Introduction
Lung cancer is one of the leading causes of cancer
mortality in the United States (1).
Early diagnosis of lung cancer is essential; if left untreated the
cancer is able to spread and affect nearby organs (2). One of the main causes of lung cancer is
the prolonged intake of tobacco smoke (3). Lung cancer can be difficult to diagnose
at earlier stages, and a quarter of people exhibit no symptoms even
following diagnosis (4). The markers
associated with cancer are of great interest to clinicians and
enable an improved understanding of tumor biology (5). At present there is no universal marker
available to detect a particular type of cancer and using markers
for cancer detection has a number of limitations. The expression of
cancer markers varies significantly between individuals and false
positives are likely to occur (6).
Cancer markers are used to diagnose the stage of
disease, assess the response to treatment and predict the
prognosis; identifying further markers may be beneficial. The
markers associated with lung cancer stem cells are of great
interest for the understanding of disease progression prior and
subsequent to treatment. Performing an analysis of lung cancer stem
cells is an accurate method to determine treatment relapse, as
cancer stem cells are not easily eliminated during therapy
(7,8).
Molecular identification of such lung cancer stem cells may be more
valuable in clinical practice as the 5-year patient survival rate
for lung cancer is <15% (9).
Currently, although no universal cancer stem cell markers are
available to identify individual cancer types, a number of cancer
stem cells including prostate, brain and colon are identified using
prominin-1 (10). The normal lung
stem cells assist in the maintenance of homeostasis upon injury
(11) and transform into cancer stem
cells following mutation (7).
The markers used for identifying lung cancer stem
cells require analysis under various pathological conditions and
validation prior and subsequent to normal treatment procedures. The
present study focused on these factors to validate the
octamer-binding protein 4 (Oct-4) marker in a mouse model.
Materials and methods
Animal experiment
The use of experimental animals throughout the
present study was approved by the Institutional Review Board at the
Guangzhou Medical University (Guangzhou, Guangdong). A total of 60
A/J mice (female, 10 weeks) were randomly chosen for the study, and
the mice were divided equally into three groups. The mice were
carefully observed for one week in laboratory conditions. The mice
were provided with readily available water and food sources. The
animals were subjected to regular observations twice a day.
Lung cancer was induced in the mice by exposing the
animals to tobacco smoke with a high nicotine content, as
previously described by Witschi et al (12). The exposed mice were housed in an
animal chamber at 25±1°C and humidity 50±5% on a 12 h light-dark
cycle. The mice were exposed to tobacco smoke carcinogen for 7 h on
alternative days. The dosage was continued for a period of 4 months
for development of an initial tumor, and mice were similarly
exposed for 6 months to develop advanced lung cancer. All mice were
given a recovery period of 2 weeks following exposure. The mice
were subsequently sacrificed by decapitation for analysis of tumors
in the lungs. For treatment, the mice that developed initial and
advanced tumors were treated daily with 15 mg/kg salirasib
(Concordia International Corp., Oakville, ON, Canada), which was
administered following tumor growth for 1 and 2 months,
respectively, via intraperitoneal injection. Control animals were
kept in filtered air conditions, without exposure to tobacco
smoke.
Immunohistochemistry
The tissue sections were initially fixed in 10%
formalin solution at 42°C for 2 days and paraffin embedded. The
tissue sections were subsequently subjected to microtome sectioning
(5 µm). The sections were placed on glass slides, de-paraffinized
and rehydrated. The endogenous peroxidase activity was blocked by
immersing the sections in freshly prepared 10%
H2O2 and 10% methanol in 1X
phosphate-buffered saline (PBS) for 20 min. The sections underwent
trypsin treatment (0.1% trypsin in 0.1% CaCl2) for 10
min to cleave the protein crosslinks to assess the antigen and
epitope. Nonspecific antigens were blocked using 4% bovine serum
albumin (BSA; Sigma-Aldrich; Merck KgaA, Darmstadt, Germany) for 2
h at room temperature. The membranes were incubated with an
anti-Oct-4 primary antibody (dilution, 1:100; cat no. ab18976;
Abcam, Cambridge, UK) overnight at 4°C. Following incubation, the
sections were thoroughly washed with 1X PBS and incubated with a
goat anti-rabbit secondary antibody (dilution, 1:3,000; cat no.
ab6721; Abcam) for 1 h at room temperature. Following washing to
prevent non-specific binding, the sections were stained with
diaminobenzidine (DAB; cat no. ab64238; Abcam).
Western blot analysis
Tissue samples from mice without lung cancer,
initial and advanced stages of the disease were dissected and
protein samples were prepared from the cell lysate. Similarly,
tissue samples following treatment with salirasib from mice without
lung cancer, initial and advanced stages of the disease were taken.
Proteins were extracted using 2X SDS sample buffer, and quantified
using the Lowry method. The extracted proteins (70 µg/lane) were
resolved on a 12% SDS-PAGE gel, as previously described (13). The protein in the gel was subsequently
transferred onto polyvinylidene difluoride membranes. Following
blocking with 5% BSA overnight at 4°C, the membranes were incubated
with the previously described anti-Oct-4 antibody (dilution, 1:400)
and a lamin B1 antibody (cat no. ab16048; dilution, 1:500; Abcam)
overnight at 4°C. The membranes were subsequently incubated with
the previously described secondary antibody (dilution, 1:3,000) for
1 h at room temperature. Following washing, the membranes were
developed with DAB and imaged and quantified using a Sigma-Aldrich
microDOC gel documentation system (cat no. Z692557; Merck
KGaA).
Results
Mice with initial and advanced stages
of lung cancer
A total of three groups of mice, each with 20 mice,
were selected. The first group served as a control, whilst the
second group exhibited the initial stages of lung cancer and the
third exhibited advanced lung cancer following exposure to tobacco
smoke. The three groups of mice were sacrificed following exposure
to tobacco smoke and a recovery period. The lung tissues samples
were dissected and subjected to histological sectioning. Clear
histological differences were observed between tissue sections of
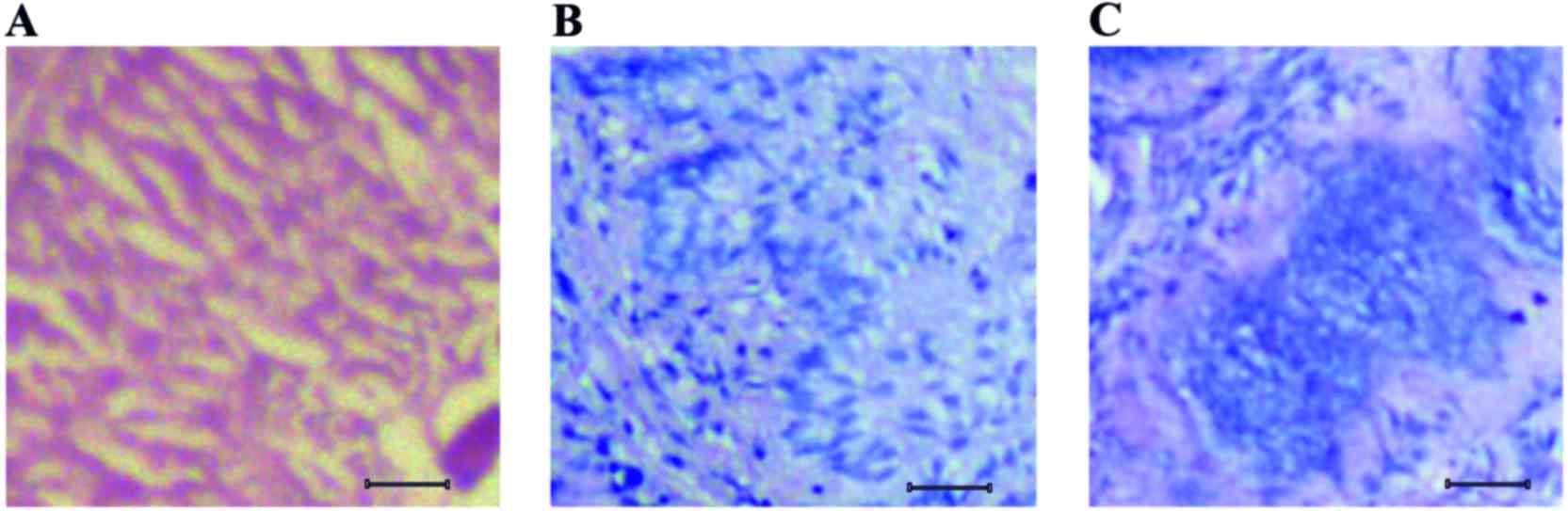
normal, initial and advanced stages of lung cancer, as shown in
Fig. 1. The section of normal lung
tissue indicated tissue layers with uniform arrangement of cells
(Fig. 1A). The tissue sections from
mice that were exposed to tobacco smoke carcinogen for 4 months
exhibited actively dividing enlarged cells that were dispersed
throughout the tissue layer (Fig.
1B). Notably, the tissue sections of mice exposed to tobacco
smoke carcinogen for 6 months exhibited advanced forms of tumor,
with aggregates of cells visible around the center of the tissue
(Fig. 1C).
Expression of Oct-4 as a cancer stem
cell marker
Although the initial stages of lung cancer following
treatment demonstrated marked improvement, it was more difficult to
observe changes in the samples with advanced lung cancer.
Therefore, a series of experiments was designed to evaluate the
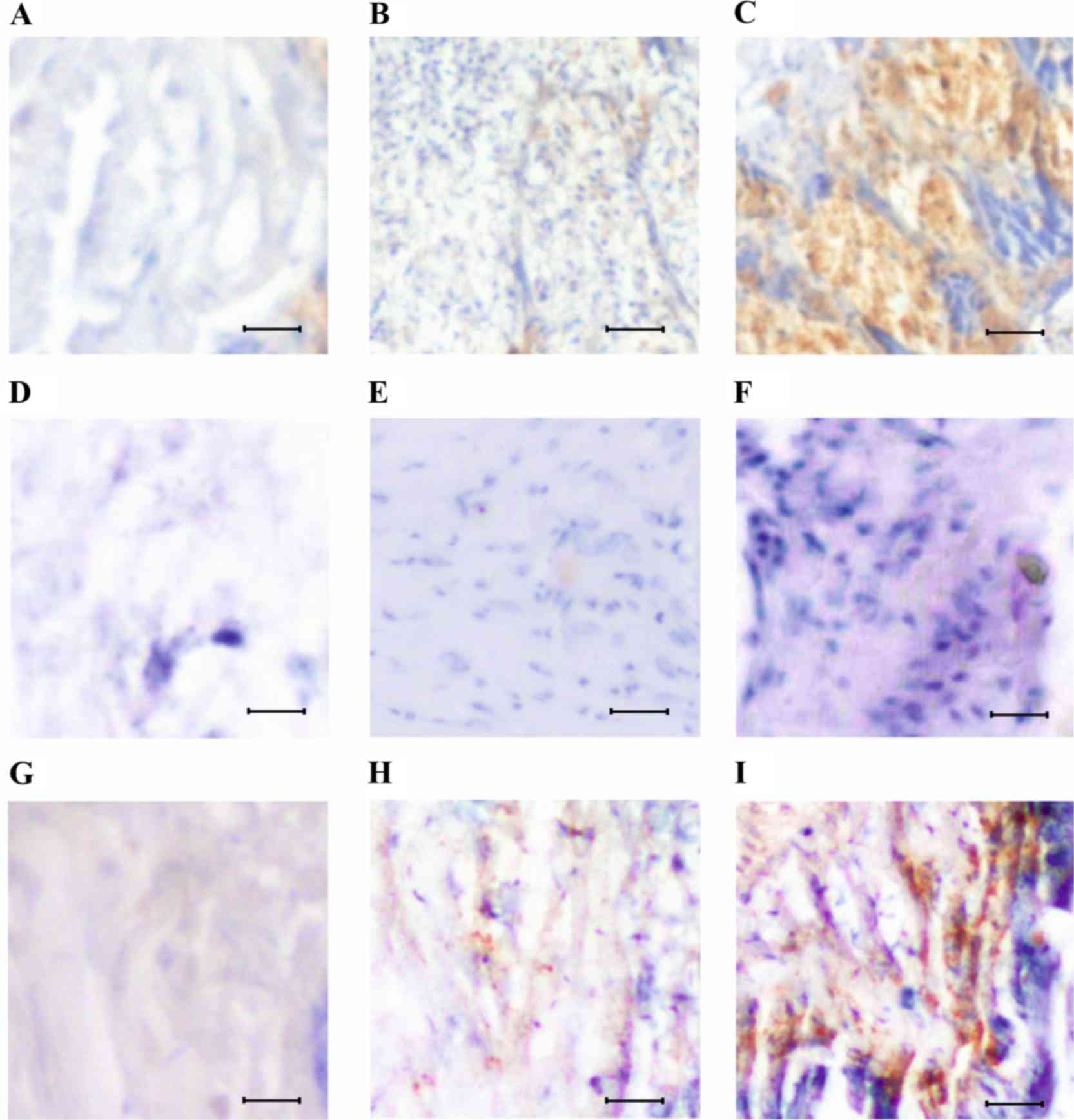
changes. Using immunohistochemical techniques, the expression of
Oct-4 was analyzed to validate the pattern of cancer stem cells in
normal, initial and advanced stages of lung cancer (Fig. 2). Low Oct-4 expression signals were
observed in the normal lung tissue sections, with a number of
Oct-4-positive cells (Fig. 2A, D and
G). The tissues sections with initial stages of lung tumor
exhibited increased Oct-4 expression, and Oct-4-positive cells were
scattered throughout the tissue layers (Fig. 2B). In the case of the tissue sections
with advanced tumors, overexpression of Oct-4 with a large number
of Oct-4-positive cells that formed aggregates was observed
(Fig. 2C).
Oct-4 as a marker to assess cancer
stem cells prior and subsequent to treatment
In order to study the pathology of lung cancer,
cancer stem cells were analysed following treatment with salirasib
for 1 month. Mice that were treated early with salirasib, following
1 month of tumor growth, exhibited reduced expression of Oct-4
(Fig. 2E and F). However, mice
treated following 2 months of tumor growth displayed increased
Oct-4 expression in initial (Fig. 2H)
and advanced stages of lung cancer (Fig.
2I). The possibility of eliminating cancer stem cells using
salirasib treatment was low in advanced stages of lung cancer.
Analyzing immunohistochemical results
using western blotting
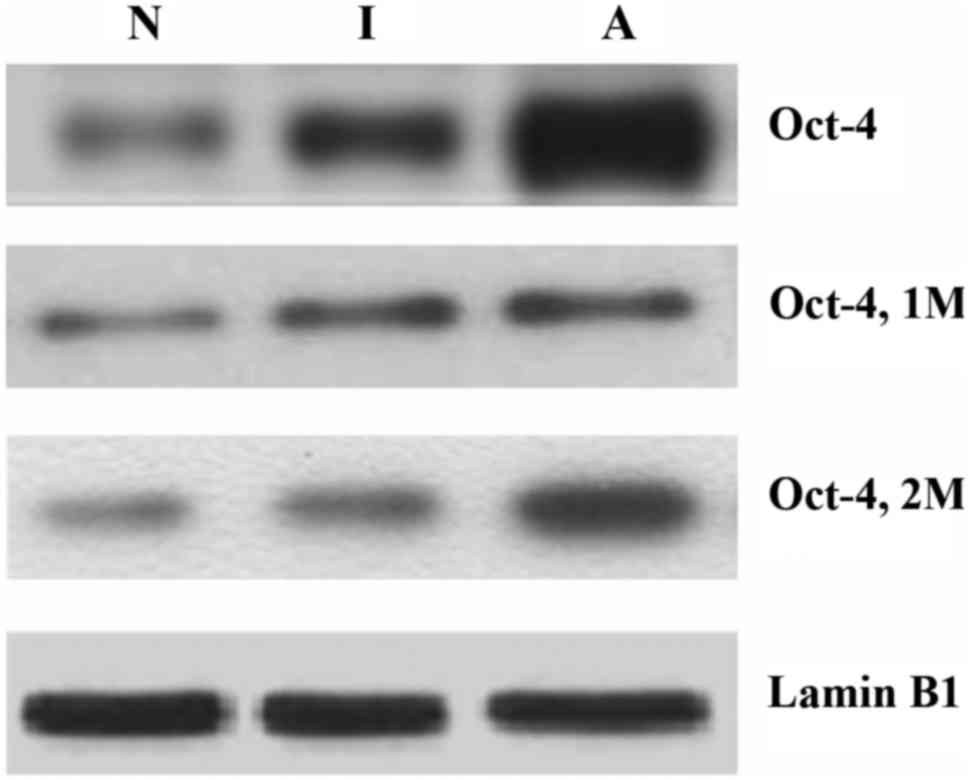
Western blot analysis further confirmed the results
from immunohistochemical staining as shown in Fig. 3. Similar to the immunohistochemical
data, western blotting revealed increased expression of Oct-4 in
advanced lung tumors and slightly reduced expression of Oct-4
following delayed salirasib treatment when compared with initially
treated tissue samples.
Discussion
The persistence of cancer stem cells following
treatment is a notable observation in studying tumor recurrence.
Cancer stem cells exhibit altered proliferation and differentiation
abilities when compared with normal stem cells (14,15).
Delaying treatment results in the emergence of advanced lung
cancer, which is difficult to control and is associated with poor
survival rates with minimal improvement (16).
Chemotherapy and radiation therapy are able to
rapidly eliminate proliferative cells, but they are less effective
at decreasing the number of cancer stem cells (17,18).
Following treatment, the quiescent chemoresistant cancer stem cells
proliferate in an aggressive fashion, and this complicates
treatment (19). The characterization
and identification of cancer stem cells can aid in the correct
assessment of disease condition and help to develop novel efficient
therapeutics against aggressive forms of cancer (10).
In the present study, initial and advanced stages of
lung cancer were successfully induced in mice by exposing the
animals to tobacco smoke and altering the exposure time. The tumor
at initial stages consisted of actively dividing cells, whereas at
advanced tumor stages the actively dividing cells proliferated in
an unlimited manner and aggregated. The cell aggregates in advanced
stages of cancer is a sign of metastatic development (20). Understanding the underlying molecular
mechanisms that regulate cancer stem cell proliferation and the
mechanisms involved in the transformation of normal stem cells into
cancer stem cells is key in developing novel effective drugs.
From the immunohistochemical data in the present
study, it was concluded that salirasib failed to have any
observable effect in the delayed treatment group. However,
salirasib may have had an effect in the early treatment group by
markedly eliminating cancer stem cells. The immunohistochemical
results were further validated by western blotting.
Overall, in the present study initial and advanced
stages of lung cancer were successfully induced using tobacco smoke
carcinogen. Lung tissue sections of initial and advanced stages of
lung cancer were differentiated using histological procedures. The
number of cancer stem cells was more likely to be markedly reduced
when treatment was administered early, (1 month following tumor
development), whereas the number of cancer stem cells was not
easily reduced when treatment was delayed (2 months following tumor
treatment).
References
|
1
|
Larsen JE and Minna JD: Molecular biology
of lung cancer: Clinical implications. Clinics Chest Med.
32:703–740. 2011. View Article : Google Scholar
|
|
2
|
Keshamouni V, Arenberg D and Kalemkerian
G: Lung Cancer Metastasis. Springer; New York, NY: 2009
|
|
3
|
Thun MJ, Hannan LM, Adams-Campbell LL,
Boffetta P, Buring JE, Feskanich D, Flanders WD, Jee SH, Katanoda
K, Kolonel LN, et al: Lung cancer occurrence in never-smokers: An
analysis of 13 cohorts and 22 cancer registry studies. PLoS Med.
5:e1852008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beckles MA, Spiro SG, Colice GL and Rudd
RM: Initial evaluation of the patient with lung cancer: Symptoms,
signs, laboratory tests, and paraneoplastic syndromes. Chest. 123
(1 Suppl):97S–104S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mumbarkar PP, Raste AS and Ghadge MS:
Significance of tumor markers in lung cancer. Indian J Clin
Biochem. 21:173–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thunnissen FB: Sputum examination for
early detection of lung cancer. J Clin Pathol. 56:805–810. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giangreco A, Groot KR and Janes SM: Lung
cancer and lung stem cells: Strange bedfellows? Am J Respir Crit
Care Med. 175:547–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witschi H, Espiritu I, Maronpot RR,
Pinkerton KE and Jones AD: The carcinogenic potential of the gas
phase of environmental tobacco smoke. Carcinogenesis. 18:2035–2042.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamidouche Z, Haÿ E, Vaudin P, Charbord P,
Schüle R, Marie PJ and Fromigué O: FHL2 mediates
dexamethasone-induced mesenchymal cell differentiation into
osteoblasts by activating Wnt/beta-catenin signaling-dependent
Runx2 expression. FASEB J. 22:3813–3822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heppner GH and Miller BE: Tumor
heterogeneity: Biological implications and therapeutic
consequences. Cancer Metastasis Rev. 2:5–23. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: An old idea - a paradigm shift. Cancer Res. 66:1883–1890.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan G, Ward R and Barton M: The
contribution of cytotoxic chemotherapy to 5-year survival in adult
malignancies. Clin Oncol (R Coll Radiol). 16:549–560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmidt P, Kopecky C, Hombach A, Zigrino
P, Mauch C and Abken H: Eradication of melanomas by targeted
elimination of a minor subset of tumor cells. Proc Natl Acad Sci
USA. 108:2474–2479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liotta LA, Kleinerman J and Saldel GM: The
significance of hematogenous tumor cell clumps in the metastatic
process. Cancer Res. 36:889–894. 1976.PubMed/NCBI
|