
(63.6–88.5) | 71.5
(55.2–82.7) | 0.245f |
|
>5 | 6 | 3 | 83.3
(27.3–97.5) | 50.0
(11.1–80.4) | 50.0
(11.1–80.4) |
|
| Wound closure after
primary resection |
|
|
|
|
| 0.069 |
| Primary
closure | 69 | 31 | 78.1
(66.4–86.2) | 60.2
(47.6–70.7) | 47.0
(34.4–58.6) |
|
|
Non-primary closure (plastic
surgical tissue transfer) | 21 | 14 | 90.5
(67.0–97.5) | 85.7
(62.0–95.2) | 69.3
(43.6–85.1) |
|
| Adjuvant
radiotherapy |
|
|
|
|
| 0.861 |
|
Yes | 27 | 13 | 81.5
(61.1–91.8) | 73.9
(52.9–86.6) | 48.4
(27.8–66.3) |
|
| No | 63 | 32 | 80.8
(68.7–88.6) | 63.0
(49.8–73.7) | 54.4
(41.1–65.9) |
|
| Adjuvant NSAID
treatment |
|
|
|
|
| 0.080 |
|
Yes | 19 | 7 | 73.7
(47.9–88.1) | 47.4
(24.4–67.3) | 36.8
(16.5–57.5) |
|
| No | 71 | 38 | 83.0
(72.1–90.0) | 71.5
(59.4–80.6) | 56.9
(44.0–67.8) |
|
| Margin status after
last resection in patients with ≥1 recurrence |
|
|
|
|
| 0.269 |
| R0 | 25 | 11 | 87.6
(66.3–95.8) | 77.9
(54.5–90.2) | 77.9
(54.5–90.2) |
|
|
R1/R2 | 20 | 12 | 89.2
(63.1–97.2) | 77.6
(50.7–91.0) | 50.2
(24.0–71.6) |
|
Treatment
The goal of surgical treatment for all patients was
function-preserving and limb-sparing resection of the primary tumor
with clear margins. The indication for adjuvant treatment was
determined at the discretion of the interdisciplinary tumor board
of our institution or the referring institutions.
A total of 27 patients received adjuvant
radiotherapy following resection of the primary tumor, with a
median overall dose of 59.7 Gy (range, 50.0–66.0 Gy), and a further
19 patients underwent first adjuvant radiotherapy subsequent to an
initial recurrence, with a median overall dose of 53.7 Gy (range
50.0–64.0 Gy). Adjuvant non-steroidal anti-inflammatory drugs
(NSAIDs) were administered following primary tumor resection in 19
patients, and a further 7 patients received NSAIDs following the
initial recurrence (ibuprofen, 1,200–1,800 mg/day; or indomethacin,
150 mg/day. NSAIDs were given for a minimum of three months (range,
3–14 months). Two patients received tamoxifen following primary
resection. Additionally, 4 patients were treated with imatinib and
1 patient with epirubicin.
Histopathological classification
All pathology slides were analyzed or reviewed for
consensus diagnosis by experienced soft tissue pathologists.
Statistical analysis
All patients were retrospectively analyzed with
regard to potential prognostic factors affecting RFS (Table I). RFS was defined as the period of
time from the date of surgery for primary disease to the date of
first recurrence. Survival rates were estimated according to the
Kaplan-Meier method with respective 95% confidence intervals (CIs),
and were compared using the log-rank test. Multivariate analyses
were performed using the Cox proportional hazards model. Variables
that were associated with P<0.10 in the univariate analysis were
included in the multivariate regression to assess independent
prognostic factors for RFS. P<0.05 was considered to indicate a
statistically significant result. All analyses were performed using
Stata software (Version 11.2; StataCorp, College Station, TX,
USA).
Analysis of surgical margins
In order to determine the impact of surgical
resection margins on RFS, the three following variables were
analyzed. In ‘margin status after primary resection’ (Table II), RFS was assessed with regard to
the resection status that was achieved following the resection of
the primary tumor in our or the referring institution. In those
patients with negative margins (R0 group) after primary resection,
the effect of the clear surgical margin width was assessed as
‘distance of closest negative surgical margin at resection of the
primary tumor (R0 group)’ (Table
II). The variable ‘margin status after last resection in
patients with ≥1 recurrence’ (Table
II) concerned the prognostic influence of the surgical margin
status that was attained at the final resection of the recurring
tumor in patients who developed ≥1 recurrence following the
resection of the primary tumor.
Results
Patient characteristics and surgical
margins
The median age at the time of initial recurrence was
38.7 years (range, 16.1–74.2 years). The patient group included in
the analysis consisted of 37 males (41.1%) and 53 females (58.9%).
Tumors were located in the lower extremities in 30 patients
(33.3%), in the upper extremities in 21 patients (23.3%), in the
intra-abdominal cavity in 14 patients (15.6%), in the head and neck
area in 7 patients (7.8%), and in the superficial trunk in 18
patients (20.0%). During follow-up, 45 patients (50%) developed ≥1
recurrence, whereas 23 patients (25.6%) had ≥2 local recurrences
(range, 2–5 recurrences). Time-to-recurrence ranged from 3 months
to 14 years (median, 17 months). No patient exhibited multifocal
disease. Mortality occurred in 1 (female) patient with 5
recurrences and macroscopic residual disease subsequent to the last
resection, following infiltration of the internal carotid artery at
6.1 years after the primary diagnosis. Only 2 patients had Gardner
syndrome.
Plastic surgical tissue transfer was necessary in 21
patients following the resection of the primary tumor;
specifically, 19 patients with soft tissue defects received local
flaps, while 2 patients with mere skin defects were transplanted
with split-thickness skin grafts. The R0 rates were 52.2% (36/69)
for patients with primary closures and 66.7% (14/21) for patients
who underwent plastic surgical tissue transfer.
Follow-up and survival
As of August 2014 (cut-off date), the reverse
Kaplan-Meier estimate of median follow-up time following primary
resection was 7.7 years (95% CI, 5.6–8.1 years) (18). The Kaplan-Meier-estimated rates of RFS
for the entire group were 52.2% (95% CI, 40.9–62.3) at 5 years and
42.7% (95% CI, 28.8–55.9) at 10 years.
Univariate analysis of survival
In the entire series, patient age and gender were
not found to be significant predictors of RFS (Table I). Similar to findings in previous
studies (1,17), tumors arising in the extremities
appeared to have a poorer prognosis compared with lesions at other
sites [5-year RFS rates, 40.0% (95% CI, 25.9–53.8%) vs. 68.0% (95%
CI, 50.4–80.4%), respectively]; however, this survival distribution
failed to reach statistical significance in the univariate analysis
(P=0.074). In contrast to the results of previous studies, tumor
size did not exhibit any effect on RFS in the present series.
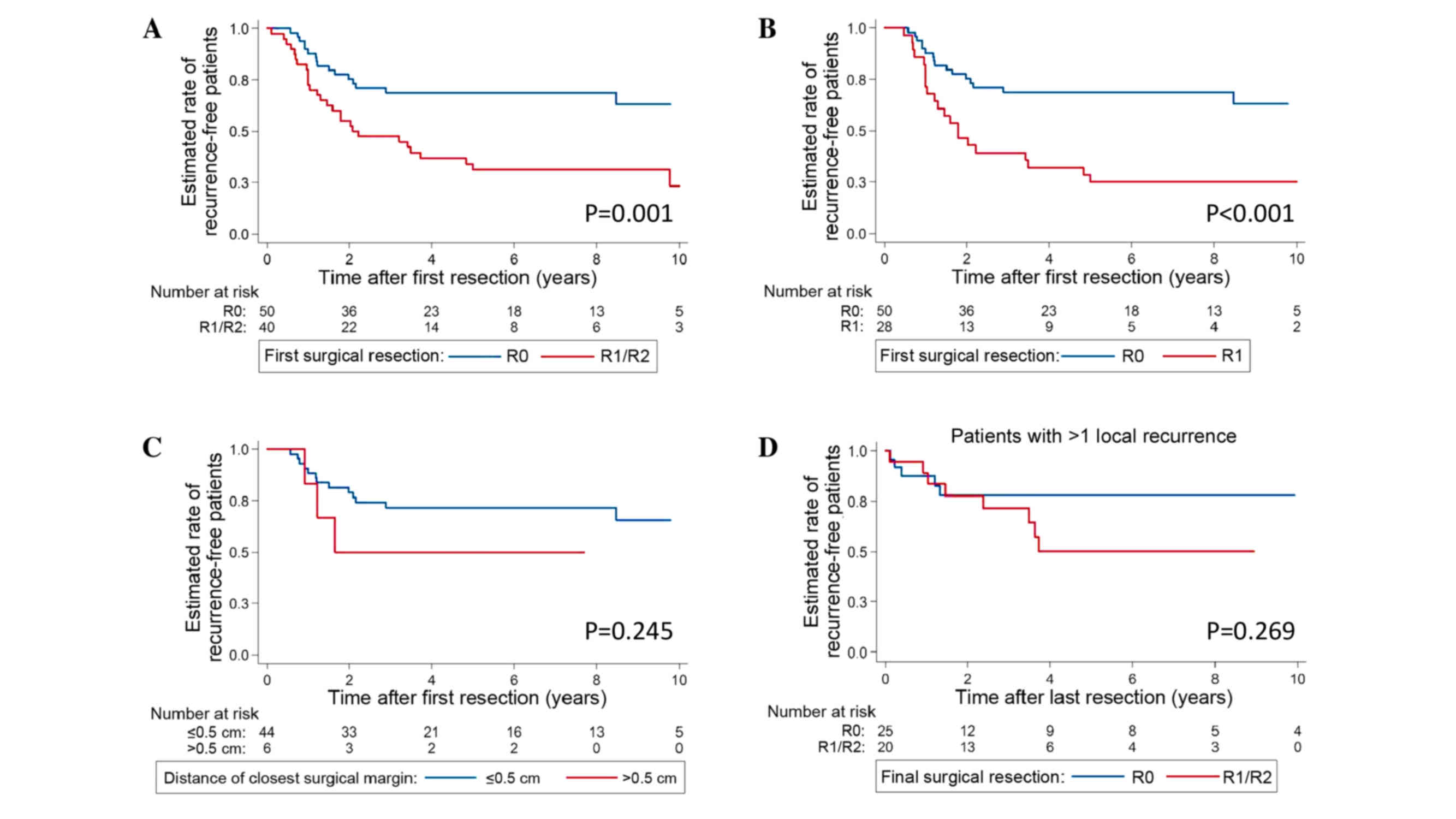
Univariate analysis identified only the surgical
margin status attained at the resection of the primary tumor as a
significant predictor of outcome. Patients who underwent complete
R0 resection of their primary tumor had a significantly improved
outcome (5-year RFS rate, 68.8%; 95% CI, 53.5–79.9%) when compared
with patients in whom incomplete R1 or R2 resection was achieved
(5-year RFS rate, 34.1%; 95% CI, 19.9–48.9%; P=0.001 vs. R0)
(Table II; Fig. 1A). Furthermore, R0 status was
associated with a more favorable RFS rate when compared only with
R1 status (5-year RFS rate, 28.6%; 95% CI, 13.5–45.6%; P<0.001
vs. R0) (Fig. 1B). R1 and R2 status
had comparably diminished RFS rates (P=0.341). Notably, surgical
margin width did not influence the RFS rates in patients who
underwent an R0 resection of their primary tumor (≤1 vs. >1 mm,
P=0.301; and ≤5 vs. >5 mm, P=0.245)] (Table II; Fig.
1C).
However, surgical margins exhibited prognostic
significance at the resection of the primary tumor only; patients
who developed ≥1 recurrence did not gain a survival benefit from an
R0 resection of the recurring tumor (5-year RFS rate, 77.9%; 95%
CI, 54.5–90.2%) compared with an R1/2 resection of the recurring
tumor (5-year RFS rate, 50.2%; 95% CI, 24.0–71.6%; P=0.269 vs. R0)
(Table II; Fig. 1D).
Regarding adjuvant treatment modalities, radiation
treatment did not result in an improved outcome compared with no
radiation treatment [5-year RFS rates, 48.4% (95% CI, 27.8–66.3%)
vs. 54.4% (95% CI, 41.1–65.9%), respectively; P=0.861). Adjuvant
treatment with NSAIDs was associated with a marginally diminished
RFS rate when compared with untreated patients [5-year RFS rates,
36.8% (95% CI, 16.5–57.5%) vs. 56.9% (95% CI, 44.0–67.8%),
respectively; P=0.080] (Table
II).
Multivariate analysis of survival
The only significant prognostic factor for RFS
according to the Cox model was the margin status attained at the
primary resection (Table III); the
hazard ratio for recurrence was 2.73 (95% CI, 1.52–4.91; P=0.001)
for patients with positive margins (R1/R2) vs. R0-resected
patients. All other variables failed to reach statistical
significance in the multivariate analysis.
 | Table III.Results of multivariate analysis on
recurrence-free survival according to Cox proportional hazards
model. |
Table III.
Results of multivariate analysis on
recurrence-free survival according to Cox proportional hazards
model.
| Category
(reference) | Hazard ratio | 95% CI | P-value |
|---|
| Margin status after
primary resection: R1/R2 (vs. R0) | 2.73 | 1.52–4.91 | 0.001 |
| Tumor site:
Extremity (vs. non-extremity) | 1.66 | 0.83–3.32 | 0.153 |
| Wound closure at
primary resection: Primary (vs. non-primary) | 1.38 | 0.64–2.96 | 0.411 |
| Adjuvant NSAID
treatment: Yes (vs. no) | 1.93 | 0.88–4.23 | 0.101 |
Discussion
In the present study, the surgical margin status
attained at the resection of the primary tumor was the only factor
that exhibited prognostic significance in the analysis of RFS;
patients with R0 margins after primary resection had a
significantly improved RFS rate compared with patients who
underwent R1 or R2 resections. Notably, narrow and wide negative
margins had similar outcomes within the R0-resected subset,
supporting a surgical approach aiming to achieve more conservative
resections, rather than radical and wide excisions. In the entirety
of the present series of patients, 6 out of 50 patients within the
R0 subgroup underwent resections with margins of >5 mm of
healthy tissue, reflecting a less radical treatment policy. In the
cases with positive margins, tumors had infiltrated critical
anatomical structures, such as large nerves of the extremities, or
were too advanced and widespread for complete resection, which
would have resulted in functional loss and increased morbidity.
Taken together, these findings suggest that a less radical surgical
approach with function-sparing resections should be employed when
feasible, without leaving microscopic or macroscopic positive
margins. However, surgical margins did not influence RFS in
patients in whom tumors had recurred.
In contrast to the current findings, none of the
large retrospective studies previously conducted by the MDACC,
MSKCC, INT and the French Sarcoma Group were able to determine a
predictive role of positive surgical margins (1,8,10,17). A
mere descriptive comparison of the four studies mentioned with the
present study does not allow a further explanation for these
contrasting results. Notably, a significant difference regarding
the RFS rates obtained between the different studies can be
detected: The overall 5-year RFS rates for patients treated
surgically in the MDACC (80%) and in the INT (76%) were markedly
higher compared with that in the present study, which reported a
5-year RFS rate of only 52.2% (95% CI, 40.9–62.3%) for the entire
series. As three out of the four centers pooled the RFS rates of R0
and R1 patients, only the MDACC data can be compared; the MDACC
study reported a 5-year RFS rate of 75% for R1/R2-resected
patients, which is markedly higher than that in the current series,
which reported a rate of 34.1% (95% CI, 19.9–48.9%) for the
corresponding patient group. This observation leads to the question
of why patients with positive margins had such poorer outcomes in
the current series compared with other studies.
A potential reason for this may be related to the
adjuvant treatments administered to patients with positive margins.
Nevertheless, adjuvant treatment modalities were not less intense
in the current patient population: From the 45 patients with
positive margins at our center, 23 (51.2%) received adjuvant
treatment (15 radiation only, 2 NSAIDs only, 2 tamoxifen only, and
4 combined treatment). The frequency of adjuvant treatment was
similar in the MDACC series, in which 36 (52.9%) out of 68 patients
with positive margins received adjuvant therapy.
A final potential explanation for the low RFS rates
in the present study may be found in the time point of recurrence
detection. It must be noted that patients at our institution are
intensely followed-up with contrast-enhanced magnetic resonance
imaging assessments every 3 months in the first 2 years, and then
every 6 months for ≥3 further years, enabling the detection of
recurrences relatively rapidly and prior to the development of
symptoms. However, we are unable to determine the true reason for
these marked outcome differences between the studies.
In conclusion, the data from the present study
suggest an improved outcome for patients with completely resected
primary tumors. Tumor biology may dictate the outcome; however,
given the diminished outcome of patients retaining positive
margins, surgical efforts must aim for function-sparing resections
with negative margins wherever feasible. In this context, close
negative margins, even those <1 mm, appear to be adequate.
However, it cannot be retrospectively concluded whether the R0
resection itself or the characteristic of ‘R0 resectability’ at the
initial surgical procedure leads to the improved outcome; it is
probable that tumors that cannot be completely resected have more
aggressive biological features than completely resectable tumors,
thus impairing the outcome more substantially. Subsequently, a
positive margin status could be a result, rather than a cause, of
biological aggressiveness, and it may not itself influence the
outcome directly.
Finally, the time point of surgical resection must
be addressed. As proposed by the European Organisation for Research
and Treatment of Cancer (EORTC) in 2015, a wait-and-see strategy
for ~1 or 2 years appears to be reasonable for patients with
asymptomatic primary tumors at non-critical sites as a frontline
approach, and can prevent unnecessary resections that may result in
lifelong morbidity (19,20). Currently, a prospective observational
study (NCT01801176) by the Institut Gustave Roussy is underway to
assess the outcome of different treatment arms formulating the role
of the wait-and-see policy in more detail. To date, the EORTC
recommends a surgical resection in cases of progression if the
expected postoperative functional impairment is limited. However,
as this can be highly subjective, the postoperative consequences
must be clearly discussed with each patient before decisions are
made.
Acknowledgements
The current study was supported by a FoRUM grant
(grant no. K090-15) from Ruhr-University Bochum (Bochum,
Germany).
References
|
1
|
Salas S, Dufresne A, Bui B, Blay JY,
Terrier P, Ranchere-Vince D, Bonvalot S, Stoeckle E, Guillou L, Le
Cesne A, et al: Prognostic factors influencing progression-free
survival determined from a series of sporadic desmoid tumors: A
wait-and-see policy according to tumor presentation. J Clin Oncol.
29:3553–3558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Camargo VP, Keohan ML, D'Adamo DR,
Antonescu CR, Brennan MF, Singer S, Ahn LS and Maki RG: Clinical
outcomes of systemic therapy for patients with deep fibromatosis
(desmoid tumor). Cancer. 116:2258–2265. 2010.PubMed/NCBI
|
|
3
|
Dômont J, Salas S, Lacroix L, Brouste V,
Saulnier P, Terrier P, Ranchère D, Neuville A, Leroux A, Guillou L,
et al: High frequency of beta-catenin heterozygous mutations in
extra-abdominal fibromatosis: A potential molecular tool for
disease management. Br J Cancer. 102:1032–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mullen JT, DeLaney TF, Rosenberg AE, Le L,
Iafrate AJ, Kobayashi W, Szymonifka J, Yeap BY, Chen YL, Harmon DC,
et al: β-Catenin mutation status and outcomes in sporadic desmoid
tumors. Oncologist. 18:1043–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazar AJ, Tuvin D, Hajibashi S, Habeeb S,
Bolshakov S, Mayordomo-Aranda E, Warneke CL, Lopez-Terrada D,
Pollock RE and Lev D: Specific mutations in the beta-catenin gene
(CTNNB1) correlate with local recurrence in sporadic desmoid
tumors. Am J Pathol. 173:1518–1527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spear MA, Jennings LC, Mankin HJ, Spiro
IJ, Springfield DS, Gebhardt MC, Rosenberg AE, Efird JT and Suit
HD: Individualizing management of aggressive fibromatoses. Int J
Radiat Oncol Biol Phys. 40:637–645. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ballo MT, Zagars GK, Pollack A, Pisters PW
and Pollack RA: Desmoid tumor: Prognostic factors and outcome after
surgery, radiation therapy, or combined surgery and radiation
therapy. J Clin Oncol. 17:158–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Merchant NB, Lewis JJ, Woodruff JM, Leung
DH and Brennan MF: Extremity and trunk desmoid tumors: A
multifactorial analysis of outcome. Cancer. 86:2045–2052. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis JJ, Boland PJ, Leung DH, Woodruff JM
and Brennan MF: The enigma of desmoid tumors. Ann Surg.
229:866–873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gronchi A, Casali PG, Mariani L, Lo Vullo
S, Colecchia M, Lozza L, Bertulli R, Fiore M, Olmi P, Santinami M
and Rosai J: Quality of surgery and outcome in extra-abdominal
aggressive fibromatosis: A series of patients surgically treated at
a single institution. J Clin Oncol. 21:1390–1397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crago AM, Denton B, Salas S, Dufresne A,
Mezhir JJ, Hameed M, Gonen M, Singer S and Brennan MF: A prognostic
nomogram for prediction of recurrence in desmoid fibromatosis. Ann
Surg. 258:347–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colombo C, Miceli R, Le Péchoux C,
Palassini E, Honoré C, Stacchiotti S, Mir O, Casali PG, Dômont J,
Fiore M, et al: Sporadic extra abdominal wall desmoid-type
fibromatosis: Surgical resection can be safely limited to a
minority of patients. Eur J Cancer. 51:186–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonvalot S, Ternès N, Fiore M, Bitsakou G,
Colombo C, Honoré C, Marrari A, Le Cesne A, Perrone F, Dunant A and
Gronchi A: Spontaneous regression of primary abdominal wall desmoid
tumors: More common than previously thought. Ann Surg Oncol.
20:4096–4102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Briand S, Barbier O, Biau D,
Bertrand-Vasseur A, Larousserie F, Anract P and Gouin F:
Wait-and-see policy as a first-line management for extra-abdominal
desmoid tumors. J Bone Joint Surg Am. 96:631–638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eastley N, Aujla R, Silk R, Richards CJ,
McCulloch TA, Esler CP and Ashford RU: Extra-abdominal desmoid
fibromatosis-a sarcoma unit review of practice, long term
recurrence rates and survival. Eur J Surg Oncol. 40:1125–1130.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin SH, Ko KR, Cho SK, Choi YL and Seo
SW: Surgical outcome of desmoid tumors: Adjuvant radiotherapy
delayed the recurrence, but did not affect long-term outcomes. J
Surg Oncol. 108:28–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lev D, Kotilingam D, Wei C, Ballo MT,
Zagars GK, Pisters PW, Lazar AA, Patel SR, Benjamin RS and Pollock
RE: Optimizing treatment of desmoid tumors. J Clin Oncol.
25:1785–1791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gronchi A, Colombo C, Le Péchoux C, Dei
Tos AP, Le Cesne A, Marrari A, Penel N, Grignani G, Blay JY, Casali
PG, et al: Sporadic desmoid-type fibromatosis: A stepwise approach
to a non-metastasising neoplasm-a position paper from the Italian
and the French Sarcoma Group. Ann Oncol. 25:578–583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasper B, Baumgarten C, Bonvalot S, Haas
R, Haller F, Hohenberger P, Moreau G, van der Graaf WT and Gronchi
A: Desmoid Working Group: Management of sporadic desmoid-type
fibromatosis: A European consensus approach based on patients' and
professionals' expertise-a sarcoma patients EuroNet and European
organisation for research and treatment of cancer/Soft tissue and
bone sarcoma group initiative. Eur J Cancer. 51:127–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|