Introduction
Chondrosarcoma is a malignant soft tissue sarcoma
with poor prognosis, and it is the second most common primary bone
tumor among all malignant bone tumors (1). At present, surgical resection remains
the primary treatment option for chondrosarcoma (2). Due to its poor response to conventional
chemotherapy and radiation therapy, chondrosarcoma is challenging
to manage (3). Therefore, novel and
effective therapeutic approaches are required for patients with
chondrosarcoma.
Puerarin, the major active ingredient extracted from
Radix puerariae, exhibits beneficial effects for
cardiovascular diseases, neurological dysfunction, diabetes,
osteoporosis, liver injury and inflammation (4). Previous studies have indicated that
puerarin exhibits anticancer effects and induces tumor cell
apoptosis in a number of cancer cell types, including colon cancer
HT-29 cells, human mantle Z138 cells, human glioblastoma cells,
stomach cancer cells and breast cancer cells (5–9).
Furthermore, increasing evidence has indicated the mechanisms
involved in the biological effect of puerarin. In hFBO1.19 cells,
puerarin attenuated the dexamethasone (DEX)-induced release of
cytochrome c and cleavage of caspase-3, which was associated
with inhibition of the c-Jun N-terminal kinase (JNK) pathway and
activation of the phosphoinositide 3-kinase
(PI3K)/threonine-protein kinase (Akt) signaling pathway (10). Puerarin increases proliferation and
differentiation and opposes cisplatin-induced apoptosis in human
osteoblastic MG-63 cells. At least partially, puerarin functions
via activation of MEK/extraellular signal-regulated kinase and
PI3K/Akt signaling pathways (11).
However, the effects of puerarin on the human chondrosarcoma cell
line SW1353 and the molecular mechanisms underlying these effects
remain unclear.
In the present study, puerarin decreased cell
viability and induced apoptosis in SW1353 cells via inhibition of
the P13K/RAC-alpha serine/Akt signaling pathway. These results
elucidated the potential molecular mechanisms underlying the
anticancer effect of puerarin in SW1353 cells, which may provide an
insight into novel therapeutic options for patients with
chondrosarcoma.
Materials and methods
Materials
The human chondrosarcoma cell line SW1353 was
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Puerarin, LY294002, PD98059,
SP600125 and SB203580 were purchased from Sigma-Aldrich (Merk KGaA,
Darmstadt, Germany). Rabbit anti-human antibodies against Bax (cat
no. 2774), Bcl-2 (cat no. 2872), Caspase-3 (cat no. 9664), Akt (cat
no. 9272), p-Akt (Ser473; cat no. 9271) and GAPDH (cat no. 2118)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
SW1353 cells were cultured in L-15 medium (Hyclone;
GE Healthcare Life Sciences Logan, UT, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and incubated at 37°C in a humidified atmosphere of 5%
CO2.
MTT assay
SW1353 cells (1×104 cells/well) were
treated with puerarin in 96-well plates (Costar; Corning
Incorporated, Corning, NY, USA) for 48 h. Then, cultures were
washed with PBS. MTT (Sigma-Aldrich; Merk KGaA) was added to each
well and the mixture was incubated at 37°C for 2 h. To dissolve the
formazan crystals, culture medium was then replaced with an equal
volume of dimethyl sulphoxide (Merck KGaA). Following agitation at
room temperature for 10 min, the absorbance of each well was
determined at 570 nm using a microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The inhibitory rate
(%)=(optical density (OD)control group-ODtest
group) × 100%/ODcontrol group.
Cell apoptosis by flow cytometry
analysis
Apoptotic cells were measured with the Annexin
V/fluorescein isothiocyanate (FITC) kit (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's protocol. Briefly,
1×106 cells were collected, washed three times with PBS
and were resuspended in 300 µl of binding buffer. A total of 5 µl
of Annexin V-FITC solution and 5 µl of propidium iodide (PI) were
added and incubated in the dark at 37°C for 15 min. Then, the
cytofluorimetric analyses were performed on a BD FACSVerse (BD
Biosciences) and data were analyzed using FACSuite software version
1.0 (BD Biosciences).
Enzymatic assay for caspase-3 and
caspase-9 activity
Caspase activity was detected using caspase-3 and
caspase-9 activity assay kits (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol. Cells were
lysed and total cellular protein extracts were quantified using a
Protein-assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
An equal amount of total protein extract was incubated at 37°C
overnight with either Ac-DEVD-pNA for the caspase-3 assay or
Ac-LEHD-pNA for the caspase-9 assay. The release of pNA was
estimated by determining the absorbance at 405 nm on a microplate
ELISA reader (Bio-Rad Laboratories, Inc.). Results are presented
with the fold change in activity compared with the untreated
control.
Western blot analysis
Cells were collected, washed and lysed with
radioimmunoprecipitation assay buffer, including 50 mM Tris-HCl
(pH=7.4), 150 mM NaCl, 1% Nonidet P-40, 0.25% NaN3, 1 mM
EDTA, 1 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml
pepstatin A, 1 mM sodium orthovanadate and 1 mM NaF. Then, the
protein concentration was determined using a BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). The proteins (20 µg) were
resolved on a 10% SDS-PAGE gel and transferred to polyvinylidene
fluoride membranes. The blots were blocked with 5% BSA
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, and probed
with rabbit anti-human antibodies against Bax, Bcl-2, Caspas-3,
Akt, p-Akt (Ser473) or GAPDH (1:1,000 dilution) for 1 h at room
temperature. After washing three times with PBS, the blots were
incubated with a horseradish peroxidase-conjugated donkey
anti-rabbit secondary antibody (1:1,000 dilution; cat no. sc2313;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at
room temperature. The signals were visualized by enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.). Images
were captured using a scanner (GE Healthcare, Chicago, IL, USA) and
proteins were quantified using Image J software version 1.42
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using SPSS statistical software
version 13.0 (SPSS, Inc., Chicago, IL, USA) and the data were
reported as the mean ± standard deviation. Statistical analysis
between two samples was evaluated using a Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Puerarin inhibits the viability of
human chondrosarcoma SW1353 cells
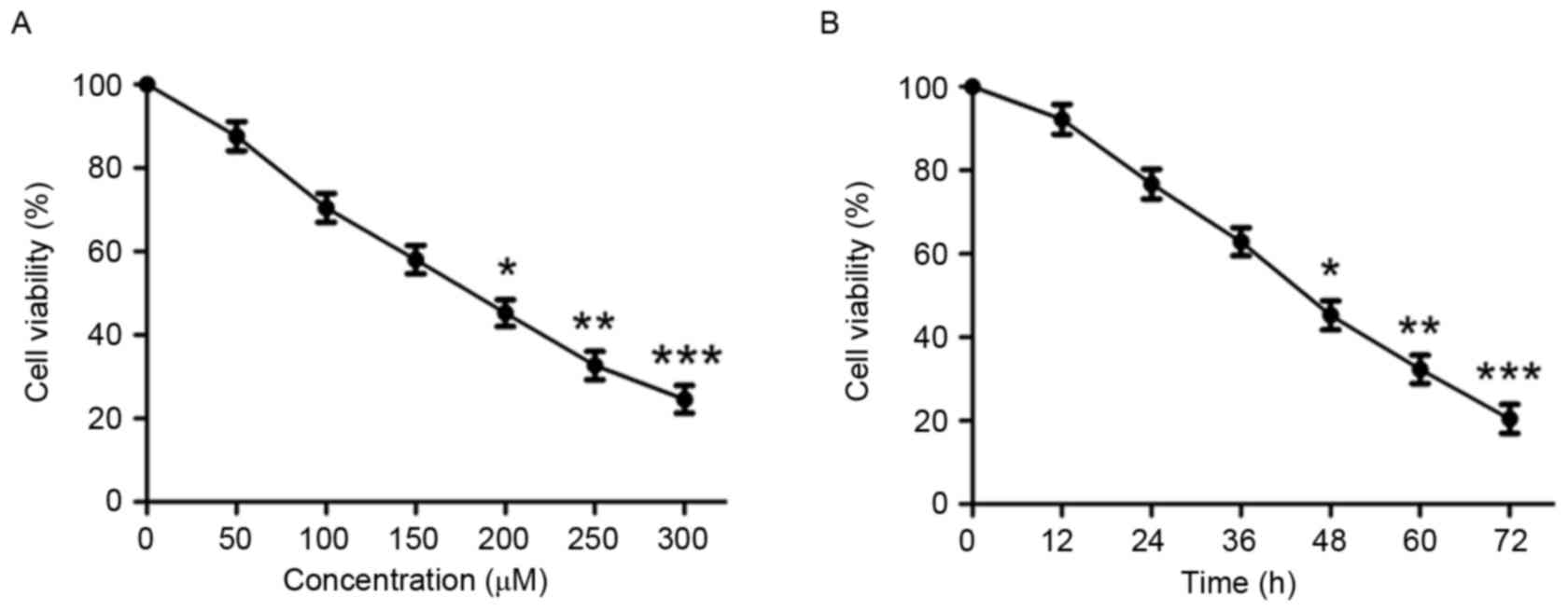
In order to investigate the effect of puerarin on
cell viability, human chondrosarcoma SW1353 cells were treated with
various concentrations of puerarin (0–300 µM) for 48 h. Puerarin
significantly reduced cell viability in a dose-dependent manner
(P<0.05, P<0.01 and P<0.001 compared with untreated
control for 200, 250 and 300 µM, respectively; Fig. 1A and Table
I). After 48 h incubation, the half-maximal inhibitory
concentration value of puerarin against SW1353 cells was 180.738
µM. Treatment of SW1353 cells for different durations indicated
that puerarin treatment significantly decreases cell viability in a
time-dependent manner (P<0.05, P<0.01 and P<0.001 compared
with untreated control for 48, 60 and 72 h, respectively; Fig. 1B).
 | Table I.Inhibitory rate of puerarin on the
proliferation of cells. |
Table I.
Inhibitory rate of puerarin on the
proliferation of cells.
| Puerarin (µM) | Optical density (570
nm) (mean ± standard deviation) | Inhibitory rate
(%) |
|---|
| 0 | 0.995±0.004 | 0 |
| 50 | 0.886±0.012 | 10.254±0.671 |
| 100 | 0.724±0.013 | 26.536±0.582 |
| 150 | 0.618±0.018 | 37.089±0.622 |
| 200 | 0.475±0.014 | 51.461±0.706 |
| 250 | 0.362±0.016 | 62.718±0.654 |
| 300 | 0.254±0.012 | 73.672±0.636 |
Puerarin induces apoptosis in SW1353
cells
To observe the effect of puerarin on cell apoptosis,
flow cytometric analysis was conducted. Puerarin treatment (48 h)
significantly increased the rate of apoptosis of SW1353 cells in a
dose-dependent manner (P<0.05, P<0.01 and P<0.001 compared
with the untreated control for 150, 200 and 250 µM, respectively;
Fig. 2A and B). Puerarin exposure
increased the apoptosis rate of SW1353 cells to 38.28% with a dose
of 200 µM.
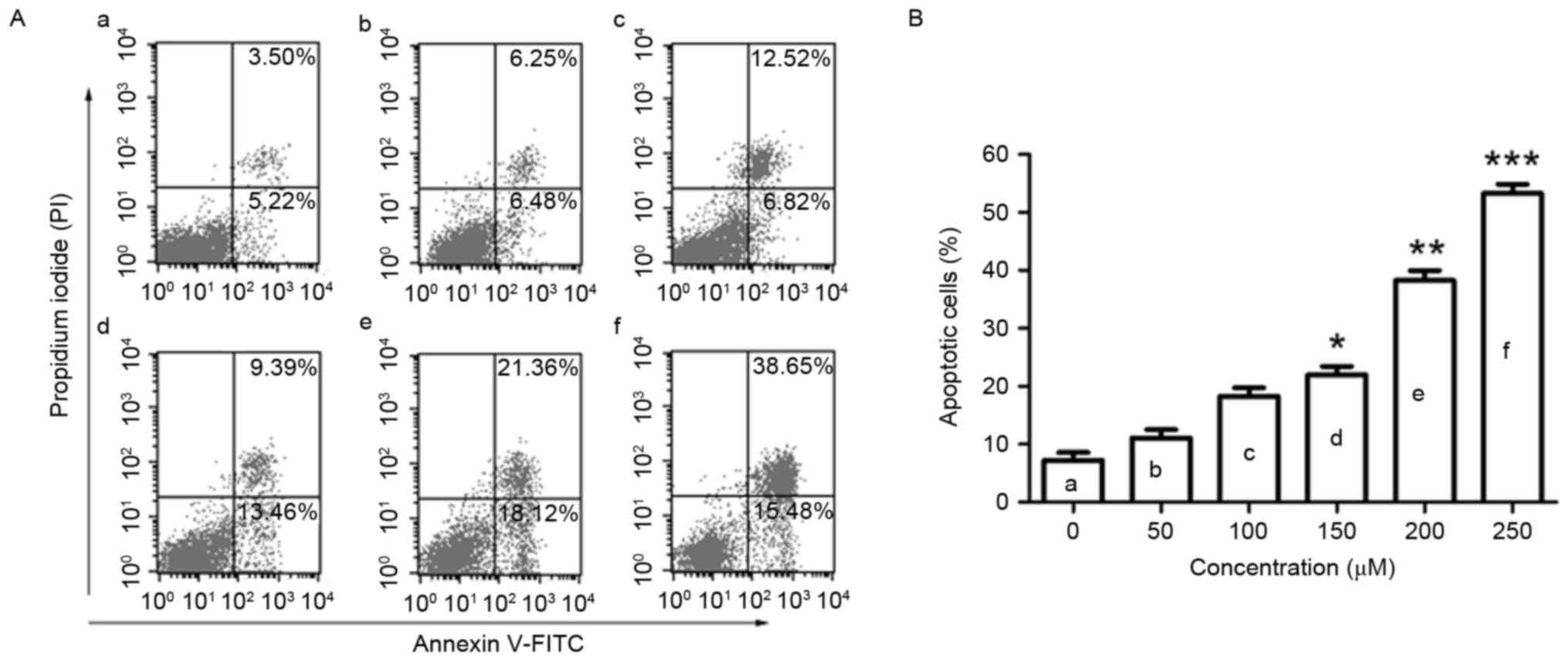 | Figure 2.Flow cytometric analysis of apoptosis
in SW1353 cells following puerarin treatment. (A) SW1353 cells were
treated with different concentrations of puerarin [(a) 0 µM, (b) 50
µM, (c) 100 µM, (d) 150 µM, (e) 200 µM, (f) 250 µM) for 48 h. A
flow cytometry assay was performed to determine the apoptosis rate,
and apoptotic cells (Annexin V+PI− and
Annexin V+PI+) are presented. (B) Percentage
of apoptotic cells. Data are presented as the mean ± standard
deviation (n=4). *P<0.05, **P<0.01 and ***P<0.001,
compared with the untreated control. PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Puerarin accelerates enzymatic
activity of caspase-3 and caspase-9
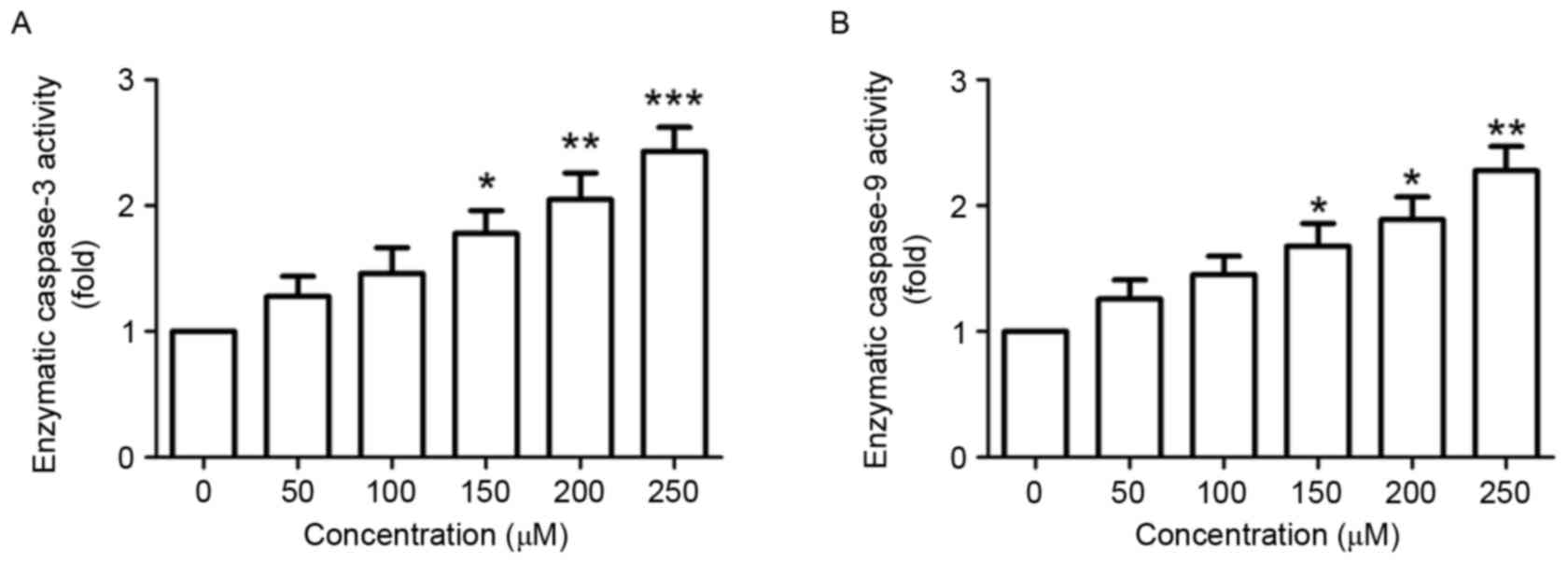
Next, the effect of puerarin on the enzymatic
activity of caspase-3 and −9 was investigated. The enzymatic
activity of caspase-3, an important marker of apoptosis (12), was significantly increased in SW1353
cells following puerarin stimulation in a dose-dependent manner
(P<0.05, P<0.01 and P<0.001 compared with the untreated
control for 150, 200 and 250 µM, respectively; Fig. 3A). Additionally, the enzymatic
activity of caspase-9 in was significantly increased in a
dose-dependent manner (P<0.05, P<0.05 and P<0.01 compared
with the untreated control for 150, 200 and 250 µM, respectively;
Fig. 3B).
Effect of puerarin on the expression
of apoptosis-associated protein in SW1353 cells
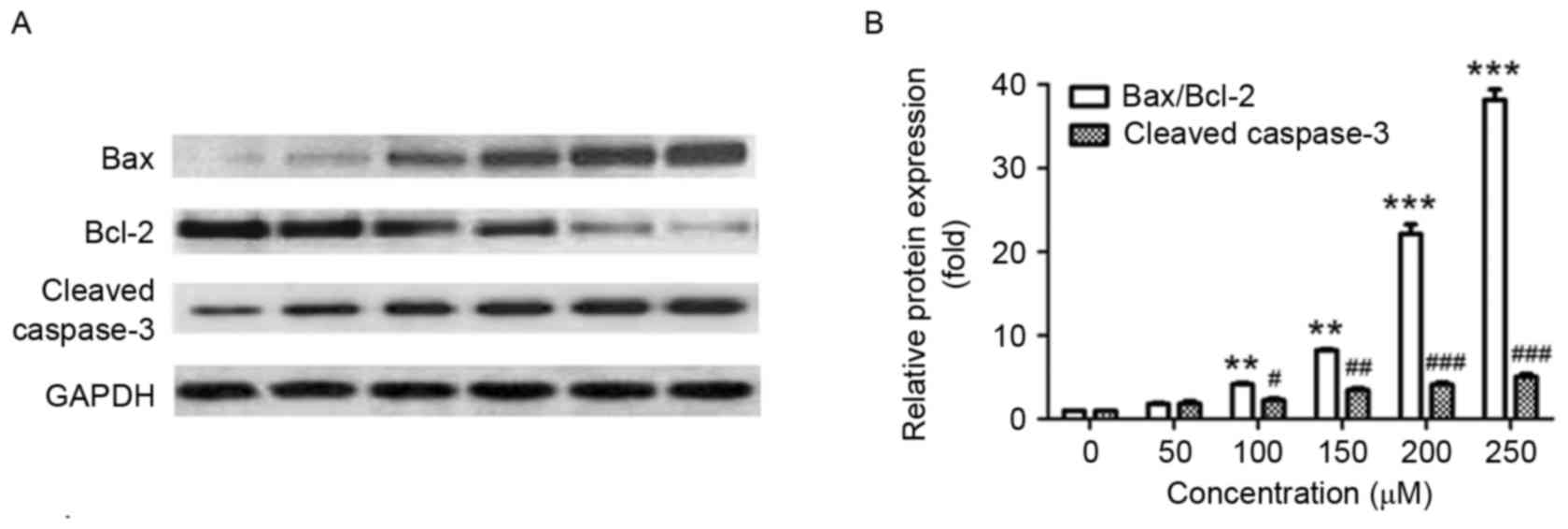
Next, the effect of puerarin on the expression of
apoptosis-associated proteins was investigated. In line with the
Annexin V-FITC/PI staining results, puerarin treatment
significantly increased the ratio of Bax/Bcl-2 compared with the
untreated control (P<0.01, P<0.01, P<0.001 and P<0.001
for 100, 150, 200 and 250 µM, respectively; Fig. 4A and B). In addition, exposure of
SW1353 cells to puerarin significantly increased the expression of
cleaved caspase-3 in a dose-dependent manner compared with the
untreated control (P<0.05, P<0.01, P<0.001 and P<0.001
for 100, 150, 200 and 250 µM, respectively; Fig. 4A and B). The Bax/Bcl2 ratio and
expression of cleaved caspase-3 increased ~20 and ~4-fold following
treatment with 200 µM puerarin, respectively (Fig. 4B).
Puerarin suppresses the P13K/Akt
signaling pathway in SW1353 cells
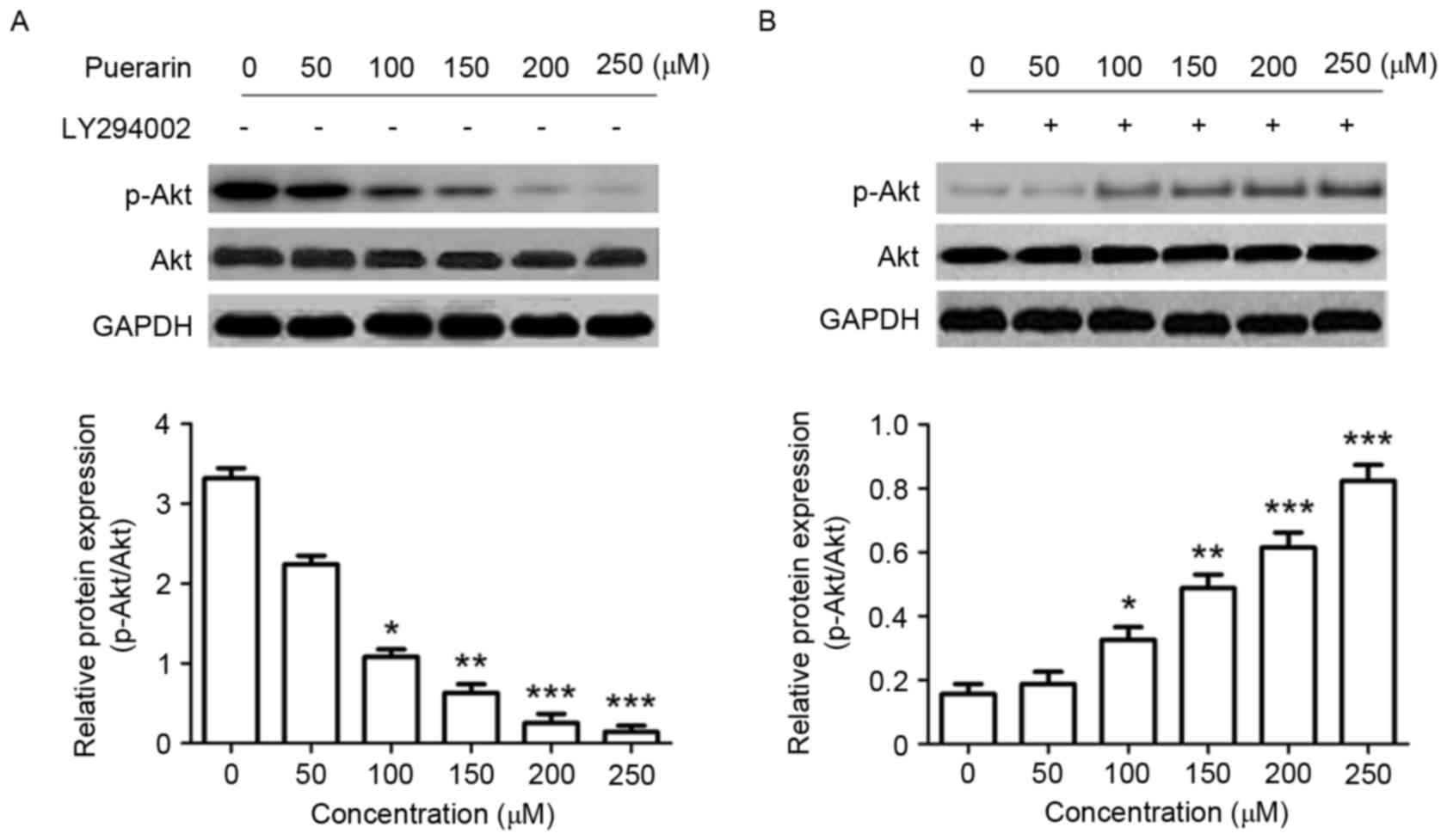
Changes in the P13K/Akt signaling pathway were
analyzed by western blotting in order to determine the underlying
molecular mechanisms of the effect of puerarin on SW1353 cells. The
expression level of p-Akt (Ser473), in addition to the ratio of
p-Akt/Akt was significantly decreased in response to puerarin
treatment compared with the untreated control (P<0.05,
P<0.01, P<0.001 and P<0.001 for 100, 150, 200, 250 µM,
respectively; Fig. 5A); however, the
P13K inhibitor LY294002 significantly abrogated this effect
(Fig. 5B).
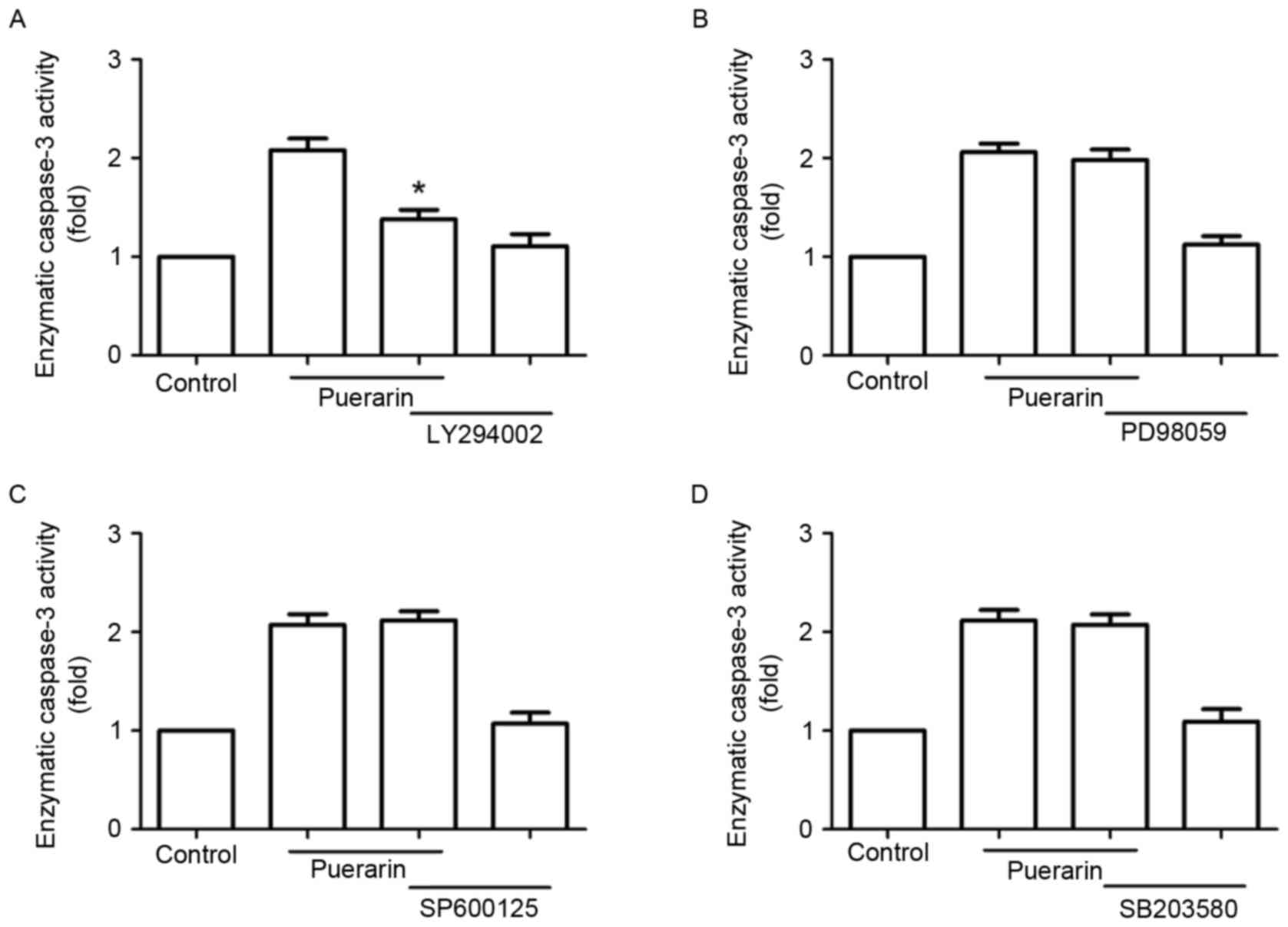
Next, SW1353 cells were treated with different
signaling inhibitors, including LY294002, PD98059, SP600125 and
SB203580 prior to treatment with puerarin. Notably, LY294002
abrogated the enzymatic activity of caspase-3 promoted by puerarin,
but not the extracellular signal-regulated kinase inhibitor
(PD98059), p38 mitogen-activated protein kinase inhibitor
(SB203580) or c-Jun N-terminal kinase inhibitor (SP600125)
(Fig. 6).
Discussion
Puerarin exhibits a variety of biological effects,
including anti-oxidant, anti-inflammatory and anti-apoptotic
activities (13). In the present
study, the antitumor activity of puerarin against human
chondrosarcoma was investigated, in addition to its possible
mechanisms of action, using SW1353 cells. The results indicated
that puerarin treatment induced apoptosis in SW1353 cells via
inhibition of the P13K/Akt signaling pathway.
Previous studies have demonstrated that puerarin
exerts protective biological effects by suppressing cell apoptosis;
puerarin treatment blocked cell apoptosis induced by
H2O2 in PC12 cells, which was associated with
a reduction in c-Myc expression, an increase in the Bcl-2/Bax ratio
and the inhibition of caspase-3 activation (14). Another study reported that puerarin
exhibited a protective effect against the lipopolysaccharide
(LPS)-induced apoptosis of H9c2 cardiomyocytes by reversing
LPS-induced downregulation of Bax and upregulation of Bcl-2
(15). In addition, puerarin afforded
protection against beta-amyloid-induced neurotoxicity by inhibiting
apoptosis in PC12 cells, which contributed to activation of the
PI3K-dependent signaling pathway in association with Akt
phosphorylation (16). Although
anti-apoptotic mechanisms have been demonstrated to be involved in
the protective effect of puerarin in the nervous and cardiovascular
system (17,18), in the present study, puerarin
demonstrated antitumor effects by reducing cell viability and
inducing cell apoptosis in SW1353 cells, which may be important for
the clinical treatment of patients with cancer.
In colon cancer HT-29 cells, puerarin treatment
regulated the expression of apoptosis-associated proteins,
including Bax, Bcl-2 and caspase-3 (5). A previous study demonstrated that the
antitumor activity of puerarin 6′-O-xyloside on the human lung
carcinoma A549 cell line, which was one of the major isoflavones of
Porites lobata. The mechanisms were associated with increased
levels of caspase-3, caspase-7, caspase-9 and Bax, and decreased
levels of Bcl-2 (19). Combined with
or without 5-fluorouracil, puerarin induced significant
proliferation suppression and apoptosis in Eca-109 esophageal
cancer cells in vitro and in vivo (20). In addition, puerarin inhibited
proliferation and induced apoptosis in U251 and U87 human
glioblastoma cell lines, and cell cycle arrest and DNA damage may
participate in the anticancer effects of puerarin (7). In SMMC-7721 hepatocellular carcinoma
cells, puerarin suppressed cell viability and induced cell
apoptosis (21). In human breast
cancer cell lines HS578T, MDA-MB-231 and MCF-7, Puerariae radix
isoflavones and their metabolites reduced cell growth and induced
cell apoptosis, suggesting that puerariae may act as a
chemotherapeutic agent against breast cancer (9). In keeping with previous reports, the
present study demonstrated that puerarin significantly inhibits the
proliferation of SW1353 cells in a time- and dose-dependent manner.
Additionally, the percentage of apoptotic cells and expression of
apoptosis-associated proteins were increased following puerarin
treatment, suggesting that induction of apoptosis may be the
primary mechanism of the anti-proliferative effect of puerarin in
SW1353 cells.
Apoptosis, also known as programmed cell death, is
characterized by two classical pathways: The death receptor pathway
and the mitochondrial pathway (22).
Caspase-9 is the initiating protein in the caspase cascade, and
caspase-3, a biomarker for cell apoptosis, is activated by
caspase-9 (23). In addition, Bax and
Bcl-2 are involved in the apoptotic process as pro-apoptotic
protein and anti-apoptotic proteins, respectively (24). The results of the present study
confirmed that apoptosis-associated mechanisms participated in the
suppressive effect of puerarin. In the present study, puerarin
significantly elevated the enzymatic activity of caspase-3 and
caspase-9. Furthermore, puerarin treatment increased the Bax/Bcl-2
ratio and promoted the expression of caspase-3 in SW1353 cells.
The molecular mechanisms underlying the effects of
puerarin pharmacological activities are complex, and different
studies led to different conclusions. A previous study demonstrated
that puerarin protected pancreatic β-cell survival via the P13K/Akt
signaling pathway (25).
Neuroprotective effects of puerarin against beta-amyloid-induced
neurotoxicity in PC12 cells were associated with activation of the
PI3K-dependent signaling pathway (26). Puerarin retarded the progression of
cardiac hypertrophy and apoptosis, which may be mediated by
blockade of the P13K/Akt and JNK signaling pathways (27). Conversely, puerarin demonstrated
anti-proliferative and pro-apoptotic effects by inhibiting the
P13K/Akt/nuclear factor κ-B cells pathway in human mantle cell
lines Z138 (6). In line with this
report, the results from the present study indicated that puerarin
inhibits activation of p-Akt, which was abrogated by the P13K
inhibitor LY294002, suggesting that the P13K/Akt signaling pathway
is involved in the anticancer effect of puerarin. These differences
observed in the effects of puerarin may be attributed to different
doses and treatment times of puerarin used in different animal and
cell models. Another reason may be that these signaling pathways
connect with each other to form networks (28), therefore they may be influenced by
each other.
In conclusion, the present study demonstrated that
puerarin treatment induced apoptosis in SW1353 cells via inhibition
of the P13K/Akt signaling pathway, which resulted in increased
expression of caspase-3 and a decreased Bcl-2/Bax ratio. These
findings suggested that puerarin may be a potential option as a
therapeutic drug for patients with chondrosarcoma.
References
|
1
|
Leddy LR and Holmes RE: Chondrosarcoma of
bone. Cancer Treat Res. 162:117–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Outani H, Hamada K, Imura Y, Oshima K,
Sotobori T, Demizu Y, Kakunaga S, Joyama S, Imai R, Okimoto T, et
al: Comparison of clinical and functional outcome between surgical
treatment and carbon ion radiotherapy for pelvic chondrosarcoma.
Int J Clin Oncol. 21:186–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maji AK, Pandit S, Banerji P and Banerjee
D: Pueraria tuberosa: A review on its phytochemical and therapeutic
potential. Nat Prod Res. 28:2111–2127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gan M and Yin X: Puerarin induced in
mantle cell lymphoma apoptosis and its possible mechanisms
involving multi-signaling pathway. Cell Biochem Biophys.
71:367–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JA, Li JQ, Shao LM, Yang Q, Liu BH,
Wu TF, Wu P, Yi W and Chen QX: Puerarin inhibits proliferation and
induces apoptosis in human glioblastoma cell lines. Int J Clin Exp
Med. 8:10132–10142. 2015.PubMed/NCBI
|
|
8
|
Yanagihara K, Ito A, Toge T and Numoto M:
Antiproliferative effects of isoflavones on human cancer cell lines
established from the gastrointestinal tract. Cancer Res.
53:5815–5821. 1993.PubMed/NCBI
|
|
9
|
Lin YJ, Hou YC, Lin CH, Hsu YA, Sheu JJ,
Lai CH, Chen BH, Lee Chao PD, Wan L and Tsai FJ: Puerariae radix
isoflavones and their metabolites inhibit growth and induce
apoptosis in breast cancer cells. Biochem Biophys Res Commun.
378:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu D, Mu S, Zhao D, Wang G, Chen Z, Ren H
and Fu Q: Puerarin attenuates glucocorticoid-induced apoptosis of
hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial
apoptotic pathways. Int J Mol Med. 36:345–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun
WJ and Gong HY: Puerarin stimulates proliferation and
differentiation and protects against cell death in human
osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt
activation. Phytomedicine. 20:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Konstantinidou AE, Givalos N, Gakiopoulou
H, Korkolopoulou P, Kotsiakis X, Boviatsis E, Agrogiannis G, Mahera
H and Patsouris E: Caspase-3 immunohistochemical expression is a
marker of apoptosis, increased grade and early recurrence in
intracranial meningiomas. Apoptosis. 12:695–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang B, Liu JH, Bao YM and An LJ:
Hydrogen peroxide-induced apoptosis in pc12 cells and the
protective effect of puerarin. Cell Biol Int. 27:1025–1031. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Y, Zhou H, Wu QQ, Li FF, Bian ZY,
Deng W, Zhou MQ and Tang QZ: Puerarin attenuates the inflammatory
response and apoptosis in LPS-stimulated cardiomyocytes. Exp Ther
Med. 11:415–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang HY, Liu YH, Wang HQ, Xu JH and Hu
HT: Puerarin protects PC12 cells against beta-amyloid-induced cell
injury. Cell Biol Int. 32:1230–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang G, Zhou L, Zhang Y, Dong M, Li X, Liu
J and Niu Y: Implication of the c-Jun-NH2-terminal kinase pathway
in the neuroprotective effect of puerarin against
1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12
cells. Neurosci Lett. 487:88–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen T, Chen H, Wang Y and Zhang J: In
vitro and in vivo antitumour activities of puerarin 6′-O-xyloside
on human lung carcinoma A549 cell line via the induction of the
mitochondria-mediated apoptosis pathway. Pharm Biol. 54:1793–1799.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Yang ZR, Guo XF, Song J, Zhang JX,
Wang J and Dong WG: Synergistic effects of puerarin combined with
5-fluorouracil on esophageal cancer. Mol Med Rep. 10:2535–2541.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|
|
24
|
Zheng TS, Hunot S, Kuida K, Momoi T,
Srinivasan A, Nicholson DW, Lazebnik Y and Flavell RA: Deficiency
in caspase-9 or caspase-3 induces compensatory caspase activation.
Nat Med. 6:1241–1247. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Shangguan Z, Liu Y, Wang J, Li X,
Yang S and Liu S: Puerarin protects pancreatic β-cell survival via
PI3K/Akt signaling pathway. J Mol Endocrinol. 53:71–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing G, Dong M, Li X, Zou Y, Fan L, Wang
X, Cai D, Li C, Zhou L, Liu J and Niu Y: Neuroprotective effects of
puerarin against beta-amyloid-induced neurotoxicity in PC12 cells
via a PI3K-dependent signaling pathway. Brain Res Bull. 85:212–218.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lavrik IN: Systems biology of apoptosis
signaling networks. Curr Opin Biotechnol. 21:551–555. 2010.
View Article : Google Scholar : PubMed/NCBI
|