Introduction
Rapid expansion of tumor mass results in inadequate
vessels, a depletion of nutrients and a local increase in necrosis.
This rapid increase in tumor growth is linked to cell death
resulting from a microenvironment that has regions of hypoxia and a
decreased extracellular pH (pHe). Extracellular acidosis (low pH)
is a tumor microenvironmental stressor that plays a critical role
in malignant transformation, progression and metastatic
dissemination (1). This
microenvironment is a complex ecology of cells and dynamic milieu
that provides pivotal clues on the mechanisms of tumor development
and progression. Tumor angiogenesis is achieved mainly through
sprouting from locally pre-existing vasculature and/or recruitment
of bone marrow-derived endothelial progenitor cells (BM-EPCs).
Emerging evidence implicates that the recruitment of BM-EPCs is
critical for tumor vasculogenesis since tumors may acquire their
vasculature by co-option of glomeruloid angiogenesis, vasculogenic
mimicry, or postnatal vasculogenesis (2–4).
Neovascularization in turn participates in supplying nutritional
support and oxygen to growing tumors (5). This relationship has been demonstrated
in a number of tumor types, including invasive breast (6), non-small cell lung (7) and prostate carcinoma (8).
Although tumor microenvironments achieve
neovascularization as the tumor grows, EPCs localize near the
periphery, reflecting the greater opportunity for adhesion in this
region owing to increased angiogenic activity and higher vascular
density (9). It has been demonstrated
that EPCs adhered preferentially near the tumor periphery,
coincident with the subsequent highest vascular density in a tumor
model using mouse embryonic EPCs (10). Including in livers with hepatocellular
carcinoma, there were more EPCs in the adjacent tissue (11). Notably, EPCs home in prior to the
arrival of tumor cells, promoting metastatic growth by forming
niches where cancer cells may locate and proliferate (12). It is proposed that tumor pHe may be
variable within a tumor with localized regions of acidity, and a pH
gradient from the periphery to the center of tumors was identified
to be coincided with the development of small, disseminated
necrosis in the tumor center (13,14). The
difference provides an avenue for the pHe and vasculogenesis of
BM-EPCs in tumors.
Since a series of experiments to identify the
effects of an acidic microenvironment utilizes pH 6.4–6.6 in
vitro (15–17), the present study aimed to detect the
vasculogenesis of BM-EPCs in vitro following cell exposure
to pHe 6.5 compared with a control of pHe 7.4. The present results
demonstrated that, compared with pHe 7.4, pHe 6.5 may significantly
induce BM-EPCs apoptosis by targeting the expression of B-cell
lymphoma 2 (Bcl2)/Bcl2 associated X-protein (Bax) and inhibiting
BM-EPCs proliferation, chemotactic migration, matrix adhesion and
tube formation through the modulation of vascular endothelial
growth factor (VEGF) receptor 2 (VEGFR2)-regulated protein kinase B
(Akt) and p38 mitogen activated protein kinase (MAPK) signaling
pathways.
Materials and methods
Isolation and cultivation of EPCs
The method of human EPCs isolation, cultivation and
identification was performed as described in a previous publication
(18). Human bone marrow was
collected from the drill holes of the pedicle during internal spine
fixation of patients with disc degenerative diseases were collected
from The First Affiliated Hospital of Sun Yat-Sen University
(Guangzhou, China) between October 2013 and September 2014 (10
patients; 5 males and 5 females; age range, 54–72 years; mean age,
61.27 years) at the time of surgery. Written informed consent for
human bone marrow collection was obtained from the patients, and
all procedures were performed in light of the guidance and approval
of the Research Ethics Committee of The First Affiliated Hospital
of Sun Yat-Sen University (no. 2008-55).
Cell culture
EPCs were cultured at 37°C with 5%
CO2/95% air in a humidified incubator, and experiments
were carried out with cells in exponential growth, cultured in an
acidic (pHe 6.5) or normal (pHe 7.4) medium for an indicated
period. The cells were plated and cultured in normal medium for 24
h prior to the medium being removed and replaced with normal (pHe
7.4) or acidic medium (pHe 6.5). The pH of the cell culture medium
was adjusted with HCl or NaOH to 6.5 or 7.4 during experiments.
Assessment of cell viability and
damage
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to evaluate living
cells by combining WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium,
monosodium salt] and 1-methoxy PMS, as described previously
(18). EPCs were cultured with EGM-2
medium in 96-well culture plates at 1×104 cells/well to
90% confluency, and then grown in medium at pHe 7.4 or pHe 6.5 for
24 h at 37°C. Subsequent to the 24-h incubation, CCK-8 was used in
line with the manufacturer's protocol and cell viability was
detected using a microplate reader at 450 nm.
Lactate dehydrogenase (LDH) is a stable cytosolic
enzyme present in numerous types of cell. LDH is released into the
media from damaged cells as a biomarker for cellular cytotoxicity
and cytolysis. The cellular viability may be assessed in terms of
LDH released from dead cells into the supernatant upon rupture of
cell membrane. For this purpose, CytoTox 96®
Non-Radioactive Cytotoxicity Assay kit (Promega Corporation,
Madison, WI, USA) was used according to the manufacturer's
protocol. Briefly, 100 µl of lysis solution was added into wells
containing the untreated control cells prior to the assay to obtain
maximum LDH release. To determine the LDH content, 50 µl of
supernatant with 50 µl of substrate solution was mixed in 96-well
plates. Subsequent to a 30 min incubation at room temperature (RT)
protected from light, the enzymatic reaction was stopped following
the addition of a stop solution. The absorbance was recorded by
spectrophotometry at 490 nm using a microplate reader. The
percentage of cytotoxicity was calculated in the light using the
following equation:
Percentage of cytotoxicity=Absorbance of
experimental samplesAbsorbance of maximum LDH releasex100
Calcein-AM/ethidium homodimer-1
cell-survival (live-dead) assay
Cell viability was determined using a
Calcein-AM/Ethidium homodimer-1 (EthD-1) Dual-Staining Assay kit
(Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The cells were cultured in medium at pH 7.4 or pH 6.5 for 24
h at 37°C. Following treatment, the culture medium was removed and
cells were gently rinsed with warm PBS. Subsequently, 2 µM
calcein-AM and 4 µM EthD-1 in 100 µl PBS was added to each culture
well and incubated at 37°C for 30 min. Fluorescence signals of the
cells were observed under a fluorescence microscope.
EPC apoptosis assay by flow cytometry
analysis
Human EPCs were cultured with EGM-2 in 24-well
culture plates at 1×105 cells/well for 24 h to ~90%
confluency, then treated with medium of pHe 7.4 or pHe 6.5 for 24 h
at 37°C. Cells were gently trypsinized, washed with PBS,
re-suspended in binding buffer, and then incubated with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) at RT
for 10 min in the dark. Flow cytometry analysis was performed to
estimate cell apoptosis and necrosis. The percentage of apoptotic
cells (Annexin V positive and PI negative) and necrotic cells
(Annexin V and PI positive) was investigated.
EPCs matrix adhesion assay
The cell-matrix adhesion assay was used as
previously described (18). Human
BM-EPCs were cultured for 24 h to 90% confluence, and treated with
pHe 7.4 or pHe 6.5 for 24 h at 37°C as above. Subsequently, BM-EPCs
at 1×104 cells/well were replated onto fibronectin (BD
Biosciences, San Jose, CA, USA) -coated 96-well culture plates, and
incubated for 30 min at 37°C. Following incubation, PBS was used to
remove non-adherent cells by washing 3 times. The adherent cells
were fixed with 4% paraformaldehyde for 20 min, washed with PBS,
and stained with 100 µl 0.1% crystal violet for 30 min at RT.
Adherent cells were counted under a phase contrast microscope by
independent investigators.
EPCs migration assay
The cell migration assay was performed as described
previously (18). Cell migration was
detected using the Transwell system (Costar; Corning Life Sciences,
Acton, MA, USA) with 6.5 mm diameter polycarbonate filters (8 µm
pore size). BM-EPCs were briefly seeded onto chemotaxis filters in
100 µl EBM-2 medium, and 600 µl of pHe 7.4 or pHe 6.5 EGM-2 medium
was added to the lower chamber to evaluate the effect of acidosis
on cellular migration.
Subsequent to a 12-h migration period, non-migrating
cells were completely removed from the top surface of the membrane
by cotton swab, and attached cells were fixed with 95% ethanol for
10 min and stained with 0.1% crystal violet. The plate was immersed
in fresh tap water to remove excess dye. Cells that have migrated
through the filter pores from the underside of the filter were then
counted in 10 random fields of view under light microscopy at ×100
magnification (Carl Zeiss Microimaging, Thornwood, NY, USA).
EPCs capillary-like tube formation
assay
The capillary-like tube formation assay was
performed as previously described (18). Matrigel (BD Biosciences, San Jose, CA,
USA) was added to each 96-well plate at 4°C and allowed to
polymerize in an incubator at 37°C for 30 min. A fraction of
1.2×104 cells/well were seeded into the Matrigel and
incubated for 18 h under the condition of pHe 7.4 or pHe 6.5 to
allow capillary tube formation. The images of the capillary network
were recorded and the tube lengths were counted using Scion Image
software 4.03 (Scion Corp., Frederick, MD, USA).
ELISA
The concentrations of VEGF, basic fibroblast growth
factor (bFGF) and interleukin-8 (IL-8) in the culture medium of
BM-EPCs were determined using a commercial human ELISA kit based on
appropriate and validated sets of monoclonal antibodies (ExCell
Biology Inc., Shanghai, China). BM-EPCs were cultured for 24 h to
90% confluency and treated with a medium of pHe 6.5 or pHe 7.4 for
24 h. Following treatment, the culture medium was collected and
stored at −80°C to be used for the ELISA assay. All experiments
were performed in at least triplicate and the absorbance was
measured at 450 nm.
Western blot analysis
Proteins were extracted from BM-EPCs using
radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The protein concentration was measured
using a BCA protein assay kit by spectrophotometry at 562 nm
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). In total, 30 µg denatured proteins were
subjected to 10% SDS-PAGE. Separated proteins were transferred to a
polyvinylidene difluoride membrane. Subsequent to being blocked
with 5% BSA buffer for 1 h, the membrane was sequentially incubated
with primary antibodies against p-VEGFR2 (#2474), VEGFR2 (#9698),
p-Akt (#4060), Akt (#4691), p-p38 MAPK (#4511), p38 MAPK (#8690)
(dilution for all, 1:1,000; all from Cell Signaling Technology,
Inc., Danvers, MA, USA) under gentle agitation overnight at 4°C.
Following washing, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody at a dilution of 1:2,000
(#7074; Cell Signaling Technology Inc.) for 1 h at RT.
Immunoreactive bands were detected using enhanced chemiluminescence
reagents (GE Healthcare Life Sciences, Marlborough, MA, USA). The
values of band intensities were quantified by Quantity One version
4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to
the respective protein loading controls. All immunoblots are
representative of ≥3 independent experiments.
Statistical analysis
Numerical data are presented as the mean ± standard
deviation from ≥3 individual experiments with cells from different
donors. Statistical comparisons between groups were performed by
one-way analysis of variance followed by the Student's t-test using
SPSS 16.0 software package (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Acidic stress induces cell death in
BM-EPCs
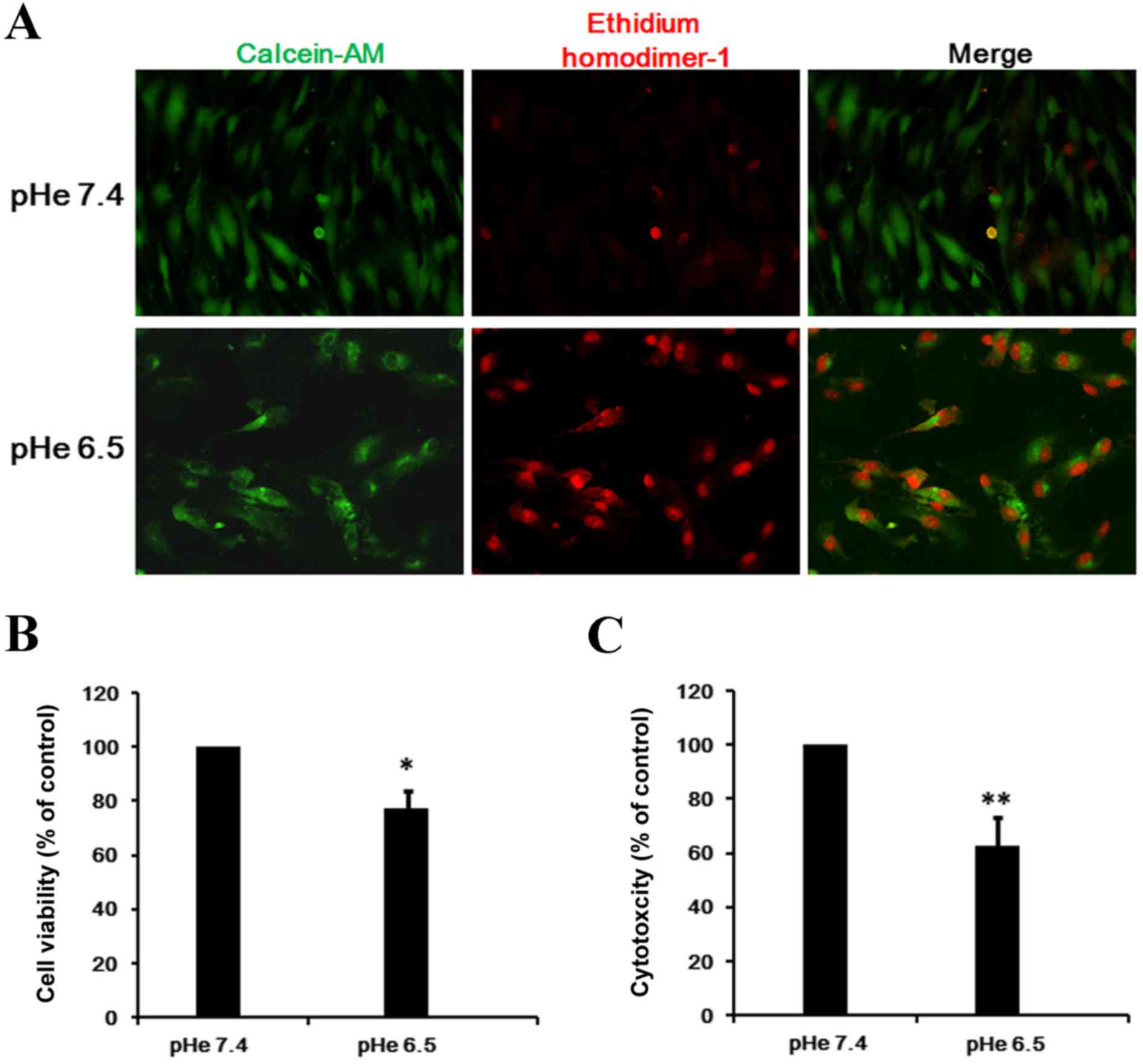
EPCs were cultured in serum-free conditions in an
acidic (pHe 6.5) or normal (pHe 7.4) medium for 48 h. The cells
were then stained with calcein AM to visualize live cells and
EthD-1 to visualize dead cells. When incubated in pHe 7.4 almost
all cells were alive, however exposure to pHe 6.5 for 24 h induced
a significant level of cell injury, as indicated by cell
contraction and nuclear membrane creasing (Fig. 1A).
In addition, the present study quantified the rate
of cell survival under acidic conditions. As presented in Fig. 1B, EPCs grown at pHe 6.5 suppressed
EPCs proliferation, and cell number after 48 h was 51.4% of pHe 7.4
(P=0.039). Furthermore, the rate of cytotoxicity significantly
increased to 61.1% following exposure to pHe 6.5 compared with
exposure to pHe 7.4, which was demonstrated by elevated levels of
LDH (Fig. 1C; P=0.004).
Acidic stress induces cell apoptosis
of BM-EPCs
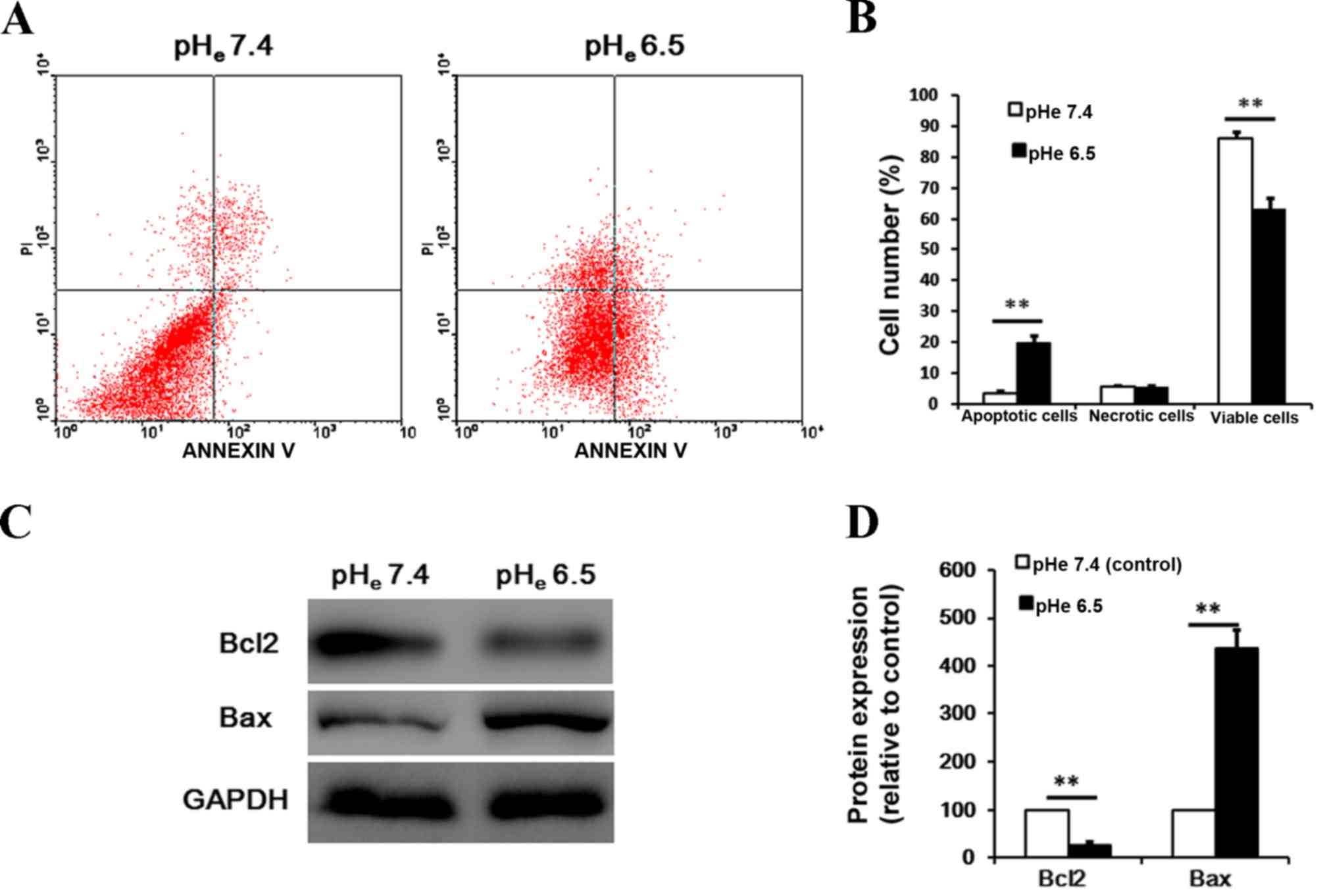
To observe the possible effects of acidic stress on
cell apoptosis, the present study examined cell apoptosis by
Annexin V-FITC and PI double staining flow cytometry. As presented
in Fig. 2A and B, the apoptosis rate
was significantly increased (from 3.2 to 19.7%) subsequent to cells
being exposed to pHe 6.5 for 24 h, compared with cells exposed to
pHe 7.4 (P<0.001). Furthermore, western blot analysis
demonstrated that acidic stress caused a profound upregulation of
pro-apoptotic Bax and a downregulation of anti-apoptotic Bcl-2
expression in BM-EPCs (Fig. 2C and D;
P<0.001).
Acidic stress inhibits migration and
cell adhesion to the extracellular matrix (ECM) of BM-EPCs
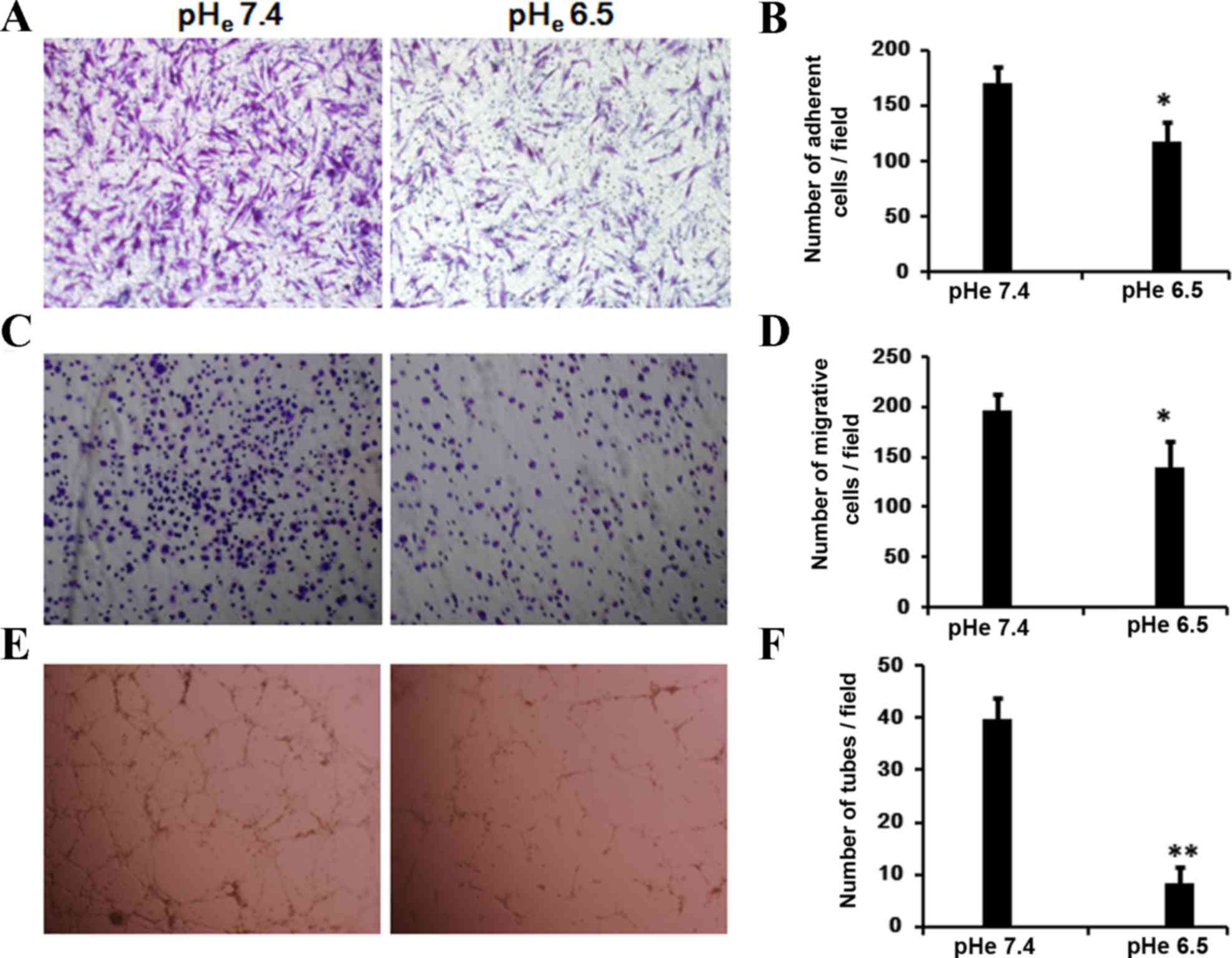
It is known that cell mobility and maintenance of
cell survival signaling are essential for tubule formation. To
evaluate the migratory abilities of BM-EPCs under the acidic stress
in vitro, the present study examined the cellular response
of BM-EPCs to migration using a Transwell assay. The cells
demonstrated an impaired ability to migrate (Fig. 3A and B) in the medium of pHe 6.5,
compared with the control group (pHe 7.4; P=0.023).
To investigate the possibility that acidic stress
affects the binding of BM-EPCs to the extracellular matrix (ECM),
cells were incubated at pHe 6.5 or pHe 7.4 for 24 h. Subsequent to
re-plating onto human fibronectin-coated culture plates, EPCs
exhibited a significant decrease in the number of adhesive cells
following a 30 min incubation (Fig. 3C
and D; P=0.028).
Acidic stress inhibits capillary-like
tube formation of BM-EPCs
The aforementioned observation that acidic stress
affects the cell mobility prompted us to examine its possible
effect on vasculogenesis, which was investigated using the
capillary tube formation assay on Matrigel. When EPCs were seeded
on growth factor reduced, two-dimensional Matrigel, defined
tube-like structures were formed in pHe 7.4 (Fig. 3E and F; P=0.002). However, the
majority of cells cultured in medium at pHe 6.5 remained in
individual clusters or ovoid colonies. These data suggest that
acidic stress inhibits capillary-like tube formation of BM-EPCs
in vitro.
Acidic stress inhibits the secretion
of VEGF, bFGF, and IL-8 in BM-EPCs
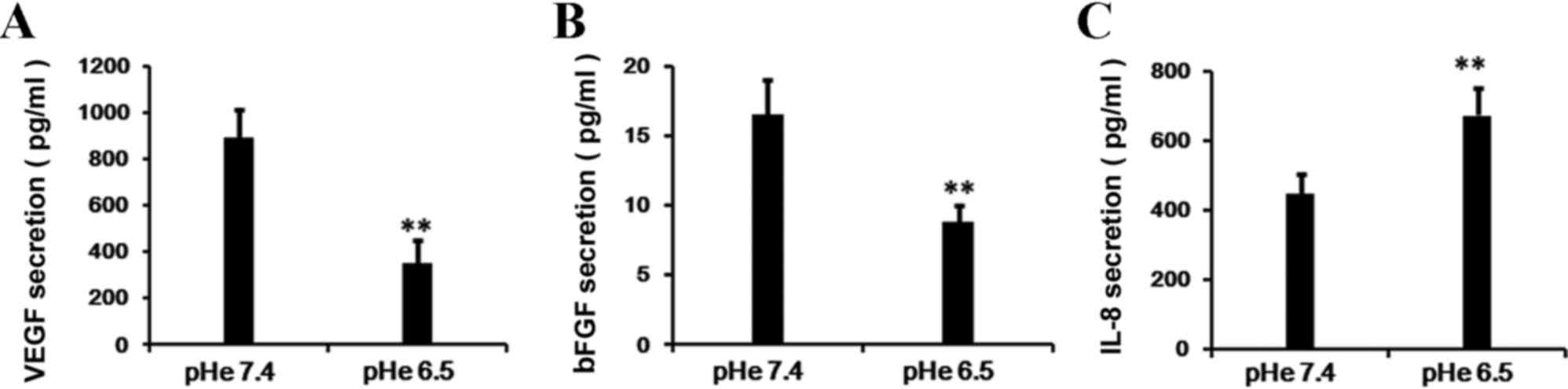
Since VEGF alters the marrow microenvironment from a
quiescent state to a pro-angiogenic and pro-tumorigenic
environment, it plays a pivotal role in the regulation of
angiogenesis as well as cell function. Therefore, the present study
assessed the secretion level of VEGF in the supernatant of BM-EPCs.
Subsequent to a 24-h incubation, the production of VEGF in the
supernatant of BM-EPCs was significantly reduced in the acidic
medium treatment (pHe 6.5) compared with the control group (pHe
7.4; Fig. 4A; P=0.005). Additional
experiments revealed that the secretion of bFGF in cells also
declined markedly following exposure to acidic stress (pHe 6.5)
compared with the normal conditions (pHe 7.4; Fig. 4B; P=0.007). However, the IL-8
secretion was activated under the acidic stress in BM-EPCs
(Fig. 4C; P=0.009).
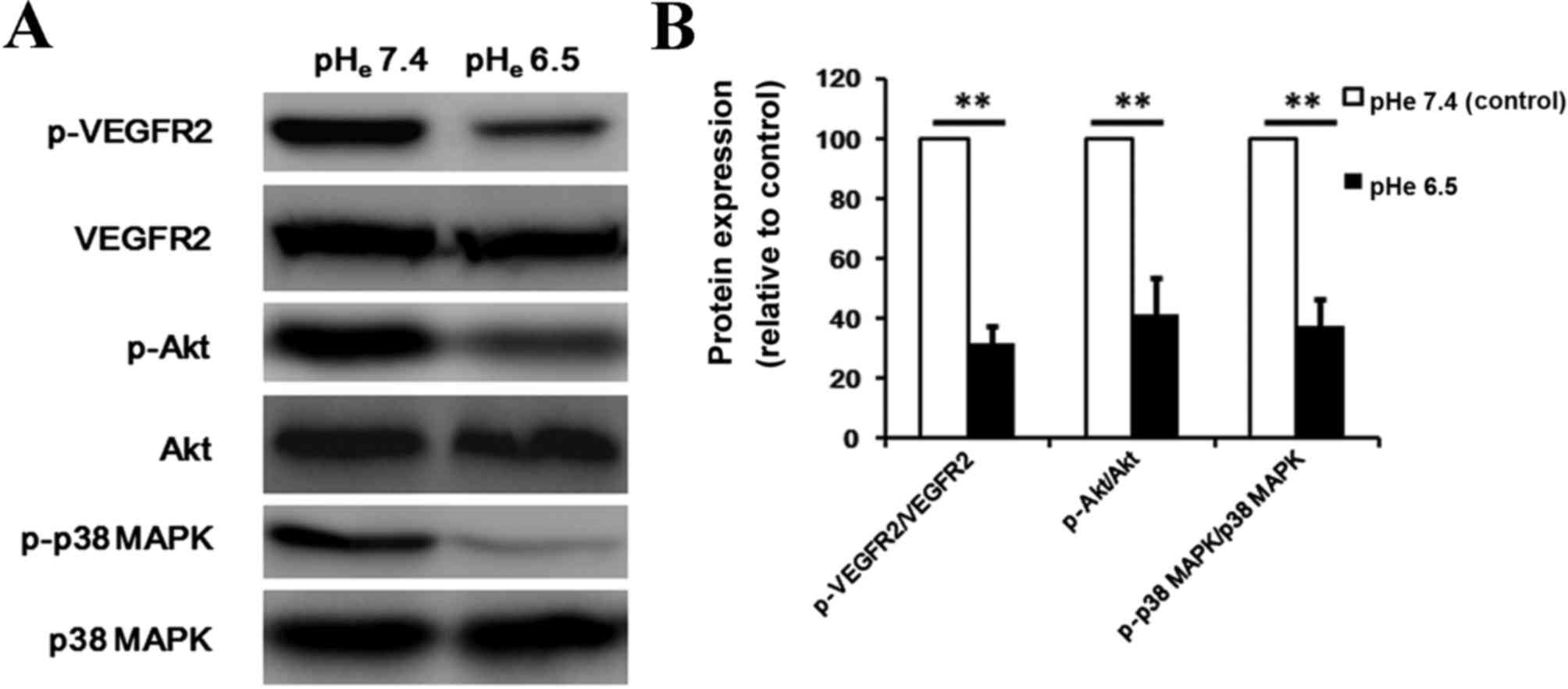
Acidic stress inhibits the
phosphorylation of VEGFR2, Akt, and p38 MAPK
Interaction of VEGF with VEGFR2 leads to the
activation of various downstream signaling molecules responsible
for EPCs proliferation, migration and survival. To additionally
delineate the mechanisms that contribute to the angiogenesis
inhibition effect of acidic stress, the present study examined the
signaling molecules involved in the VEGF pathway using western blot
analysis. Under the conditions used in the present experiments, the
phosphorylation of VEGFR2 was suppressed by acidic stress (Fig. 5A; P=0.001). Thus, the anti-angiogenic
property of acidic extracellular conditions may be in part due to
the inhibition of VEGFR2. Additionally, upon exploration of the key
pathway components that drive the endothelial cell function in
angiogenesis, the present study observed that acidic extracellular
conditions may effectively repress VEGF-triggered activation of the
signaling cascade, including phosphorylated Akt (P=0.001) and
phosphorylated p38 MAPK (P=0.001) in BM-EPCs (Fig. 5). This result supports that acidic
stress may suppress tumor angiogenesis by blocking these signaling
pathways.
Discussion
Acidosis was identified as an important stress
factor triggering apoptosis in coronary endothelial cells under
ischemic conditions through activation of caspase-12 in a previous
study (19). In the present study, it
was identified that acidic stress may induce cell apoptosis, and
inhibit cell proliferation, matrix adhesion, and migration and
markedly reduce VEGF expression and the capacity to incorporate
into the functional vascular networks in BM-EPCs.
Tumor neovascularization is a precisely coordinated
process characterized by vessel expansion in the early phases and
extensive neovessel formation in rapidly growing tumors (20). Importantly, it has been suggested that
vasculogenesis by EPCs as well as angiogenesis plays a critical
role in the production of blood vessels in tumor microenvironments
(18,21). A kinetic analysis of EPC contribution
as a function of tumor growth demonstrated that EPCs are recruited
to the tumor periphery preceding neovessel formation, and then EPCs
differentiate into endothelial cells and incorporate into a subset
of sprouting tumor neovessels luminally (4). EPCs localize near the periphery,
reflecting the greater opportunity for adhesion in this region
owing to increased angiogenic activity and higher vascular density
(9). Interestingly, within the
necrotic areas of a tumor, glycolysis and cessation of
CO2 production and proton-binding structures are exposed
to alleviate the acidosis of the tissue during sustained necrosis
(22). The present results suggest
that acidic stress may suppress the angiogenesis of EPCs that may
result in the inhibition of neovasculogenesis in tumor, and pHe may
be the reason of EPCs localize near the periphery and higher
vascular density in periphery region of a tumor.
It is reported that hypercarbic acidosis enhances
the mRNA expression of VEGF and the secretion of bFGF (23). The effect of acidosis on the
inhibition of endothelial cell function may be explained by
different mechanisms other than enhanced expression of VEGF and
bFGF, including diminished affinity of the growth factors for their
associated receptors, diminished receptor numbers, or inhibition of
the intracellular signals triggered by the agonist-receptor
interaction (24–26). The present study observed that the
level of VEGF production dramatically decreased in BM-EPCs
following inoculation in the condition of pHe 6.5 compared with pHe
7.4, which may be through the inhibition of VEGFR-2 expression.
Conflicting results have been produced in previous
studies designed to investigate whether acidic stress (pHe <7.0)
may promote invasive growth and metastatic dissemination of tumors
(27). Human melanoma cells cultured
in an acidic environment in vitro were identified to show
enhanced invasiveness compared with the normal condition (28), and increasing the pH of metastatic
breast tumors was associated with reduced formation of spontaneous
metastases in vivo (29).
However, enhanced invasiveness was not observed following acidosis
exposure in similar experiments with rodent fibrosarcoma cell lines
(30). However, emerging evidence
suggests that solid tumors are commonly characterized by a unique
pathophysiological microenvironment, and extracellular acidosis
(low pH) is a typical tumor microenvironmental stressor (31–33). Acute
acidosis has been reported to inhibit proliferation and increase
apoptosis of tumor cells (34,35), as
well as inhibit angiogenesis in the aortic ring vessel outgrowth
model (36). The present results
demonstrated that severe acidosis led to marked cell death, which
may inhibit cellular activities including cell-matrix adhesion,
chemotactic migration and capillary tube formation in BM-EPCs.
VEGF is one of the most potent angiogenic cytokines
that has strong abilities to promote proliferation of vascular
cells. It was identified that EPCs provide both instructive
(release of angiogenic cytokines) and structural (vessel
incorporation and stabilization) functions that facilitate the
initiation of vessel formation at the site of tumor
neovasculogenesis. The recruitment of EPCs to the tumor bed
contributed to the initiation of a proangiogenic program.
Meanwhile, it was also identified that the activation of VEGFR2,
Akt and p38 MAPK was dramatically attenuated by the extracellular
acidosis. Therefore, the present data demonstrates that acidosis
inhibits cell functional activities, which may be due to a
mechanism that blocks the VEGF/VEGFR2 axis, specifically targeting
Akt and p38 MAPK signaling pathways.
In summary, the present study demonstrates that
acidic stress inhibits the angiogenic functions of BM-EPCs that are
required for tumor neovascularization to occur. Additional studies
on the characterization of molecular mechanisms by which various
pHe conditions modulate the function and gene expression of BM-EPCs
will be necessary to reveal the pathophysiological relevance of
these findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272938 and
81402227), and the National Natural Science Foundation of Guangdong
(grant no. 2014A030310157).
References
|
1
|
Kallinowski F, Schlenger KH, Runkel S,
Kloes M, Stohrer M, Okunieff P and Vaupel P: Blood flow,
metabolism, cellular microenvironment, and growth rate of human
tumor xenografts. Cancer Res. 49:3759–3764. 1989.PubMed/NCBI
|
|
2
|
De Palma M and Naldini L: Role of
haematopoietic cells and endothelial progenitors in tumour
angiogenesis. Biochim Biophys Acta. 1766:159–166. 2006.PubMed/NCBI
|
|
3
|
Spring H, Schuler T, Arnold B, Hämmerling
GJ and Ganss R: Chemokines direct endothelial progenitors into
tumor neovessels. Proc Natl Acad Sci USA. 102:18111–18116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi
JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA,
Benezra R and Mittal V: Bone marrow-derived endothelial progenitor
cells are a major determinant of nascent tumor neovascularization.
Genes Dev. 21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collet G, Skrzypek K, Grillon C, Matejuk
A, El Hafni-Rahbi B, Lamerant-Fayel N and Kieda C: Hypoxia control
to normalize pathologic angiogenesis: Potential role for
endothelial precursor cells and miRNAs regulation. Vascul
Pharmacol. 56:252–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macchiarini P, Fontanini G, Hardin MJ,
Squartini F and Angeletti CA: Relation of neovascularisation to
metastasis of non-small-cell lung cancer. Lancet. 340:145–146.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
9
|
Davidoff AM, Ng CY, Brown P, Leary MA,
Spurbeck WW, Zhou J, Horwitz E, Vanin EF and Nienhuis AW: Bone
marrow-derived cells contribute to tumor neovasculature and, when
modified to express an angiogenesis inhibitor, can restrict tumor
growth in mice. Clin Cancer Res. 7:2870–2879. 2001.PubMed/NCBI
|
|
10
|
Vajkoczy P, Blum S, Lamparter M,
Mailhammer R, Erber R, Engelhardt B, Vestweber D and Hatzopoulos
AK: Multistep nature of microvascular recruitment of ex
vivo-expanded embryonic endothelial progenitor cells during tumor
angiogenesis. J Exp Med. 197:1755–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang
L, Chen J and Ding Y: Identification and clinical significance of
mobilized endothelial progenitor cells in tumor vasculogenesis of
hepatocellular carcinoma. Clin Cancer Res. 13:3814–3824. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dome B, Hendrix MJ, Paku S, Tóvári J and
Tímár J: Alternative vascularization mechanisms in cancer:
Pathology and therapeutic implications. Am J Pathol. 170:1–15.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kallinowski F and Vaupel P: pH
distributions in spontaneous and isotransplanted rat tumours. Br J
Cancer. 58:314–321. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerweck LE and Seetharaman K: Cellular pH
gradient in tumor versus normal tissue: Potential exploitation for
the treatment of cancer. Cancer Res. 56:1194–1198. 1996.PubMed/NCBI
|
|
15
|
Hjelmeland AB, Wu Q, Heddleston JM,
Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan
A and Rich JN: Acidic stress promotes a glioma stem cell phenotype.
Cell Death Differ. 18:829–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dietl K, Renner K, Dettmer K, Timischl B,
Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart
LA, Oefner PJ, et al: Lactic acid and acidification inhibit TNF
secretion and glycolysis of human monocytes. J Immunol.
184:1200–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar S, Reusch HP and Ladilov Y: Acidic
pre-conditioning suppresses apoptosis and increases expression of
Bcl-xL in coronary endothelial cells under simulated ischaemia. J
Cell Mol Med. 12:1584–1592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang S, Peng L, Tang Y, Zhang L, Guo W,
Zou X and Peng X: Hypoxia of PC-3 prostate cancer cells enhances
migration and vasculogenesis in vitro of bone marrow-derived
endothelial progenitor cells by secretion of cytokines. Oncol Rep.
29:2369–2377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar S, Kasseckert S, Kostin S, Abdallah
Y, Schafer C, Kaminski A, Reusch HP, Piper HM, Steinhoff G and
Ladilov Y: Ischemic acidosis causes apoptosis in coronary
endothelial cells through activation of caspase-12. Cardiovasc Res.
73:172–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryschich E, Schmidt J, Hämmerling GJ, Klar
E and Ganss R: Transformation of the microvascular system during
multistage tumorigenesis. Int J Cancer. 97:719–725. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn GO and Brown JM: Role of endothelial
progenitors and other bone marrow-derived cells in the development
of the tumor vasculature. Angiogenesis. 12:159–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaupel PW, Frinak S and Bicher HI:
Heterogeneous oxygen partial pressure and pH distribution in C3H
mouse mammary adenocarcinoma. Cancer Res. 41:2008–2013.
1981.PubMed/NCBI
|
|
23
|
D'Arcangelo D, Facchiano F, Barlucchi LM,
Melillo G, Illi B, Testolin L, Gaetano C and Capogrossi MC:
Acidosis inhibits endothelial cell apoptosis and function and
induces basic fibroblast growth factor and vascular endothelial
growth factor expression. Circ Res. 86:312–318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dairaghi DJ, Oldham ER, Bacon KB and
Schall TJ: Chemokine receptor CCR3 function is highly dependent on
local pH and ionic strength. J Biol Chem. 272:28206–28209. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
French AR, Tadaki DK, Niyogi SK and
Lauffenburger DA: Intracellular trafficking of epidermal growth
factor family ligands is directly influenced by the pH sensitivity
of the receptor/ligand interaction. J Biol Chem. 270:4334–4340.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmedtje JF Jr, Liu WL and Chen Y: pH is
critical to the regulation of expression of the beta 2-adrenergic
receptor gene in hypoxia. Biochim Biophys Acta. 1314:25–33. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rofstad EK: Microenvironment-induced
cancer metastasis. Int J Radiat Biol. 76:589–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martínez-Zaguilán R, Seftor EA, Seftor RE,
Chu YW, Gillies RJ and Hendrix MJ: Acidic pH enhances the invasive
behavior of human melanoma cells. Clin Exp Metastasis. 14:176–186.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robey IF, Baggett BK, Kirkpatrick ND, Roe
DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby
RA and Gillies RJ: Bicarbonate increases tumor pH and inhibits
spontaneous metastases. Cancer Res. 69:2260–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuvier C, Jang A and Hill RP: Exposure to
hypoxia, glucose starvation and acidosis: Effect on invasive
capacity of murine tumor cells and correlation with cathepsin (L +
B) secretion. Clin Exp Metastasis. 15:19–25. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tomida A and Tsuruo T: Drug resistance
mediated by cellular stress response to the microenvironment of
solid tumors. Anticancer Drug Des. 14:169–177. 1999.PubMed/NCBI
|
|
32
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.PubMed/NCBI
|
|
33
|
Flacke JP, Kumar S, Kostin S, Reusch HP
and Ladilov Y: Acidic preconditioning protects endothelial cells
against apoptosis through p38- and Akt-dependent Bcl-xL
overexpression. Apoptosis. 14:90–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Putney LK and Barber DL: Na-H
exchange-dependent increase in intracellular pH times G2/M entry
and transition. J Biol Chem. 278:44645–44649. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smallbone K, Maini PK and Gatenby RA:
Episodic, transient systemic acidosis delays evolution of the
malignant phenotype: Possible mechanism for cancer prevention by
increased physical activity. Biol Direct. 5:222010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burbridge MF, West DC, Atassi G and Tucker
GC: The effect of extracellular pH on angiogenesis in vitro.
Angiogenesis. 3:281–288. 1999. View Article : Google Scholar : PubMed/NCBI
|