Introduction
Colorectal cancer (CRC) is the third leading cause
of morbidity and the fourth cause of mortality in all types of
cancer globally, with ~1.2 million new cases and ~600,000
mortalities recorded annually (1).
The rate of incidence of CRC has stabilized in the United States
and numerous other western countries; however, it is increasing
rapidly in economically transitioning areas, including eastern
European countries and most parts of Asia (2,3). The
increased incidence rate of CRC in economically transitioning areas
is thought to be due a shift towards western dietary and lifestyle
factors, including a diet rich in unsaturated fats and red meat,
increased total energy intake, excessive alcohol consumption and
reduced physical activity (4). At
present, the majority of patients with early (stage I and II) CRC
are treated by surgery to remove the cancer, while patients with
late (stage III and IV) CRC often undergo chemotherapy (5), and the toxic effect of chemotherapy
remains a challenge (6). Therefore,
it is important to clarify the molecular mechanisms underlying the
development and progression of CRC in order to develop novel drugs,
which may increase the survival rate for patients with CRC.
There are numerous pathways involved in the
pathogenesis of CRC, including Wnt/β-catenin, p53, transforming
growth factor b, mitogen activated protein kinase, phosphoinositide
3-kinase, epidermal growth factor receptor, Notch,
RhoA/rho-associated protein kinase and toll-like receptor (7,8).
Hyperactivation of the Wnt/β-catenin signaling pathway is
considered to be the most important driving force behind CRC
(9). Any genetic defects occurring to
members of Wnt/β-catenin pathway, including LDL receptor related
protein 5/6, Axin, dishevelled (Dvl), adenomatous polyposis coli
(APC), glycogen synthase kinase 3 β and β-catenin, will result in
the nuclear accumulation of β-catenin. β-catenin forms complexes
with the transcription cofactor T-cell factor (TCF)/lymphoid
enhancer factor (LEF) to promote the expression of its target genes
(c-Myc, cyclin D1 and survivin) which promotes the initiation and
development of CRC (10).
Resistance to apoptosis, caused by an imbalance of
pro-apoptotic members including B-cell lymphoma 2 (Bcl-2), B-cell
lymphoma-extra large (Bcl-XL), myeloid cell leukemia
sequence 1 and the anti-apoptotic partners Bcl-2-like protein 11
(Bim), Bcl-2-associated X, apoptosis regulator (Bax),
p53-upregulated modulator of apoptosis (Puma), hyperactivation of
nuclear factor-κB (NF-κB), inactivation or mutation of p53, or the
overexpression of certain oncoproteins (including c-Myc and Ras),
are also important factors in the progression of CRC (11,12). Thus,
attempts to inverse these abnormal alterations simultaneously
represents the rationale behind current strategies for CRC
chemotherapy.
As a result of the increase in the use of targeted
therapies, studies into the use of natural products as anticancer
drugs were abandoned by numerous large pharmaceutical companies
between 1997 and 2007. However since 2007, 12 natural product
derivatives, including temsirolimus, everolimus, ixabepilone,
vinflunine, romidepsin, trabectedin, cabazitaxel, abiraterone
acetate, eribulin mesylate, homoharringtonine, carfilzomib and
ingenol mebutate, have been approved for the treatment of cancer
(13). The discovery of these natural
products has encouraged multiple groups to continue the
investigation of natural compounds with anticancerous activities.
Ent-kaurane diterpenoid is a small secondary metabolite
molecule with comprehensive antineoplasmic activities, which has
attracted the interest of bio-organic chemists (14,15). In a
previous study by our group on the active constituents of Pteris
semipinnata, a traditional Chinese medicine, several
ent-kaurane diterpenoids were isolated, and their antitumor
activities and mechanisms were studied (16).
The present study focused on screening more active
ent-kaurane diterpenoids from P. semipinnata
through systemic chemical constituent separation. This separation
resulted in the isolation of a novel ent-kaurane
diterpenoid, termed pterisolic acid G (PAG), which exhibited
significant inhibitory effects on the viability of human CRC HCT116
cells. The present study reported the isolation and identification
of PAG, as well as the molecular mechanisms behind its activity.
The present results demonstrate that PAG is a novel
chemotherapeutic agent, which simultaneously inhibits viability and
induces apoptosis in human CRC HCT116 cells.
Materials and methods
Materials
The aerial parts, those completely exposed in air,
of P. semipinnata were collected in Zhangjiang,
China, and the identify of these were validated by Professor
Zhang-Pin Gou (Department of Pharmacology, Guangdong Medical
University, Zhanjiang, China). A voucher specimen (no. 20140812)
was deposited in the Guangdong Key Laboratory for Research and
Development of Natural Drugs (Zhanjiang, China). RPMI-1640 medium
was purchased from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Fetal bovine serum (FBS) was obtained from
Gibco; Thermo Fisher Scientific, Inc. Propidium iodide (PI) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Hoechst 33342 was obtained from Beyotime Institute of Biotechnology
(Haimen, China).
Antibodies against glycogen synthase kinase-3
(GSK-3β; cat no. 610201; dilution, 1:2,000), GSK-3β (p-Try216; cat
no. 612312; dilution, 1:1,000), β-catenin (cat no. 610,154;
dilution, 1:1,000) and c-Myc (cat no. 551101; dilution, 1:500) were
obtained from BD Biosciences (San Jose, CA, USA). Antibodies
against GSK-3β (p-Ser9; cat no. 9322; dilution, 1:2,000), β-actin
(cat no. 4967; dilution, 1:3,000), survivin (cat no. 2808;
dilution, 1:1,000), Bcl-2 (cat no. 2870; dilution, 1:1,000),
Bcl-XL (cat no. 2764; dilution, 1:1,000), Puma (cat no.
12450; dilution, 1:500) and Bax (cat no. 5023; dilution, 1:1,000)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Antibodies against Dvl-2 (cat no. sc-13974; dilution,
1:1,000), cyclin D1 (cat no. sc-753; dilution, 1:500) and p53 (cat
no. sc-6243; dilution, 1:2,000) were from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The histone 3 antibody (cat no. orb136531;
dilution, 1:3,000) was bought from Biorbyt, Ltd. (Cambridge, UK).
The caspase-3 (pro and active) antibody (cat no. NB100-56708;
dilution, 1:2,000) was purchased from Novus Biologicals, LLC
(Littleton, CO, USA). Purified anti-poly ADP ribose polymerase
(PARP; dilution, 1:2,000) was obtained from BioLegend, Inc. (San
Diego, CA, USA). NF-κB-p65 (cat no. 21014; dilution, 1:1,000) and
NF-κB-p65 [phospho (p-) Ser536; cat no. 11014; dilution, 1:500]
antibodies were purchased from Signalway Antibody LLC (College
Park, MD, USA).
General chemi-analysis
experiments
Nuclear magnetic resonance (NMR) spectra were
recorded using a Bruker AV-500 spectrometer at room temperature,
according to the manufacturer's instructions. High-resolution
electrospray ionization mass spectrometry data were acquired using
an Agilent 6210 liquid chromatography/mass selective detector
time-of-flight mass spectrometer (Agilent Technologies, Inc., Santa
Clara, CA, USA) at room temperature, according to the
manufacturer's instructions. Analytical high-performance liquid
chromatography (HPLC) and UV spectra were performed on an Agilent
1200 series and a C18 reversed-phase column (4.6×250 mm,
5.0 µM; Cosmosil; Agilent Technologies, Inc.; 25°C, 1 ml/min,
CH3CN-H2O, 19:81, v/v), according to the
manufacturer's instructions. Preparative HPLC was performed on a
Gilson 305 pump, a Varian Prostar 345 UV detector and a
C18 reversed-phase column (20×250 mm, 5.0 µM; Cosmosil;
Agilent Technologies, Inc.; 25°C, 20 ml/min,
CH3CN-H2O; 19:81, v/v; internal standards
were not used), according to the manufacturer's instructions.
Purification of PAG
The dried aerial sections of P.
semipinnata (5.0 kg) were powdered and extracted 3 times
with 95% ethyl alcohol at room temperature (50 l each time). The
solution was concentrated under vacuum at 50°C to yield 495 g
residue. The residue was suspended in distilled water, and
subsequently successively partitioned with n-hexane, ethyl acetate
(EtOAc) and n-butanol. Following removal of the solvent, 100 g
EtOAc extract was subjected to a Sephadex LH-20 column to remove
flavonoids using CH3OH as the eluent. Total terpenoid
(TT, 80 g) was yielded upon concentrating the CH3OH
dripped ahead. TT (70 g) was separated by silica gel column eluting
with gradient mixtures of CHCl3-CH3OH (100:0
to 100:20, v/v) to obtain 6 fractions (Fraction A to fraction F).
Fraction B (10.5 g) was subjected to chromatography using a
reversed-phase C18 silica gel column with gradient
CH3OH-H2O (10:90 to 60:40, v/v) to yield 16
sub-fractions (B1 to B16). PAG (100.3 mg) was further purified by
preparative HPLC (CH3CN-H2O, 19:81, v/v) from
1.3 g of sub-fraction B9.
Cell culture
Human CRC HCT116 cells (CCL-247; American Type
Culture Collection, Manassas, VA, USA) were cultured with RPMI-1640
medium supplemented with 10% heat-inactivated FBS in an atmosphere
of 5% CO2 at 37°C. Cells were subcultured when they
reached 90% confluence.
Cell viability assay
Cell Counting Kit-8 (CCK-8; catalog no. CK04;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) and trypan
blue dye exclusion (TBE) assays were used to evaluate the effect of
PAG on HCT116 cell viability according to the manufacturer's
protocol. Briefly, 2×103 cells were seeded in each well
of 96-well microplates and exposed to different concentrations
(3.125, 6.25, 12.5, 25, 50 and 100 µM) of PAG for 24, 48 and 72 h.
Dimethyl sulfoxdide was added to cells as a negative control.
Cellular viability was measured as a percentage of the viability of
control cells. At 4 h prior to culture termination, 10 µl CCK-8
reagent was added to each well. After 2–3 h incubation at 37°C, the
optical density was read on a 96-well microplate reader at a
wavelength of 490 nm. For the TBE test, a total of 2×105
cells/ml were seeded in 24-well plates and treated with PAG at
concentrations of 0, 3.125, 6.25, 12.5 and 25 µM for 24 h. Cells
stained with 0.04% trypan blue (catalog no. T8154; Sigma-Aldrich;
Merck KGaA) for 5 min at room temperature. Cell numbers were
assessed by cell counting under an inverted light microscope
(TS100; magnification, ×100; Nikon Corporation, Tokyo, Japan). Dead
cells were stained blue and live cells were not.
Cell cycle and apoptosis analysis by
flow cytometry
Following culture with or without PAG for 24 h,
HCT116 cells were pelleted by centrifugation at 200 × g for 5 min
at room temperature, washed with PBS, then fixed overnight in 75%
ethanol at 4°C and subsequently washed twice in cold PBS. Prior to
fluorescence-activated cell sorting (FACS) analysis using a flow
cytometer (Epics, XL-ECL; Beckman Coulter, Inc., Brea, CA, USA),
cells were labelled with PI work buffer (1X PBS; 50 µg/ml PI; 2.5
µg/ml RNase) at room temperature for 1 h in the dark. Cell-cycle
distribution and apoptosis rate were analyzed with equipped
software (EXPO32 ADC software, version 1.1; Beckman Coulter,
Inc.).
Hoechst 33342 staining
PAG-treated cells were fixed by a mixture of
methanol and glacial acetic acid (v/v, 3:1) at room temperature for
15 min and washed with PBS twice. Hoechst 33342 dye (2.5 µg/ml) was
then added, and cells were incubated for 3–5 min in the dark at
room temperature. Fluorescent images including 0, 12.5, 25 and 50
µM PAG-triggered apoptotic cells were captured using a fluorescence
microscope (DM2500; 200-fold magnification; Leica Microsystems,
Inc., Buffalo Grove, IL, USA). The average cell number of a
microscopic field was 500–560, and the typical images were selected
as representative figures.
Reactive oxygen species (ROS)
measurement
Cells were pretreated with 10 µM
dichloro-dihydro-fluorescein diacetate (DCFH-DA; S0033; Beyotime
Institute of Biotechnology, Haimen, China) in the presence or
absence of PAG for 20 min at 37°C. Cells were then washed 3 times
with RPMI-1640 medium and ROS generation was measured by flow
cytometry, according to the manufacturer's protocol.
Western blot analysis
Proteins were extracted from the cultured cells
using a RIPA lysis buffer supplemented with freshly added 100 mM
phenylmethylsulfonyl fluoride on ice for 30 min. The cell lysates
were centrifuged at 15,000 × g for 10 min at 4°C. Supernatants were
quantified using a BCA protein assay kit, and total protein (50–80
µg/lane) was subjected to SDS-PAGE (10, 12 or 15% gels), according
to the molecular weight of target proteins. Subsequently, protein
was transferred to a nitrocellulose membrane and blocked with 5%
skim milk at room temperature for 1 h. The membrane was incubated
with corresponding primary antibody mentioned above [antibodies
against β-catenin, histone 3, β-actin, Dvl-2, p-Try216-GSK-3β,
p-Ser9-GSK-3β, GSK-3β, cyclin D1, c-Myc, caspase-3 (pro and
active), PARP, survivin, Bcl-2, Bcl-XL, Puma, Bax, Bim, p53,
p-Ser536-NF-κB-p65 and NF-κB-p65)] at 4°C overnight and washed 3
times (10 min each) with PBS-Tween-20 (PBST). Subsequently, the
membrane was incubated with peroxidase-conjugated goat anti-rabbit
IgG (H+L) secondary antibody (cat no. ZB-2301; dilution,
1:1,000–1:4,000) or peroxidase-conjugated goat anti-mouse IgG (H+L)
secondary antibody (cat no. ZB-2305; dilution, 1:1,000–1:4,000)
(all from OriGene Technologies, Inc., Rockville, MD, USA) at room
temperature for 1 h, washed 2 times with PBST (10 min each) and
washed once with PBS. The blots were visualized using enhanced
chemiluminescence reagents (WBKLS0050; EMD Millipore, Billerica,
MA, USA) in a dark room, followed by developing the blots on X-ray
film. β-actin was used as the internal control in western blot
analysis.
Statistical analysis
GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA) was used to perform the statistical
analysis. Comparisons between multiple groups were performed using
one-way analysis of variance with Tukey's post hoc intergroup
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of PAG
The novel compound was obtained as a white amorphous
powder (methanol), and had a molecular formula of
C20H28O6 as determined by
HR-ESI-MS at m/z 387.1816 [M+Na]+(calculated for
C20H28O6Na, 387.1778). The UV
revealed λmax at 238 nm indicating the presence of an α,
β-unsaturated ketone carbonyl chromophore (17). The 13C NMR and DEPT-135
spectra identified 20 carbons, including an α, β-unsaturated ketone
group at δC 207.0 (C), 154.9 (C) and 116.7
(CH2), a carboxylic carbon at δC 184.4 (C), 2
methyls at δC 26.2 (CH3) and δC
19.8 (CH3), 5 methenes at δC 17.9
(CH2), 25.3 (CH2), 25.9 (CH2),
29.3 (CH2) and δC 31.8 (CH2), 6
methines at δC 47.5 (CH), 46.0 (CH), 36.8 (CH), 70.8
(OCH), 71.6 (OCH) and 85.4 (OCH), and 3 quaternary carbons at
δC 40.2 (C), 42.5 (C) and δC 56.3 (C).
The 1H-NMR spectrum exhibited legible
signals as follows: 2 olefinic protons at δH 5.86, 5.41
(each broad singlet) due to an exocyclic methylene group, 3
oxygenated methine protons at δH 3.50 (1H, d, J=3.2 Hz),
4.27 (1H, d, J=5.6 Hz) and 5.21 (1H, d, J=5.8 Hz), and 2 methyl
singlets at δH 1.30 (3H, s) and 0.77 (3H, s). The
aforementioned structural features revealed that the compound
should be a 15-oxo-ent-kuar-16-en-19-oic acid derivative
(18).
With the aid of 1H-1H
correlation spectroscopy (COSY), heteronuclear single quantum
coherence spectroscopy and heteronuclear multiple bond correlation
(HMBC) experiments, all the 1H and 13C NMR
signals were assigned as presented in Table I. The HMBC correlations between
δH 3.50 (H-1) and δC 19.8 (C-20)/25.3
(C-3)/47.5 (C-5)/40.2 (C-10); between δH 5.21 (H-6) and
δC 40.2 (C-10)/47.5 (C-5)/71.6 (C-7); and between
δH 4.27 (H-7) and δC 207.0 (C-15)/29.3
(C-14)/56.3 (C-8)/85.4 (C-6), revealed the presence of hydroxy
groups at C-1, C-6 and C-7, respectively. The assignment of C19-oic
acid was further confirmed by the HMBC correlations between
δH 1.30 (H-18)/1.61 (H-3)/1.98 (H-3)/2.31 (H-5) and
δC 184.4 (C-19). Determined using the Bruker AV-500
spectrometer, the 13C chemical shift of
18-CH3 at δ 26.2 suggested its β-orientation (19) (α-18-CH3 at ~δ16.7)
(20), which was supported by the
absence of rotational overhauser effect spectroscopy (ROESY)
correlation between δH 1.04 (Me-20) and δH
1.39 (Me-18). The ROESY correlations between H-5 and H-9, and H-9
and H-1 indicated that these protons were cofacial and β-oriented.
The β-H-6 and α-H-7 orientations were confirmed by ROESY
correlations between H-6 and Me-18, and H-7 and Me-20,
respectively. Detailed key 1H-1H COSY, HMBC
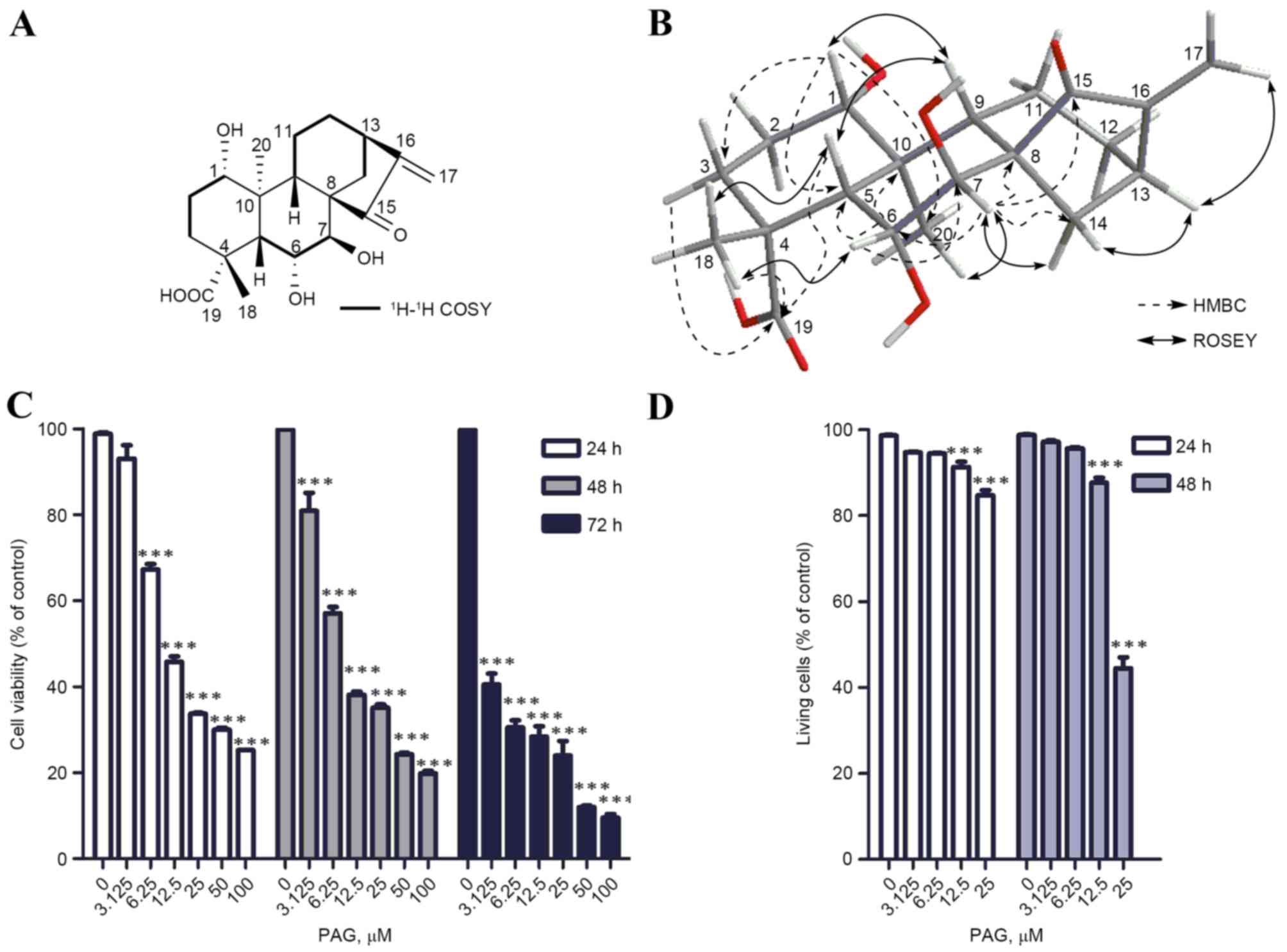
and ROESY information is presented in Fig. 1A and B. Accordingly, the structure of
the compound was elucidated as ent-1β, 6α,
7β-trihydroxy-15-oxo-9 (11),
16-kauradien-19α-oic acid, and termed PAG (Fig. 1A).
 | Table I.1H NMR and 13C NMR spectroscopic data
of PAG (CD3OD, J in Hz). |
Table I.
1H NMR and 13C NMR spectroscopic data
of PAG (CD3OD, J in Hz).
| Position | δC | δH |
|---|
| 1 | 70.8 CH | 3.50 (t, 3.2) |
| 2 | 25.9
CH2 | 1.73 (m) |
|
|
| 1.50 (m) |
| 3 | 25.3
CH2 | 1.98 (ddd, 14.4,
6.5, 2.6) |
|
|
| 1.61 (m) |
| 4 | 42.5 C | – |
| 5 | 47.5 CH | 2.31 (d, 6.0) |
| 6 | 85.4 CH | 5.21 (d, 5.8) |
| 7 | 71.6 CH | 4.27 (d, 5.6) |
| 8 | 56.3 C | – |
| 9 | 46.0 CH | 1.92 (dd, 13.1,
4.3) |
| 10 | 40.2 C | – |
| 11 | 17.9
CH2 | 1.54 (m) |
|
|
| 1.59 (m) |
| 12 | 31.8
CH2 | 1.50 (m) |
|
|
| 2.28 (m) |
| 13 | 36.8 CH | 3.02 (dd, 9.1,
4.5) |
| 14 | 29.3
CH2 | 2.02 (d, 11.8) |
|
|
| 2.08 (dd, 11.9,
4.6) |
| 15 | 207.0 C | – |
| 16 | 154.9 C | – |
| 17 | 116.7
CH2 | 5.86 (s) |
|
|
| 5.41 (s) |
| 18 | 26.2
CH3 | 1.30 (s) |
| 19 | 184.4 C | – |
| 20 | 19.8
CH3 | 0.77 (s) |
PAG inhibits the viability of HCT116
cells
The inhibitory effect of PAG on HCT116 cell
viability was measured using a CCK-8 assay and a TBE test. As
presented in Fig. 1C, PAG exhibited
significant cytotoxicity to HCT116 cells in a dose- and
time-dependent manner with IC50 values at 20.43, 16.15
and 4.07 µM for 24, 48 and 72 h, respectively. The results of the
TBE assay also demonstrated that PAG sharply induced HCT116
cellular death in a concentration-dependent manner following 24 and
48 h treatment (Fig. 1D). At 72 h, no
stained cells were observed since the cells were lysed by PAG (data
not shown).
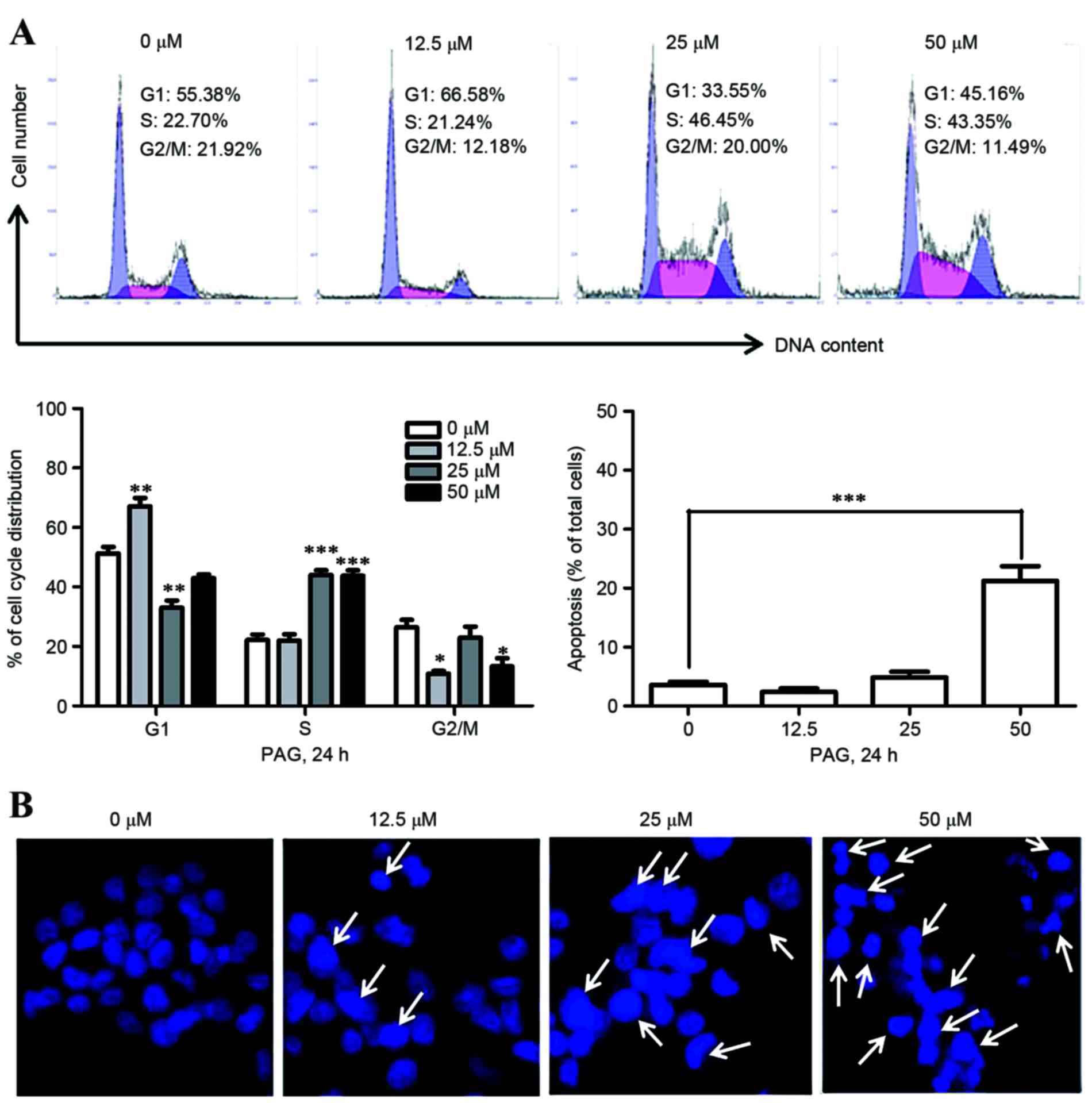
Effects of PAG on the cell cycle and
apoptosis in HCT116 cells
The present study subsequently explored whether the
cell cycle and apoptosis were affected by PAG using FACS analysis
following PI staining. The results revealed that PAG affected cell
cycle distributions without a clear dose-dependent effect overall.
However, it was worth noting that PAG markedly increased the S
population at 25 and 50 µM following 24 h treatment. Analysis of
apoptosis revealed dose-dependent apoptotic cell death in HCT116
cells following PAG treatment (Fig.
2A). Taking these low percentages of apoptosis detected by flow
cytometry into consideration, the rate of apoptosis was then
observed by fluorescence microscopy subsequent to staining with
Hoechst 33342 dye. As presented in Fig.
2B, blue fluorescence in HCT116 cells treated with the same
concentrations of PAG as FACS analysis for 24 h was visibly
increased. Together, these results demonstrated that PAG induced
cellular apoptosis in HCT116 cells.
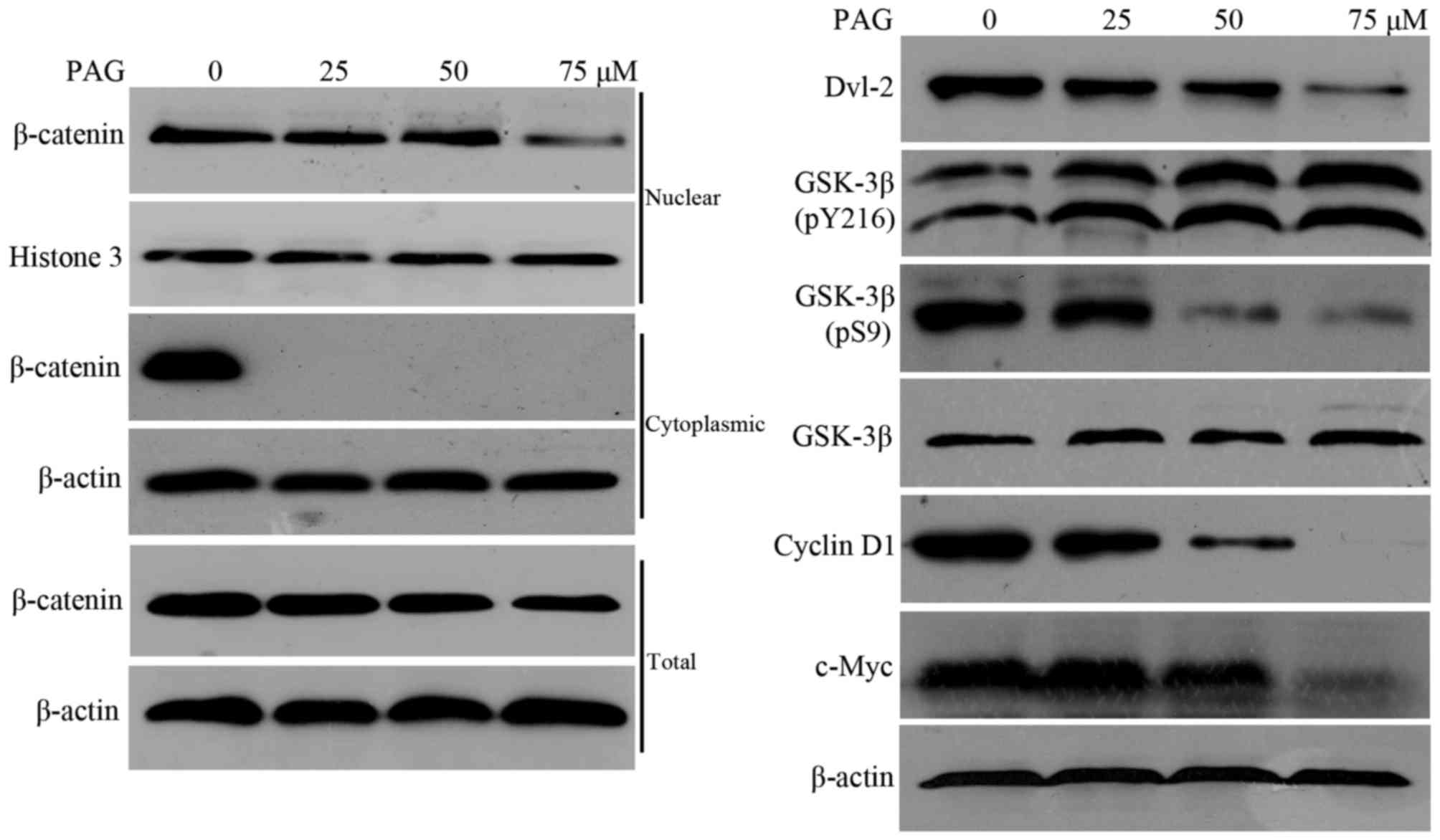
PAG suppresses the
Dvl-2/GSK-3β/β-catenin pathway in HCT116 cells
From the aforementioned information, it was clear
that PAG significantly inhibited cellular viability in HCT116
cells, however the mechanisms behind this remain unclear.
Previously, two ent-kaurane diterpenoids have been reported
to execute antitumor functions in CRC cell lines by inhibiting the
Wnt/β-catenin pathway (21,22). This led the present study to
investigate the interference of PAG on this signaling. Therefore,
nuclear, cytoplasmic and total β-catenin, as well as its target
proteins c-Myc and cyclin D1, were detected by western blot
analysis. The results demonstrated that nuclear and total
β-catenin, along with c-Myc and cyclin D1, were decreased by PAG in
a dose-dependent manner, while cytoplasmic β-catenin was not
detected by western blot analysis in all 3 treated concentrations
of PAG, compared with the untreated control (Fig. 3).
These results suggested that PAG may depress nuclear
accumulation and decrease the total content of β-catenin by
promoting cytoplasmic β-catenin degradation to inhibit cell
viability. β-catenin degradation is initiated by amino-terminal
serine/threonine phosphorylation, which is performed by GSK-3β in a
complex with the tumor suppressor proteins (Axin), APC and casein
kinase-1α (CK1α) (23). Therefore,
further investigations were performed in order to evaluate the
effects of PAG on GSK-3β, p-Ser9-GSK-3β (inhibitory form of GSK-3β)
and p-Tyr216-GSK-3β (active form of GSK-3β) (24). The upregulation of GSK-3β and
p-Tyr216-GSK-3β, and the downregulation of p-Ser9-GSK-3β (Fig. 3) implied that PAG enhanced GSK-3β
activity. The recruitment of Dvl to Frizzled may result in the
deactivation of GSK-3β by phosphorylation of Ser9 and the
dephosphorylation of Tyr216 (25,26),
resulting in the disassembly of the β-catenin destruction complex
(Axin/APC/GSK-3β/CK1α) (27),
ultimately leading to the nuclear accumulation of β-catenin in the
canonical Wnt/β-catenin pathway. The effect of PAG on Dvl-2 was
then examined. The results revealed that PAG downregulated Dvl-2
protein levels (Fig. 3). In summary,
the present results indicated that PAG inhibited the viability of
HCT116 cells by suppressing the Dvl-2/GSK-3β/β-catenin pathway.
Mechanisms underlying cellular
apoptosis induced by PAG
Western blot analysis revealed an increase in the
cleavage of PARP (85 kDa) and active-caspase-3 (17 kDa) in a
concentration-dependent manner by PAG, which coincided with the
decline of PARP (116 kDa) and pro-caspase-3 (35 kDa; Fig. 4A), and confirmed the presence of
caspase-dependent apoptosis. To investigate how PAG induced the
apoptosis of HCT116 cells, investigations were performed on
apoptosis-associated proteins. As presented in Fig. 4B, PAG decreased the levels of
anti-apoptotic proteins including survivin, Bcl-2 and
Bcl-XL, and increased the levels of pro-apoptosis
proteins including Puma, Bax and Bim. The decrease of the
anti-apoptotic factors NF-κB p65 and p-p65, as well as the increase
of a the tumour suppressor p53, were also detected following PAG
treatment (Fig. 4C).
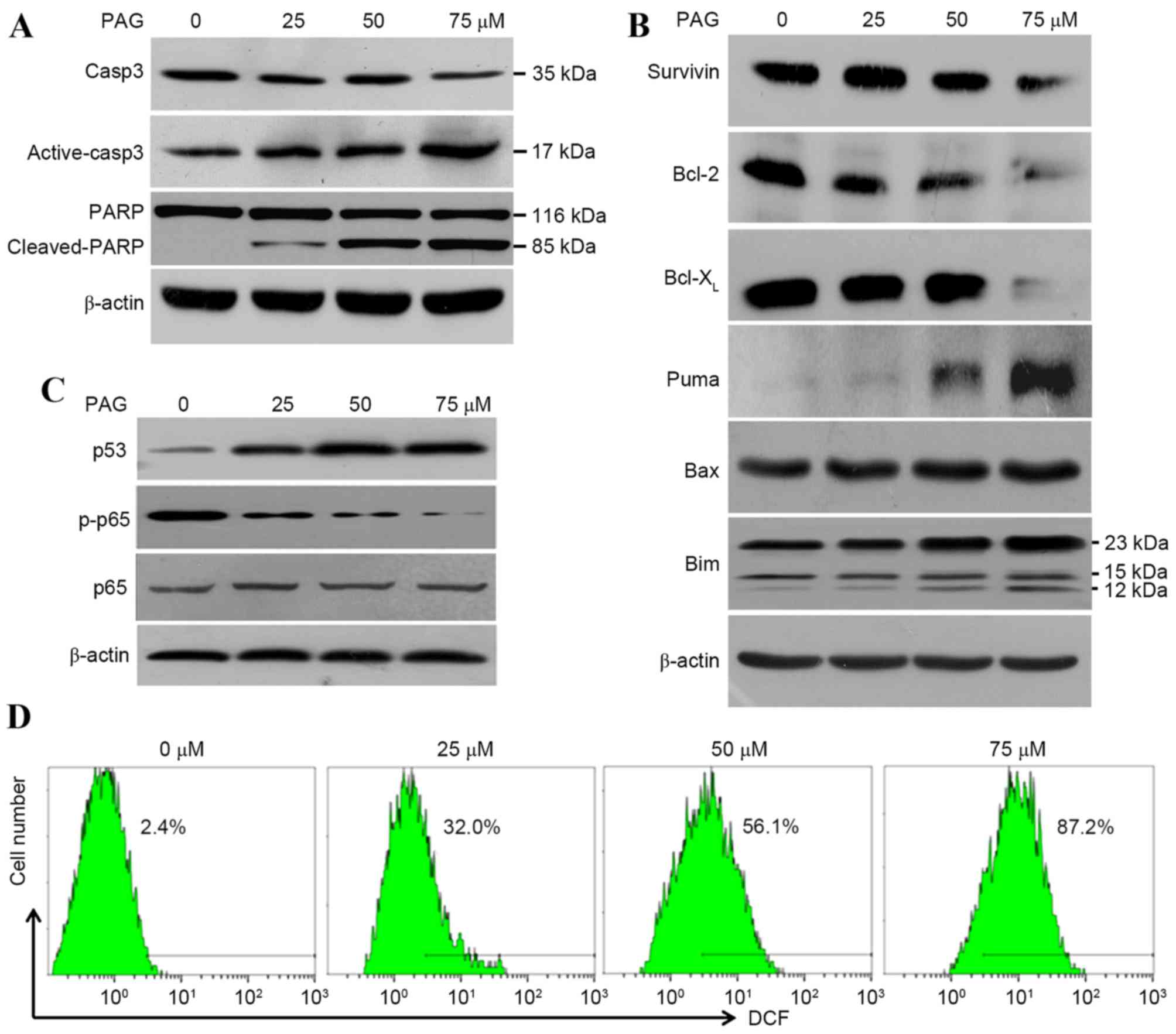 | Figure 4.PAG triggered cellular apoptosis in
HCT116 cells by increasing p53 expression, decreasing NF-κB p65
activity and promoting the generation of ROS. Cells were exposed to
PAG at the indicated concentrations for 24 h. Cell lysates were
analyzed by (A) western blot for markers, (B) associated proteins
and (C) inducers of apoptosis. (D) Cells treated with 0, 25, 50 and
75 µM PAG for 6 h were stained with dichloro-dihydro-fluorescein
diacetate, and the ROS levels were measured by flow cytometry.
β-actin was used as a control. PAG, pterisolic acid G; ROS,
reactive oxygen species; Casp3, caspase 3; PARP, poly ADP ribose
polymerase; p-, phosphorylated; Bcl-2, B-cell lymphoma-2;
Bcl-XL, B-cell lymphoma-extra large; Puma,
p53-upregulated modulator of apoptosis; Bax, Bcl-2-associated X,
apoptosis regulator; Bim, Bcl-2-like protein 11. |
Additionally, DCFH-DA detection was performed
following PAG treatment, since it has been previously demonstrated
that ent-kaurane diterpenoid induces apoptosis by increasing
intracellular ROS generation (28).
The increase of intracellular ROS levels by PAG in a
concentration-dependent manner (Fig.
4D) confirmed this result. In summary, the results of the
present study suggested that PAG induced cellular apoptosis in
HCT116 cells by decreasing NF-κB p65 activity, increasing p53
expression and promoting the generation of ROS.
Discussion
Over the past decades, chemotherapy techniques for
metastatic (m) CRC has developed from the use of a single agent to
a combination of cytotoxic therapies and target-specific agents.
Although the median survival time has improved, the 5-year overall
survival rate for patients with mCRC remains poor (6). Therefore, it is important to develop
effective therapeutic agents for the treatment of CRC, particularly
mCRC.
The screening of small molecule inhibitors of the
Wnt/β-catenin signaling pathway is regarded as a promising strategy
for CRC chemotherapy, since the ectopic activation of this pathway
is involved in CRC pathogenesis and development (29). Theoretically, these small molecule
inhibitors achieve their effect by targeting cytoplasmic proteins
(Dvl, Tankyrase, Porcupine, CK1, Axin, APC and GSK-3),
transcriptional factors (TCF/LEF) or co-activators (CBP, p300,
Pygo, Bcl-9) of the Wnt/β-catenin cascade. A number of synthetic
compounds targeting the Wnt/β-catenin signaling pathway have been
identified, and a number of these have been subject to clinical
trials (30). Natural products
including ginsenosides (31),
berberine (32), hydnocarpin
(33), murrayafoline A (34), tetrandrine, curcumin, epigallocatechin
gallate, baicalin, magnolol and resveratrol (35), are also antagonists of the
Wnt/β-catenin signaling pathway. Ent-kaurane diterpenoids,
11α, 12α-epoxyleukamenin E (21) and
Henryin (22), isolated from
Isodon rubescens var. lushanensis and Salvia
cavaleriei, respectively, are able to interfere with the
Wnt/β-catenin signaling pathway by impairing β-catenin/TCF4
transcriptional complexes, exerting antitumor activities in CRC
cells.
Similarly, the novel ent-kaurane diterpenoid
PAG identified in the present study may also disrupt the same
pathway to exhibit its antineoplastic effect; however, the exact
mechanism behind this may differ from what has been indicated in
previous studies. The present study revealed that PAG decreases
nuclear accumulation and promotes cytoplasmic degradation of
β-catenin by decreasing Dvl-2 expression and increasing GSK-3β
activity. This suggests that PAG inhibited the viability of HCT116
cells by suppressing the Dvl-2/GSK-3β/β-catenin pathway.
Targeting apoptosis pathways is also an effective
method for cancer chemotherapy (36).
A number of antitumor agents promote cancer cell apoptosis via
NF-κB, p53, ROS, death receptor, phosphatase and tensin homolog,
genotoxicity and T lymphocyte cytotoxicity (37). Activated NF-κB suppresses apoptosis by
enhancing the expression of anti-apoptotic genes, including
Bcl-XL, X-inhibitor of apoptosis protein (IAP), IAP1,
IAP2, radiation-inducible immediate-early gene-1 L and the Bcl-2
family member Bfl-1, which in turn results in an advancement in
tumor development (38,39). Based on this, the pro-apoptotic
function of PAG may be partially due to the resultant decreased
NF-κB p65 and p-p65 expression. In contrast to NF-κB, p53 promotes
apoptosis by inducing the expression of Bcl-2 family members and
increasing the permeability of the outer membrane of the
mitochondria (12,40,41).
Therefore, increased p53 expression in HCT116 cells may also
contribute to the pro-apoptotic effect of PAG. ROS overexpression,
which triggers cell apoptosis, necrosis or autophagy, is a
mechanism common to all non-surgical therapeutic approaches for
cancers, including chemotherapy, radiotherapy and photodynamic
therapy (42). Thus, the PAG-induced
increase of intracellular ROS levels may be associated with its
pro-apoptosis activity. In general, PAG induces cellular apoptosis
of HCT116 cells by suppressing NF-κB p65 activity, stimulating p53
expression and promoting ROS generation.
In conclusion, the present study purified a novel
ent-kaurane diterpenoid, PAG, from P.
semipinnata and investigated its anti-neoplastic activities
in human CRC HCT116 cells. Mechanistic studies revealed that PAG
not only inhibited the viability of HCT116 cells by suppressing the
Dvl-2/GSK-3β/β-catenin pathway, but also induced apoptosis of
HCT116 cells by stimulating p53 expression, downregulating NF-κB
p65 activation and promoting intracellular ROS generation. The
results of the present study suggested that PAG, as a novel
inhibitor of the Wnt/β-catenin pathway and inducer of apoptosis,
should be further investigated via in vivo experiments and
comprehensive mechanistic studies in order to examine its potential
use as a novel therapeutic agent for the treatment of CRC.
Acknowledgements
The present study was supported by grants from the
National Science and Technology Pillar Program of China (grant no.
2013BAI11B05), the National Natural Science Foundation of China
(grant no. 81503221 and 81503226), the Shenzhen Technology
Development Project (grant no. CXZZ20150402104158173) and the
Undergraduates ‘Climbing’ Program of Guangdong Province (grant no.
pdjh2015b0299).
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merla A and Goel S: Novel drugs targeting
the epidermal growth factor receptor and its downstream pathways in
the treatment of colorectal cancer: A systematic review. Chemother
Res Pract. 2012:3871722012.PubMed/NCBI
|
|
7
|
Perkins G and Laurent-Puig P: Colorectal
cancer biology. Rev Prat. 65:802–806. 2015.(In French). PubMed/NCBI
|
|
8
|
Zeng Y, Xie H, Qiao Y, Wang J, Zhu X, He
G, Li Y, Ren X, Wang F, Liang L and Ding Y: FMNL2 regulates
Rho/ROCK pathway to promote actin assembly and cell invasion of
colorectal cancer. Cancer Sci. 106:1385–1393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang X, Tan J, Li J, Kivimäe S, Yang X,
Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, et al: DACT3 is an
epigenetic regulator of Wnt/beta-catenin signaling in colorectal
cancer and is a therapeutic target of histone modifications. Cancer
Cell. 13:529–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meek DW: Regulation of the p53 response
and its relationship to cancer. Biochem J. 469:325–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basmadjian C, Zhao Q, Bentouhami E, Djehal
A, Nebigil CG, Johnson RA, Serova M, de Gramont A, Faivre S,
Raymond E and Désaubry LG: Cancer wars: Natural products strike
back. Front Chem. 2:202014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CX, Yin QQ, Zhou HC, Wu YL, Pu JX, Xia
L, Liu W, Huang X, Jiang T, Wu MX, et al: Adenanthin targets
peroxiredoxin I and II to induce differentiation of leukemic cells.
Nat Chem Biol. 8:486–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun HD, Huang SX and Han QB: Diterpenoids
from Isodon species and their biological activities. Nat Prod Rep.
23:673–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye H, Liang NC and Zheng XB: Progress in
research on anti-tumor effect of 5F isolated from Pteris
semipinnata L. Nat Pro Res Dev. 26:2082–2087. 2014.
|
|
17
|
Schubert WM and Sweeney WA: The effect of
ring strain on the ultraviolet spectra of α,β-unsaturated carbonyl
compounds. J Am Chem Soc. 77:2297–2300. 1955. View Article : Google Scholar
|
|
18
|
Wang F, Li YJ, Ren FC, Wei GZ and Liu JK:
Pterisolic acids A-F, new ent-kaurane diterpenoids from the fern
Pteris semipinnata. Chem Pharm Bull (Tokyo). 59:484–487. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hutchison M, Lewer P and MacMillan J:
Carbon-13 nuclear magnetic resonance spectra of eighteen
derivatives of ent-kaur-16-en-19-oic acid. J Chem Soc, Perkin
Trans. 1:2363–2366. 1984. View Article : Google Scholar
|
|
20
|
Santos HS, Barros FW, Albuquerque MR,
Bandeira PN, Pessoa C, Braz-Filho R, Monte FJ, Leal-Cardoso JH and
Lemos TL: Cytotoxic diterpenoids from Croton argyrophylloides. J
Nat Prod. 72:1884–1887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Q, Yao G, Zhang M, Guo G, Hu Y, Jiang
J, Cheng L, Shi J, Li H, Zhang Y and Liu H: A novel ent-kaurane
diterpenoid executes antitumor function in colorectal cancer cells
by inhibiting Wnt/β-catenin signaling. Carcinogenesis. 36:318–326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Pu J, Jiang S, Su J, Kong L, Mao B,
Sun H and Li Y: Henryin, an ent-kaurane diterpenoid, inhibits Wnt
signaling through interference with β-catenin/TCF4 interaction in
colorectal cancer cells. PLoS One. 8:e685252013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Y, Kanno T and Nishizaki T: Acute
restraint stress impairs induction of long-term potentiation by
activating GSK-3β. Neurochem Res. 40:36–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aschenbach WG, Ho RC, Sakamoto K, Fujii N,
Li Y, Kim YB, Hirshman MF and Goodyear LJ: Regulation of
dishevelled and beta-catenin in rat skeletal muscle: An alternative
exercise-induced GSK-3beta signaling pathway. Am J Physiol
Endocrinol Metab. 291:E152–E158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S, Lee J, Park J and Chung J: BP75,
bromodomain-containing M(r) 75,000 protein, binds dishevelled-1 and
enhances Wnt signaling by inactivating glycogen synthase kinase-3
beta. Cancer Res. 63:4792–4795. 2003.PubMed/NCBI
|
|
27
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao YJ, Bai HY, Li ZH, Zou J, Chen JW,
Zheng F, Zhang JX, Mai SJ, Zeng MS, Sun HD, et al: Longikaurin A, a
natural ent-kaurane, induces G2/M phase arrest via downregulation
of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in
hepatocellular carcinoma cells. Cell Death Dis. 5:e11372014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weng W, Feng J, Qin H and Ma Y: Molecular
therapy of colorectal cancer: Progress and future directions. Int J
Cancer. 136:493–502. 2015.PubMed/NCBI
|
|
30
|
Zhang X and Hao J: Development of
anticancer agents targeting the Wnt/β-catenin signaling. Am J
Cancer Res. 5:2344–2360. 2015.PubMed/NCBI
|
|
31
|
Bi X, Xia X, Mou T, Jiang B, Fan D, Wang
P, Liu Y, Hou Y and Zhao Y: Anti-tumor activity of three
ginsenoside derivatives in lung cancer is associated with
Wnt/β-catenin signaling inhibition. Eur J Pharmacol. 742:145–152.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albring KF, Weidemuller J, Mittag S,
Weiske J, Friedrich K, Geroni MC, Lombardi P and Huber O: Berberine
acts as a natural inhibitor of Wnt/β-catenin
signaling-identification of more active 13-arylalkyl derivatives.
Biofactors. 39:652–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee MA, Kim WK, Park HJ, Kang SS and Lee
SK: Anti-proliferative activity of hydnocarpin, a natural lignan,
is associated with the suppression of Wnt/β-catenin signaling
pathway in colon cancer cells. Bioorg Med Chem Lett. 23:5511–5514.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi H, Gwak J, Cho M, Ryu MJ, Lee JH, Kim
SK, Kim YH, Lee GW, Yun MY, Cuong NM, et al: Murrayafoline A
attenuates the Wnt/beta-catenin pathway by promoting the
degradation of intracellular beta-catenin proteins. Biochem Biophys
Res Commun. 391:915–920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tarapore RS, Siddiqui IA and Mukhtar H:
Modulation of Wnt/β-catenin signaling pathway by bioactive food
components. Carcinogenesis. 33:483–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai L and Wang S: Targeting apoptosis
pathways for new cancer therapeutics. Annu Rev Med. 65:139–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kucharczak J, Simmons MJ, Fan Y and
Gelinas C: To be, or not to be: NF-kappaB is the answer-role of
Rel/NF-kappaB in the regulation of apoptosis. Oncogene.
22:8961–8982. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin A and Karin M: NF-kappaB in cancer: A
marked target. Semin Cancer Biol. 13:107–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li XL, Zhou J, Chen ZR and Chng WJ: P53
mutations in colorectal cancer-molecular pathogenesis and
pharmacological reactivation. World J Gastroenterol. 21:84–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dashzeveg N and Yoshida K: Cell death
decision by p53 via control of the mitochondrial membrane. Cancer
Lett. 367:108–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J and Yi J: Cancer cell killing via
ROS: To increase or decrease, that is the question. Cancer Biol
Ther. 7:1875–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|