Introduction
Chemotherapy is one of the most effective and
widespread methods for the treatment of cancers (1). Five-fluorouracil might be the
outstanding representative among these methods (2). Five-fluorouracil is an effective
treatment for cancers of the colon, breast, stomach, head and neck
and is particularly effective in the management of liver cancer
(3,4).
The mechanism of the cytotoxicity of 5-FU has been ascribed to the
misincorporation of fluoronucleotides into RNA and DNA and to the
inhibition of the nucleotide synthetic enzyme thymidylate synthase
(5). When used as a monotherapy for
hepatocellular carcinoma (HCC) patients, 5-FU results in
improvements of median survival times by 14 weeks (6). However, 5-FU treatment results in
numerous side effects due to its poor selectivity for tumor cells
over normal cells; 5-FU not only effectively inhibits the
proliferation of tumor cells but also kills normal cells.
Clinically, 5-FU treatment leads to the dysfunction of organs that
include the heart, liver and kidney. Treatment with 5-FU also
induces myelosuppression and immunosuppression (7–10).
Moreover, the administration of routine doses of 5-FU of patients
is typically accompanied by diarrhea, nausea, vomiting, poor
appetite and low blood counts (11).
Therefore, it is essential to develop new drugs to prevent the
unwanted side effects induced by 5-FU in cancer patients while
simultaneously enhancing its efficacy against tumors (12).
Calcineurin (Cn) is the only
Ca2+/calmodulin (CaM)-dependent serine/threonine protein
phosphatase. Cn is a heterodimer composed of a 61-kDa catalytic
subunit (CnA) and a 19-kDa regulatory subunit (CnB) (13,14).
Recent research has shown that Cn is necessary for the inhibition
of tumor outbreaks, and genetic and pharmacological suppression of
the function of the calcineurin/nuclear factor of activated T cells
(NFAT) promotes tumor formation in mouse skin (15). Cn inhibitors, such as cyclosporin A
(CsA), might increase the risk of squamous cell carcinomas in organ
transplant recipients to a greater extent than in the normal
population (16). CnB was
traditionally thought to regulate the phosphatase activity of the
calcineurin A subunit (17,18). However, in recent years, it has been
shown that CnB has functions that are independent of its role as
the regulatory subunit of CnA. For example, CnB is indispensible
for the positive selection of thymocytes; however, CnB is
unnecessary for negative selection (18). CnB is also necessary for enabling
centrioles to retain pricentriolar material (PCM) and to organize
the interphase aster in Drosophila Melanogaster neuroblasts
(19). Moreover, CnB can potentiate
the activation of procaspase-3 by accelerating its proteolytic
maturation (20). Previous work in
our lab has revealed that intraperitoneal injection of CnB prolongs
the survival of mice with H22 ascitic tumors and inhibits the
growth of S180 sarcomas in a mouse xenograft model (21). The antitumor effect of CnB is closely
related to its function in immune regulation because we also found
that CnB can mature and activate dendritic cells and enhance
antigen presentation and thus function as a novel adjuvant of
cancer vaccines (22) and the
Engerix-B® HBV vaccine (23). Additionally, CnB can activate
macrophages by binding to integrinαM, and then promotes the
expression and secretion of TNF-related apoptosis-inducing ligand
(TRAIL), a specific pro-apoptotic cytokine (24,25).
Further, synergistic interaction between CnB and IFN-γ enhances
macrophage antitumor activity by polarize tumor associated
macrophages to M1-phenotype (26).
The toxicity of CnB has also been evaluated; acute toxicity
experiments have indicated that mice can endure at least 50-fold
the normal CnB dose (21,23). Because CnB is an innovative genetic
engineering antitumor drug candidate with very low toxicity, and it
can activate immune system so we were interested in determining
whether combination therapies with CnB and clinical
chemotherapeutic drugs, like 5-FU, could produce enhanced antitumor
activity with reduced side effects on immune systems, for instance,
myelosuppression and immunosuppressive.
Materials and methods
Ethics statement
The experimental procedures were approved by the
Animal Ethics Committee of Beijing Normal University and were
performed in strict accordance with institutional guidelines. All
efforts were made to minimize the number of animals used and their
suffering.
Materials
Recombinant human CnB protein was produced in our
laboratory (the amino acid sequences of the human, mouse and rat
CnB proteins are identical). Endotoxin was removed using Cellufine™
ETclean S endotoxin-removing beads (Chisso Corporation, Japan). The
purity of CnB was greater than 98%, and LPS contamination was below
4 EU/mg (27). Anti-Ki-67 (a
proliferation marker) and 5-FU were purchased from Canspec
Scientific Instruments Corporation (Shanghai, China). All other
reagents were of standard laboratory grade.
Animals and tumor transplantation
Specific pathogen-free female CD-1 (ICR) mice
weighting 18–20 g were purchased from the Vital River Laboratories
(Beijing, China) (28). All animals
were housed in microisolator cages with autoclaved food and bedding
to minimize exposure to viral and microbial pathogens, and all
procedures were handled according to protocols approved by the
Institutional Animal Care and Use Committee (23). Hepatoma 22 cells were obtained from
H22 ascite-bearing mice, diluted with 0.9% normal saline (NS)
solution to 5×106/ml and then transplanted s.c. (at 0.2
ml/mouse) via an injection syringe into the left armpits of the ICR
mice with aseptic manipulations (29).
Drug preparation and treatment
CnB was suspended in endotoxin-free water, and 5-FU
was diluted in 0.9% NaCl. For the combination therapy, CnB and 5-FU
were simultaneously dissolved in endotoxin-free water. CD-1 (ICR)
mice were randomly divided into 4 groups (10 mice in each group),
and the different groups were injected i.p. with different drugs.
The CnB group and the combination therapy group were administered
CnB (5 mg/kg) doses three times (1 time/day) prior to S.C. tumor
implantation to activate their immune systems. The 5-FU group was
administered 15 mg/kg of 5-FU once every 2 days following S.C.
tumor implantation. The day after the S.C. tumor implantation,
interval dosing was initiated in the combination therapy group;
only CnB (5 mg/kg) was administered the first day, and the combined
drugs (5 mg/kgCnB+15 mg/kg5-FU) were injected on the following
day.
Antitumor effects
The antitumor effects of the drugs were determined
by calculating the volumes of the solid tumors and the tumor
inhibition rates. Approximately 6 days after S.C. tumor
implantation, nearly all of the solid tumors had become larger, and
the smallest tumor was the size of a rice grain. Subsequently, the
tumor volumes were recorded every two days. The long and short axes
of each solid tumor were measured with calipers, and the tumor
volumes were calculate using the formula 1/2 × length ×
width2 (30). After the
tumors were harvested (as described in the next paragraph), the
tumor weights were measured using electric scales. The tumor
inhibition rates were calculated according to the following
formula: (mean tumor weight of the control group-mean tumor weight
of the drug treated group)/mean tumor weight of the control group
×100% (21).
Routine blood examinations
Approximately 15 days after the S.C. tumor
implantations, when the average tumor volume of the negative
control (NaCl) group exceeded 1000 mm3, anesthetize the
mouse by exposure to 2–3% isoflurane, then blood from retro orbital
plexus of each mouse was collected into an EDTA anticoagulation
tube, and the numbers of platelets and white blood cells were
measured using a blood testing instrument. Subsequently, the mice
were sacrificed by cervical dislocation.
Splenic index
The spleens were harvested and weighed after the
mice were sacrificed (as described in the preceding paragraph). The
splenic index was calculated according to the following formula:
organ weight (mg)/body weight(g).
Body weight
The mean body weights of each group were measured
before and after the experiment. The ratio of body weight gain was
calculated according to the following formula: (mean weight after
the experiment-mean weight before the experiment)/mean weight
before the experiment ×100%.
Histological and immunohistochemical
tissue staining
The tumors and organs (liver and kidneys) were
harvested, fixed in 10% formalin, embedded in paraffin and sliced
into 4-µm-thick slices. The slices were stained with hematoxylin
and eosin (H&E) according to routine methods. Once sufficiently
dyed, each slice was observed using a conventional light
microscope. Immunostaining was also performed by deparaffinizing
and by rehydrating the 4-µm-thick sections. Then, 3% or 5%
H2O2 was used to block the activity of
endogenous peroxidases, and the sections were blocked with 5%
serum. All sections were incubated with an antimouse Ki-67
monoclonal antibody (1:200) at 37°C for 90 min. After being washed
3 times with PBS (10 min/time), the sections were incubated with a
secondary antibody at 37°C for 22 min and then washed three times
with PBS (10 min/time) and visualized with 3,3′diaminobenzidine
tetrahydrochloride (DAB) as a chromogen substrate. Finally, the
tissue sections were lightly counterstained with hematoxylin,
cleared and mounted.
Statistical analyses
The data are expressed as the means and standard
deviations (SDs). All the data were analyzed using
independent-sample t tests. P<0.05 was considered statistically
significant. The statistical analyses were performed using Graphpad
Prism Software.
Results
Combined CnB and 5-FU exerts an
enhanced antitumor effect in hepatoma 22-transplanted mice
First, we sought to determine the antitumor activity
of the combination therapy. Thus, we studied the effects of CnB in
combination with 5-FU on tumor growth in these mice. The results
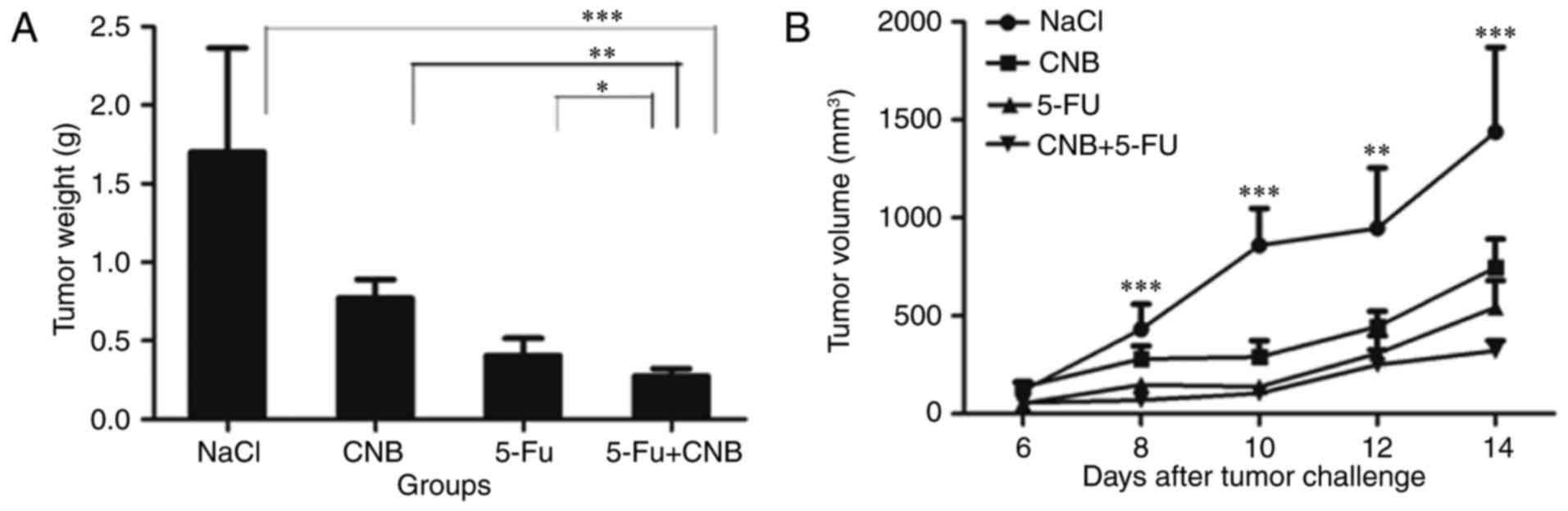
are shown in Fig. 1. Fig. 1A shows that the mean volume of the
tumors in the combination therapy group was much smaller than that
in the negative control (NaCl) group (***P<0.001, **P<0.01).
The antitumor effect observed in the combination group was also
greater than those observed in the single-agent groups. In addition
to observing the growth of the tumors by measuring their volume
every two days, we also cut out the solid tumors and calculated the
mean tumor weights for the different therapeutic groups. Fig. 1B shows that the tumor inhibiting
effects were significantly enhanced when CnB and 5-FU were combined
(*P<0.05 compared to 5-FU and **P<0.01 compared to CnB). The
tumor inhibition rate of the combination therapeutic group was
83.60%, which was approximately 30% greater than that of the CnB
group (54.60%) and also higher than that of the 5-FU group
(76.20%). The tumor volume and tumor weight data strongly
illustrate the enhanced antitumor effect of the CnB and 5-FU
combination therapy.
Pathological examination of the tumor
tissues reveals that the combination therapy leads to increased
tumor cell death
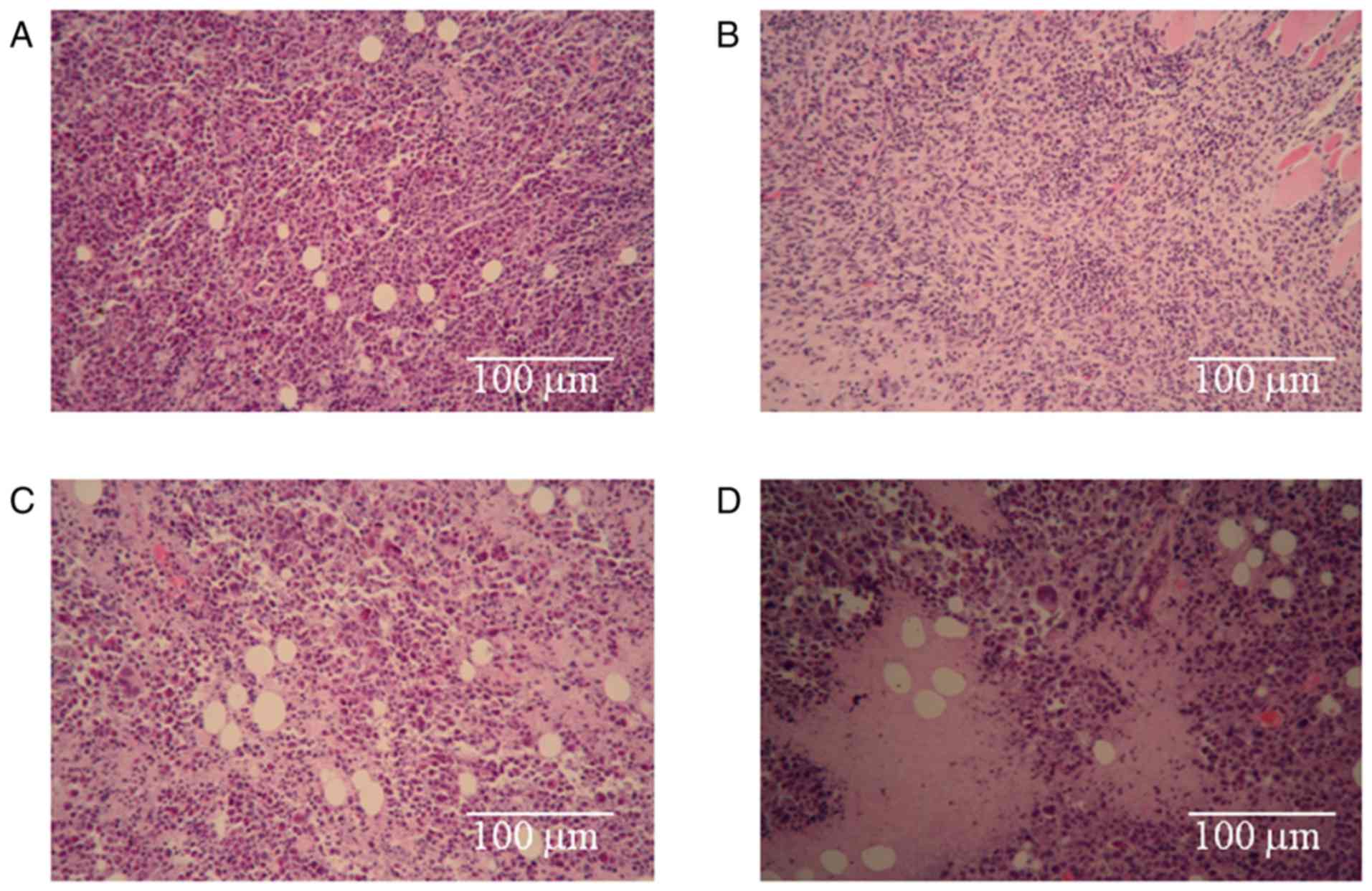
To investigate the morphologies the H22 cells
treated with the different drugs, pathological examinations of the
tumor tissues were performed. The results are shown in Fig. 2. The arrangement of the H22 cells in
the negative control (NaCl) group (Fig.
2A) was tight, and a well-developed proliferative ability was
present. The staining was bright, deep and consistent, and no
necrotic areas were observed in this group. The most obvious
observations in the CnB group (Fig.
2B) were the looseness of the tumor and the smaller sizes of
the cells compared to those of the negative control (NaCl) group.
Apparently necrotic areas were observed in the 5-FU treatment group
(Fig. 2C), and necrotic cellular
debris were scattered around. The necrotic area in the combination
group (Fig. 2D) was much larger than
that in the single-treatment 5-FU group, and necrotic cellular
debris was also obvious in this group. These results prove that the
treatments with CnB, 5-FU and the combination therapy led to the
death of H22 tumor cells and that the death rate in the combination
group was greater than those of the single-treated CnB and 5-FU
groups.
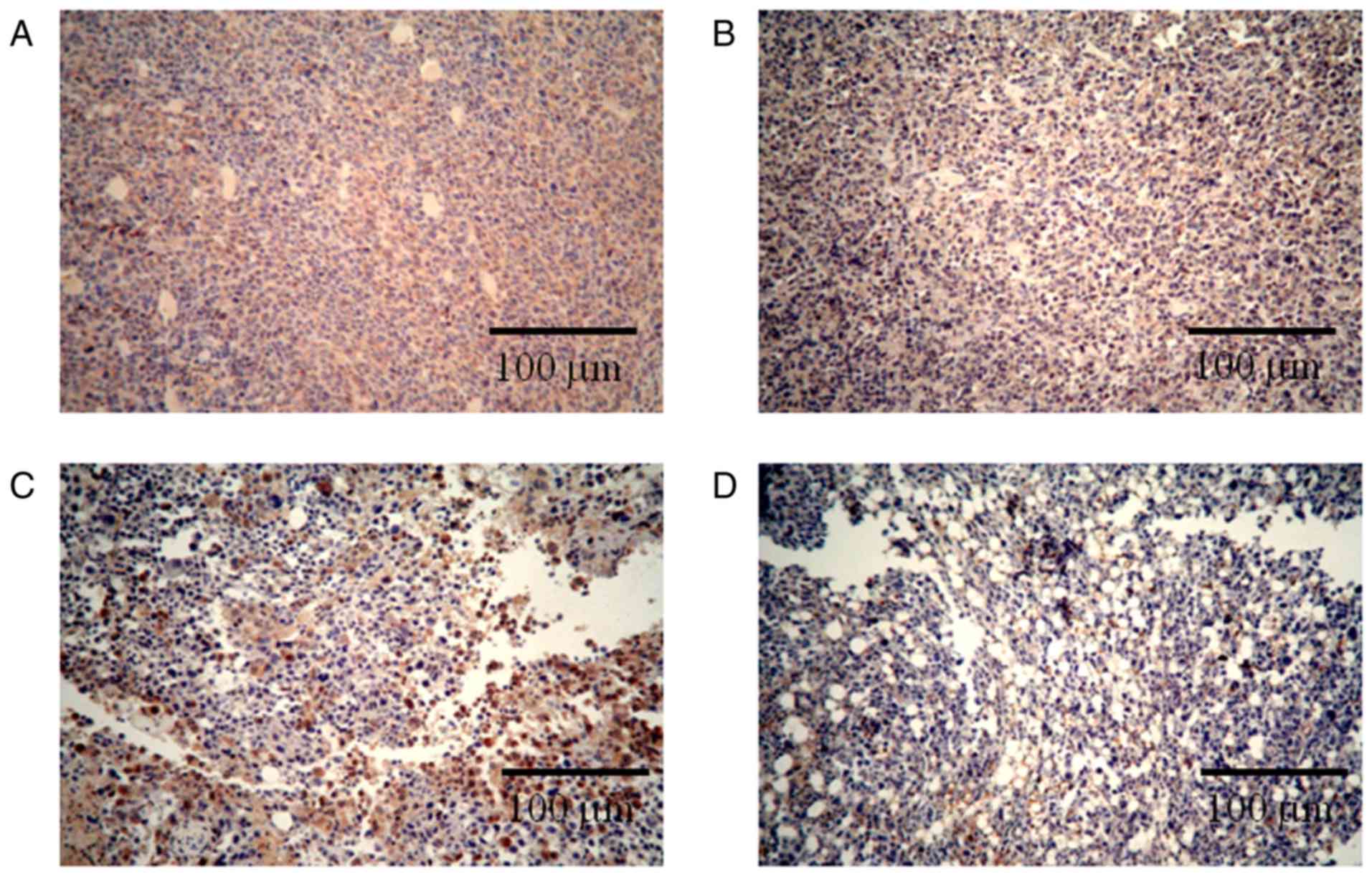
Immunohistochemical staining for Ki-67 revealed that
the combination therapy enhanced the inhibition of tumor cell
proliferation. Ki-67 is a nuclear protein that is only expressed
during the active phases of the cell cycle and is a known
proliferative and prognostic marker in both the laboratory and the
clinic (31). Reduction in the
expression of Ki-67 represent the anti-proliferation activities of
different drugs (32,33). As seen in Fig. 3, the number of Ki-67-positive cells in
the negative control (NaCl) group (Fig.
3A) was much higher than the numbers observed in the
single-treated CnB and 5-FU groups (Fig.
3B, C); in the combination group, most of the cells did not
express Ki-67 (Fig. 3D). These
results indicate that the combination CnB and 5-FU therapy
significantly inhibited the proliferation of H22 tumor cells and
was more effective than either of the single treatments with CnB or
5-FU.
CnB ameliorated the body weight loss
induced by 5-Fu
The body weights of each therapeutic group were
recorded before and after the experiments. Compared to the negative
control (NaCl) group, 5-FU resulted in significant inhibition of
the increase in body weight (weight gain was reduced by
approximately 50% compared to the NaCl group) (Table I). In contrast, CnB treatment alone
group exhibited a significant increase in body weight (35.6%
greater than that of the NaCl group). When the H22-transplanted
mice were treated with CnB and 5-FU together, the mean body weights
exhibited significant growth compared to those observed in the 5-FU
group (69.2%), which indicates that CnB greatly improved the 5-FU
side effect of weight loss.
 | Table I.CnB ameliorated the adverse effects
of 5-FUa. |
Table I.
CnB ameliorated the adverse effects
of 5-FUa.
| Groups | Ratio of body
weight gain (%) | Spleen index
(mg/g) | Platelet
(×109/l) | WBC
(×109/l) |
|---|
| Nacl | 13.29 |
10.64±2.18 |
976.56±203.71 |
12.16±6.09 |
| CnB | 18.02 |
10.52±2.34 |
1001.27±269.14c* |
11.50±2.88e** |
| 5-FU | 6.48 |
9.01±1.88b* |
815.50±168.72d* |
6.56±1.59f** |
| CnB+5-FU | 10.97 |
11.09±1.71 |
1005.82±229.38 |
11.17±3.08 |
CnB reduced the toxicity of 5-Fu as
indicated by organ weight
The spleen is the largest peripheral immune organ,
is the site of residence of mature T and B lymphocyte cells and is
the most important site of immune responses. The splenic index
directly reflects immune function. As shown in Table I, the immunosuppressor 5-FU
significantly reduced the splenic index. In contrast, the splenic
indices of the CnB-alone and negative control (NaCl) groups were
maintained at the same level. Furthermore, following the addition
of CnB, the splenic index of the combination therapeutic group was
significantly increased (*P<0.05). This result strongly
indicates that CnB improved the immunosuppressive effect induced by
5-Fu.
CnB ameliorated the abnormalities in
the hematological parameters
Clinically, some of the most apparent side effects
of chemotherapeutic treatment are declines in hematological
parameters. These declines might have a negative effect on patient
immunity and can even lead to many complications. As seen in
Table I, the hematological parameters
of the 5-FU-treated group sharply dropped. The platelet numbers
exhibited a 20% decrease compared to those observed in the negative
control (NaCl) group. Furthermore, the WBC numbers of the 5-FU
group were only half of those observed in the negative control
(NaCl) group. However, CnB significantly ameliorated the
abnormalities of the platelet and WBC numbers that were induced by
5-Fu (platelets: *P<0.05 CnB compared with 5-FU, WBC:
**P<0.01 CnB compared with 5-FU). Following the addition of CnB,
the platelet and WBC numbers reached levels that were similar to
those observe in the negative control (NaCl) group. Significant
differences were observed between the 5-FU group and the
combination group (platelets: *P<0.05 5-FU compared to the 5-FU+
CnB group, WBC: **P<0.01 5-FU compared to the 5-FU+CnB group).
These results indicated that CnB rescued the changes in the
hematological parameters that were induced by 5-Fu.
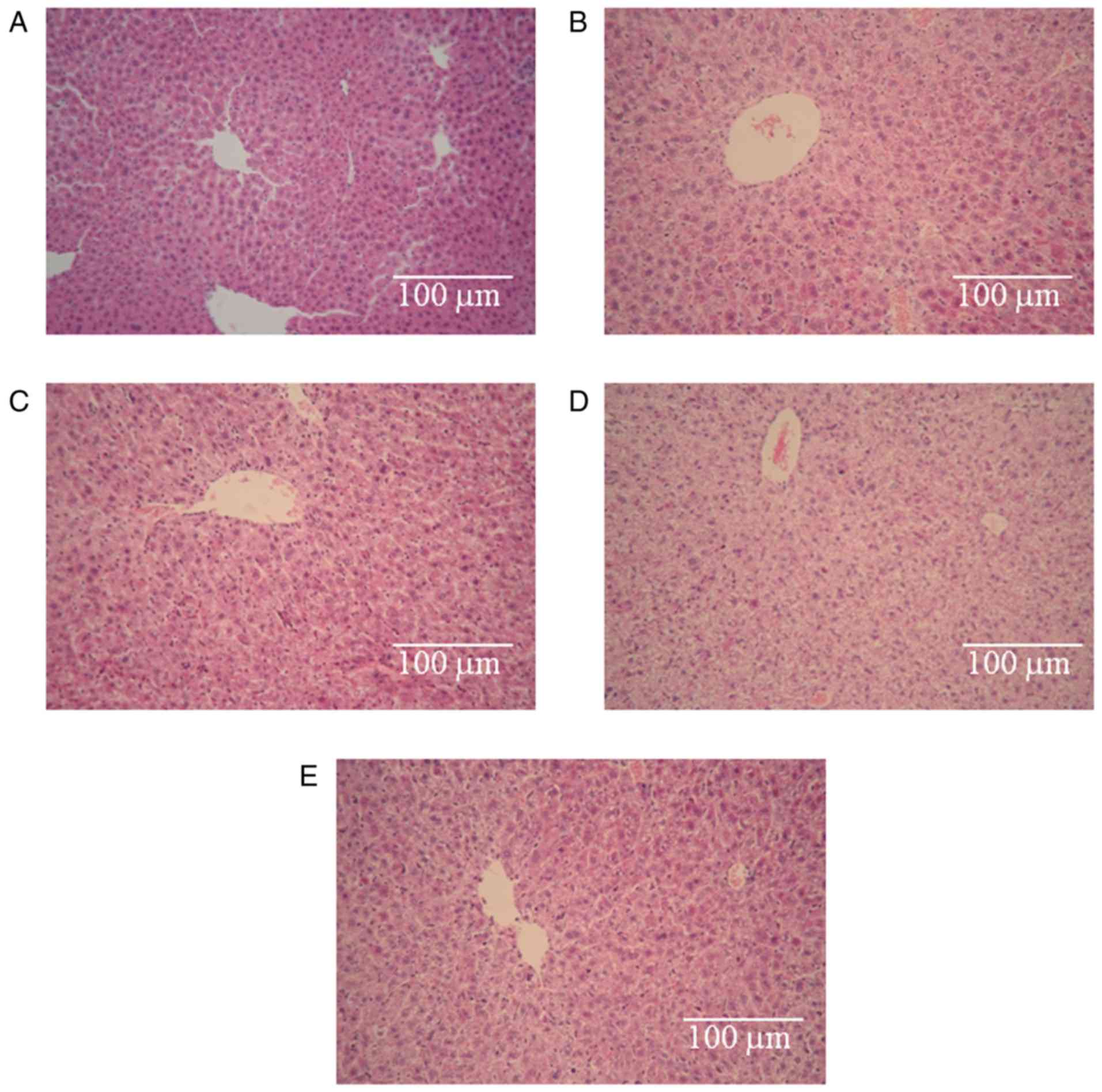
CnB ameliorated the hepatotoxicity
caused by 5-FU
Light microscopy observations revealed that the
control hepatic tissues exhibited normal large polygonal cells with
prominent round nuclei, eosinophilic cytoplasm, a few spaced
hepatic sinusoids arranged between the hepatic cords, with a fine
arrangement of Kupffer cells (Fig.
4A) (34). Fig. 4B, C and E illustrate some degree of
degeneration of the hepatic cords, loose cytoplasm and focal
inflammatory cell infiltration. The degrees of damage observe in
the three groups were identical. However, pronounced
histopathological abnormalities were observed in the 5-FU group
(Fig. 4D). These abnormalities
involved the dissolution of the hepatic cords, which appeared as
empty vacuoles aligned by strands of necrotic hepatocytes. The
hepatotoxicity in this group was much greater than the
hepatotoxicities observed in the other groups. Based on these
results, we conclude that the hepatotoxicity induced by 5-FU was
obvious, no hepatotoxicity was observed in the CnB group, and CnB
ameliorated the hepatotoxicity caused by 5-FU.
Discussion
Clinically, combination therapy has become a general
approach to cancer treatment (35).
The goal of combination therapy is to augment the antitumor effects
and to reduce the side effects and toxicities of drugs to the
fullest extent possible.
Five-fluorouracil is widely used for a range of
cancers. However, despite many efforts, systemic single-agent
treatments have exhibited poor efficacy (36–39) and
have only been able to achieve objective response rates of
approximately 10% (40).
Additionally, some patients discontinue therapy due to serious
adverse effects (10,35,41).
Although 5-FU in combination with other chemotherapeutic agents
improves response rates and survival new therapeutic strategies are
urgently required (5).
As an innovative genetic engineering antitumor
protein, CnB exhibits efficacious antitumor activity and has low
toxicity (21). Comparatively, CnB is
easy to express and to purify (42),
and the cost of CnB is lower than that of other protein drugs.
Unlike some antitumor agents, that have to be extracted from
Chinese herbs (43,44), CnB does not have any disadvantages for
the environment.
Regarding the drawbacks of 5-FU and the merits of
CnB, we speculate that combination therapy will result in good
effects. Our results revealed that combination treatment of CnB and
5-Fu produced a significant augmentation of the antitumor effect in
H22-transplanted ICR mice. When the mice were treated
simultaneously with CnB and 5-FU, the tumor volumes were clearly
reduced compared to those of the single-treated CnB and 5-FU
groups. Moreover, the tumor inhibition rate of the combination
group exceeded 80%, whereas these rates in the CnB and 5-FU
single-agent groups were approximately 70 and 50%, respectively.
Significant differences were observed between the combination group
and single-agent groups (P<0.01). The pathological examinations
of the tumor tissues from the negative control (NaCl) group
revealed that the H22 cells grew vigorously in a compact
arrangement, whereas different amounts of necrotic cellular debris
were observed in the single-treated CnB and 5-FU groups.
Furthermore, the necrotic areas in the combination group were much
larger. Additionally, Ki-67 nuclear protein immunohistochemical
staining further demonstrated a decline in the numbers of positive
proliferation cells after animals were treated with combined CnB
and 5-FU compared with the single-agent groups. These findings
indicate that CnB effectively increased the ability of 5-FU to
inhibit cell proliferation in tumors.
Adverse drug reactions are a major clinical problem,
and a meta-analysis involving 1219 patients with colorectal cancer
revealed that grade 3 to 4 toxicity is encountered in 31–34% of the
patients who receive 5-FU and that 0.5% of these patients
experience lethal toxicity (45,46).
Recent data suggest that combination therapy not only significantly
improves the quality of life of cancer patients (39) but might also improve their responses
potentially their survival (47).
Indeed, the clinical experience accumulated over the last 30 years
of cancer management suggests that combinations of different agents
offer the best possible therapeutic efficacies (10,48). In
our experiment, the reduced side effects of combination therapy
were evaluated by analyzing the body weights, immune organs
indices, peripheral blood platelet and WBC cell numbers, and the
pathologies of the livers of the mice. Our results revealed that
5-FU evidently reduced body weight gain, immune organ growth and
the numbers of platelets and WBC cells. However, CnB ameliorated
all of these adverse effects. Significant improvements were
observed in the animals that were treated with both CnB and 5-FU
(P<0.01). Moreover, H&E staining of the mouse livers
revealed that CnB also improved the microstructural damage induced
by 5-FU. We also performed pathological examinations of the
kidneys; however, no significant differences were observed between
the groups. We suggest that this lack of difference arose because
liver is more sensitive to 5-FU than kidney and the toxicity of
5-FU in the blood is reduced upon arrival at the kidney due to
previous detoxification in the liver.
In conclusion, our current study revealed that the
combination of CnB and 5-FU had a more potent inhibitory effect on
H22 tumor growth. Furthermore, the side effects were not increased
by the combination therapy; rather the CnB significantly attenuated
the toxicity induced by 5-FU. We speculate that the immune
regulation function of CnB played an important role in this process
that involved promoting the cytotoxic effects of immunocytes on
tumor cells, aiding the repair of damaged tissues, and maintaining
homeostasis in the bodies of the mice. The high efficiency and low
toxicity of CnB makes it a promising novel antitumor drug.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China, the National Important Novel
Medicine Research Project, the International Cooperation Project
and the Fundamental Research Funds for the Central
Universities.
References
|
1
|
Zhao T, Mao G, Zhang M, Zou Y, Feng W, Gu
X, Zhu Y, Mao R, Yang L and Wu X: Enhanced antitumor and reduced
toxicity effect of Schisanreae polysaccharide in 5-Fu treated
Heps-bearing mice. Int J Biol Macromol. 63:114–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao Z, Liao L, Chen X, Lan L, Hu H, Liu Z,
Chen L, Huang S and Du J: Enhancement of antitumor activity of
low-dose 5-fluorouracil by combination with Fuzheng-Yiliu granules
in hepatoma 22 tumor-bearing mice. Integr Cancer Ther. 12:174–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonetti RG, Liberati A, Angiolini C and
Pagliaro L: Treatment of hepatocellular carcinoma: A systematic
review of randomized controlled trials. Ann Oncol. 8:117–136. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng MR, Li Q, Wan T, He B, Han J, Chen
HX, Yang FX, Wang W, Xu HZ, Ye T and Zha BB: Galactosylated
chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer
growth and its side effects. World J Gastroenterol. 18:6076–6087.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boucher E, Corbinais S, Brissot P,
Boudjema K and Raoul JL: Treatment of hepatocellular carcinoma
(HCC) with systemic chemotherapy combining epirubicin, cisplatinum
and infusional 5-fluorouracil (ECF regimen). Cancer Chemother
Pharmacol. 50:305–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan Y, Lu Y, Wang D, Liu J, Song X, Zhang
W, Zhao X, Nguyen TL and Hu Y: Effect of epimedium
polysaccharide-propolis flavone immunopotentiator on
immunosuppression induced by cyclophosphamide in chickens. Cell
Immunol. 281:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao HF, Chen YJ and Yang YC: A novel
polysaccharide of black soybean promotes myelopoiesis and
reconstitutes bone marrow after 5-flurouracil- and
irradiation-induced myelosuppression. Life Sci. 77:400–413. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wang Y, Liu X, Yuan Y and Yue T:
Free radical scavenging and immunomodulatory activities of
Ganoderma lucidum polysaccharides derivatives. Carbohydr Polym.
91:33–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou S, Kou G, Fan X, Wang H, Qian W, Zhang
D, Li B, Dai J, Zhao J, Ma J, et al: Eradication of hepatoma and
colon cancer in mice with Flt3L gene therapy in combination with
5-FU. Cancer Immunol Immunother. 56:1605–1613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SH, Lee Y, Han SH, Kwon SY, Kwon OS,
Kim SS, Kim JH, Park YH, Lee JN, Bang SM, et al: Systemic
chemotherapy with doxorubicin, cisplatin and capecitabine for
metastatic hepatocellular carcinoma. BMC Cancer. 6:32006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Wu B, Zhang X, Xu M, Chang H, Lu X
and Ren X: Purification of a polysaccharide from Boschniakia
rossica and its synergistic antitumor effect combined with
5-Fluorouracil. Carbohydr Polym. 89:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crabtree GR: Generic signals and specific
outcomes: Signaling through Ca2+, calcineurin, and NF-AT. Cell.
96:611–614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manalan AS and Klee CB: Activation of
calcineurin by limited proteolysis. Proc Natl Acad Sci USA. 80:pp.
4291–4295. 1983; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Nguyen BC, Dziunycz P, Chang S,
Brooks Y, Lefort K, Hofbauer GF and Dotto GP: Opposing roles for
calcineurin and ATF3 in squamous skin cancer. Nature. 465:368–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Euvrard S, Kanitakis J and Claudy A: Skin
cancers after organ transplantation. N Engl J Med. 348:1681–1691.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heit JJ, Apelqvist AA, Gu X, Winslow MM,
Neilson JR, Crabtree GR and Kim SK: Calcineurin/NFAT signalling
regulates pancreatic beta-cell growth and function. Nature.
443:345–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neilson JR, Winslow MM, Hur EM and
Crabtree GR: Calcineurin B1 is essential for positive but not
negative selection during thymocyte development. Immunity.
20:255–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Januschke J, Reina J, Llamazares S,
Bertran T, Rossi F, Roig J and Gonzalez C: Centrobin controls
mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat
Cell Biol. 15:241–248. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saeki M, Irie Y, Ni L, Itsuki Y, Terao Y,
Kawabata S and Kamisaki Y: Calcineurin potentiates the activation
of procaspase-3 by accelerating its proteolytic maturation. J Biol
Chem. 282:11786–11794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei Q, Lian ML, Jing FZ, Zhang N, Yan MS,
Chen Y and Gao QS: Studies of calcineurin B subunit from genetic
engineering for use in medicine. Drug Dev Res. 56:40–43. 2002.
View Article : Google Scholar
|
|
22
|
Li J, Guo J, Su Z, Hu M, Liu W and Wei Q:
Calcineurin subunit B activates dendritic cells and acts as a
cancer vaccine adjuvant. Int Immunol. 23:327–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu M, Su Z, Yin Y, Li J and Wei Q:
Calcineurin B subunit triggers innate immunity and acts as a novel
Engerix-B HBV vaccine adjuvant. Vaccine. 30:4719–4727. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su Z, Xin S, Xu L, Cheng J, Guo J, Li L
and Wei Q: The calcineurin B subunit induces TNF-related
apoptosis-inducing ligand (TRAIL) expression via CD11b-NF-κB
pathway in RAW264. 7 macrophages. Biochem Biophys Res Commun.
417:777–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Su Z, Xin S, Cheng J, Li J, Xu L
and Wei Q: The calcineurin B subunit (CnB) is a new ligand of
integrin αM that mediates CnB-induced Apo2L/TRAIL expression in
macrophages. J Immunol. 188:238–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su Z, Yang R, Zhang W, Xu L, Zhong Y, Yin
Y, Cen J, DeWitt JP and Wei Q: The synergistic interaction between
the calcineurin B subunit and IFN-γ enhances macrophage antitumor
activity. Cell Death Dis. 6:e17402015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su Z, Xin S, Li J, Guo J, Long X, Cheng J
and Wei Q: A new function for the calcineurin b subunit:
Antiplatelet aggregation and anticoagulation. IUBMB Life.
63:1037–1044. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Li L, Huang Y and Wei Q:
Calcineurin subunit B upregulates β-interferon production by
phosphorylation of interferon regulatory factor 3 via Toll-like
receptor 4. Cancer Sci. 103:515–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia L, Xu B, Guo W, Ge ZM, Li RT and Cui
JR: Enhanced antitumor effect of TM208 in combination with 5
fluorouracil in H22 transplanted mice. J Chine Pharmace Sci.
20:615–626. 2011.
|
|
30
|
Rolny C, Mazzone M, Tugues S, Laoui D,
Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et
al: HRG inhibits tumor growth and metastasis by inducing macrophage
polarization and vessel normalization through downregulation of
PlGF. Cancer Cell. 19:31–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antoon JW, White MD, Slaughter EM, Driver
JL, Khalili HS, Elliott S, Smith CD, Burw ME and Beckman BS:
Targeting NFκB mediated breast cancer chemoresistance through
selective inhibition of sphingosine kinase-2. Cancer Biol Ther.
11:678–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hosaka N, Ichikawa Y, Ishikawa T,
Nagashima Y, Kunisaki C, Takahashi M, Moriwaki Y, Akiyama H,
Yamaguchi S, Ota M, et al: Correlation of immunohistochemical p53
labeling index with inhibition rate in chemosensitivity test in
gastric and colon cancer. Anticancer Res. 21:229–235. 2000.
|
|
33
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Sayyad HI, Ismail MF, Shalaby F,
Abou-El-Magd R, Gaur RL, Fernando A, Raj MH and Ouhtit A:
Histopathological effects of cisplatin, doxorubicin and
5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol
Sci. 5:466–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmoll HJ and Arnold D: Update on
capecitabine in colorectal cancer. Oncologist. 11:1003–1009. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hejna M and Zielinski CC: Nonsurgical
management of gallbladder cancer: Cytotoxic treatment and
radiotherapy. Expert Rev Anticancer Ther. 1:291–300. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knox JJ, Hedley D, Oza A, Siu LL, Pond GR
and Moore MJ: Gemcitabine concurrent with continuous infusional
5-fluorouracil in advanced biliary cancers: A review of the
Princess Margaret Hospital experience. Ann Oncol. 15:770–774. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Todoroki T: Chemotherapy for gallbladder
carcinoma-a surgeon's perspective. Hepatogastroenterology.
47:948–955. 2000.PubMed/NCBI
|
|
39
|
Harrop R, Drury N, Shingler W, Chikoti P,
Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven
N and Hawkins RE: Vaccination of colorectal cancer patients with
TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and
irinotecan) is safe and induces potent immune responses. Cancer
Immunol Immunother. 57:977–986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kobayashi K, Tsuji A, Morita S, Horimi T,
Shirasaka T and Kanematsu T: A phase II study of LFP therapy (5-FU
(5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive
(Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer.
6:1212006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nowak AK, Chow PK and Findlay M: Systemic
therapy for advanced hepatocellular carcinoma: A review. Eur J
Cancer. 40:1474–1484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Q and Lee EY: Expression and
reconstitution of calcineurin A and B subunits. Biochem Mol Biol
Int. 41:169–177. 1997.PubMed/NCBI
|
|
43
|
Shoemaker M, Hamilton B, Dairkee SH, Cohen
I and Campbell MJ: In vitro anticancer activity of twelve Chinese
medicinal herbs. Phytother Res. 19:649–651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang W, Hemm I and Bertram B: Recent
development of antitumor agents from Chinese herbal medicines; part
I. Low molecular compounds. Planta Med. 69:97–108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lazarou J, Pomeranz BH and Corey PN:
Incidence of adverse drug reactions in hospitalized patients: A
meta-analysis of prospective studies. Jama. 279:1200–1205. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Kuilenburg AB, Meinsma R, Zonnenberg
BA, Zoetekouw L, Baas F, Matsuda K, Tamaki N and van Gennip AH:
Dihydropyrimidinase deficiency and severe 5-fluorouracil toxicity.
Clin Cancer Res. 9:4363–4367. 2003.PubMed/NCBI
|
|
47
|
Kornek G, Raderer M, Schüll B, Fiebiger W,
Gedlicka C, Lenauer A, Depisch D, Schneeweiss B, Lang F and
Scheithauer W: Effective combination chemotherapy with paclitaxel
and cisplatin with or without human granulocyte colony-stimulating
factor and/or erythropoietin in patients with advanced gastric
cancer. Br J Cancer. 86:1858–1863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liljenfeldt L, Gkirtzimanaki K, Vyrla D,
Svensson E, Loskog AS and Eliopoulos AG: Enhanced therapeutic
anti-tumor immunity induced by co-administration of 5-fluorouracil
and adenovirus expressing CD40 ligand. Cancer Immunol Immunother.
63:273–282. 2014. View Article : Google Scholar : PubMed/NCBI
|