Introduction
Esophageal cancer is the eighth most common cancer
and the sixthleading cause of cancer-associated mortality worldwide
(1). Approximately 70% of global
esophageal cancer cases occur in China, with esophageal squamous
cell carcinoma (ESCC) accounting for the vast majority of cases
(>90%) (2). Currently,
multi-modality treatment improves the quality of life and prolongs
the survival time of patients with ESCC. However, the 5-year
survival rate remains poor owing to the limited clinical
opportunities for the early diagnosis and treatment of ESCC.
Therefore, there is an urgent requirement to pursue novel
diagnostic indicators, prognostic biomarkers, therapeutic targets
and therapeutic approaches for ESCC treatment.
Calcyphosine (CAPS), a Ca2+-binding
protein, was initially isolated from the canine thyroid cDNA
library as a substrate that can be phosphorylated by protein kinase
A in a Cyclic adenosine monophosphate (cAMP)-dependent manner
(3,4).
CAPS was also detected in humans and other mammals such as cows and
rabbits, and even in certain invertebrates, such as sponges;
however, it was determined to be absent from mice and five other
rodents (5–7). To date, three subtypes of CAPS shave
been reported: Type-I CAPS, type-II CAPS and CAPS 2. A previous
study revealed that type-I CAPS may be specific to mammals, type-II
CAPS widely exists in metazoan speciesand CAPS 2 is unique to human
beings (8). The synthesis and
phosphorylation of type-I CAPS are upregulated by thyrotropin and
cyclic AMP analogues that can promote cell proliferation and
maintain expression of the differentiated thyrocyte phenotype, and
are downregulated by 12-O-tetradecanoylphorbol-13-acetate
(TPA) and epidermal growth factor, which repress cell
differentiation (9). As a member of
the EF hand motif family, CAPS contains four EF-hand domains for
calcium binding (10). Although the
exact function of CAPS remains unclear, its Ca2+-binding
phosphorylatory abilities may implicate it in cross-signaling
between calcium-phosphatidylinostitol and cAMP cascades (11).
In recent years, attention has been drawn to the
associations between CAPS protein expression and various diseases,
including certain types of cancer. For example, CAPS was
significantly down regulated in the bronchoalveolar lavage fluid of
sulfur mustard-exposed patients when compared with healthy controls
(12). Previous studies also showed
that CAPS was overexpressed in ovarian cancer (13), ependymoma (14), endometrial cancer (15), lung cancer (16) and colorectal cancer (17). Another previous study revealed that
CAPS promoted cancer progression and may be a prognostic indicator
in colorectal cancer patients (17).
However, the expression and role of CAPS in ESCC require further
investigation. The present study investigated CAPS expression in
ESCC tumor tissues, and examined the association between CAPS
expression, clinicopathological features and survival outcomes for
patients with ESCC. To the best of our knowledge, this is the first
study of the clinical relevance of CAPS in ESCC to date.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University (Zhangzhou,
China) and written informed consent was obtained from each patient
involved in the present study.
Tumor samples
A total of 104 fresh samples of tumor tissues were
immediately harvested from patients (40 women, 64 men; mean age,
62.36 years and range 42–80 years) with ESCC who underwent surgical
resections between November 2013 and January 2015 in the Department
of Thoracic Surgery, the First Affiliated Hospital of Zhengzhou
University. None of the patients had received preoperative
chemotherapy or radiotherapy. Tumor tissue samples were obtained
from operative specimens, washed twice with PBS and maintained in
RNA wait (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) at −80°C until the time of analysis, following
evaluation by a pathologist. Clinical and pathological
characteristics were obtained from clinical database and pathology
records. A total of 64 formalin-fixed paraffin-embedded ESCC
tissues and 4 corresponding adjacent non-cancerous tissues with
available follow-up information were obtained from the Department
of Pathology, the First Affiliated Hospital of Zhengzhou University
between October 2008 and December 2010, and were used for
immunohistochemical analysis. The clinicopathological features were
analyzed according to age, gender, tumor invasion depth,
histological grade, lymph node metastasis and Tumor-Node-Metastasis
(TNM) stage (18).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from ESCC tissue specimens
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
First-strand cDNA was synthesized from 1 µg of total RNA using the
Revert Aid First Strand c-DNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc., Pittsburg, PA, USA). Briefly, 1 µg total
RNA samples were incubated at 42°C with 2 µl 5X gDNA eraser buffer,
1 µl gDNA eraser and RNase-free dH2O for 2 min, then the
enzyme mix was added and the solution was incubated at 37°C for 15
min. The CAPS mRNA levels were quantified in duplicate using a
Stratagene Mx3005P (Agilent Technologies, Santa Clara, California,
USA). The Premix Tap kit (Takara Bio, Inc., Otsu, Japan) was used
to perform the qPCR reaction according to the manufacturer's
instruction; GAPDH was used as a loading control. PCR thermocycling
conditions were as follows: Incubation at 95°C for 2 min followed
by 40 cycles of denaturation at 96°C for 15 sec and annealing at
60°C for 1 min. Eachsample was obtained from three independent
experiments and used for analysis of relative mRNA expression
normalized by GAPD Husing the 2−ΔΔCq method (19). The synthetic primers for CAPS and
GAPDH were obtained from Sangon Biotech (Shanghai, China) and the
primers sequences are shown in Table
I.
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Genes | Primers | Product, bp |
|---|
| CAPS |
| 190 |
|
Forward |
5′-AGGCACCTTCCACTAGCAACAG-3′ |
|
|
Reverse |
5′-CCATGCTTGGTCTGGGCTCT-3′ |
|
| GAPDH |
| 271 |
|
Forward |
5′-AAGGTCATCCCTGAGCTGAA-3′ |
|
|
Reverse |
5′-TGACAAAGTGGTCGTTGAGG-3′ |
|
CAPS staining
For CAPS staining, 4-mm sections of formalin-fixed
paraffin embedded tissues were cut and stained. The slides were
heated in an oven at 65°C for 30 min, deparaffinized in dimethyl
benzene for 5 min twice, rehydrated in graded alcohol (100, 100,
95, 95, 70, 70, 50 then 50%) for 5 min each in turn and washed with
TBST. Following antigen retrieval, antigenicity was performed by
heating the tissue in citrate buffer; tissues were then blocked
with 3% H2O2 for 30 min at room temperature
followed by incubation with 10% goat serum (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for another 30 min at room
temperature. Samples were then incubated with CAPS primary antibody
(cat. no. ab186741, 1:200 dilution; Abcam, Cambridge, UK) in dark
box at 4°C overnight, followed by treatment with secondary antibody
(cat. no. sc2040; IgG-B, 1:200 dilution; Santa Cruz Biotechnology,
Inc.) for 15 min at 37°C. For the negative control, samples were
incubated with PBS instead of specific antibody. Following washing
with PBS 4 times, the sections were incubated with biotinylated
horseradish peroxidase-labeled streptavidin (Origene Technologies,
Inc., Beijing, China) at 4°C overnight. The reaction was visualized
with 3,3-diaminobenzidine (DAB; Origene Technologies, Inc.) as a
peroxidase substrate and the sections were counter-stained with
Meyer's hematoxylin at room temperature for 3 min. The slides were
visualized using a bright-field light microscope, and at least 5
consecutive non-overlapping fields were viewed (magnification,
×200). The detection of nuclear and/or cytoplasmic staining in any
percentage of tumor cells was considered positive. Complete absence
of staining was considered as negative for CAPS. Immunostaining was
scored by two independent pathologists according to the
immunoreactive score (IRS). The proportion of positive tumor cells
examined was scored as 0 (no positive tumor cells), 1 (1–40%), 2
(41–75%) or 3 (>75%). Staining intensity was scored as 0 (no
staining), 1 (week staining), 2 (intermediate staining) or 3
(strong staining). The stain signal was evaluated by the
multiplication values (IRS) of the two scores.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS 22.0
software (IBM Corp., Armonk, NY, USA) and the GraphPad Prism 5.0
software package (GraphPad Software, Inc., La Jolla, CA, USA). A
paired t-test was applied to compare the difference in CAPS
expression between tumor tissues and adjacent normal tissues. A
Mann-Whitney U test was used to compare other groups with one
another in terms of CAPS expression. The associations between CAPS
expression and clinicopathological parameters of ESCC patients were
analyzed via χ2 and Fisher's exact probability tests.
The Kaplan-Meier method and log-rank test were used where indicated
to plot the overall survival curve and analyze the association of
patient survival with CAPS expression. In addition, univariate and
multivariate analyses were conducted to evaluate the prognostic
value of CAPS expression in patients with ESCC using the Cox
proportional hazards regression model. P<0.05 was considered to
indicate a statistically significant difference.
Results
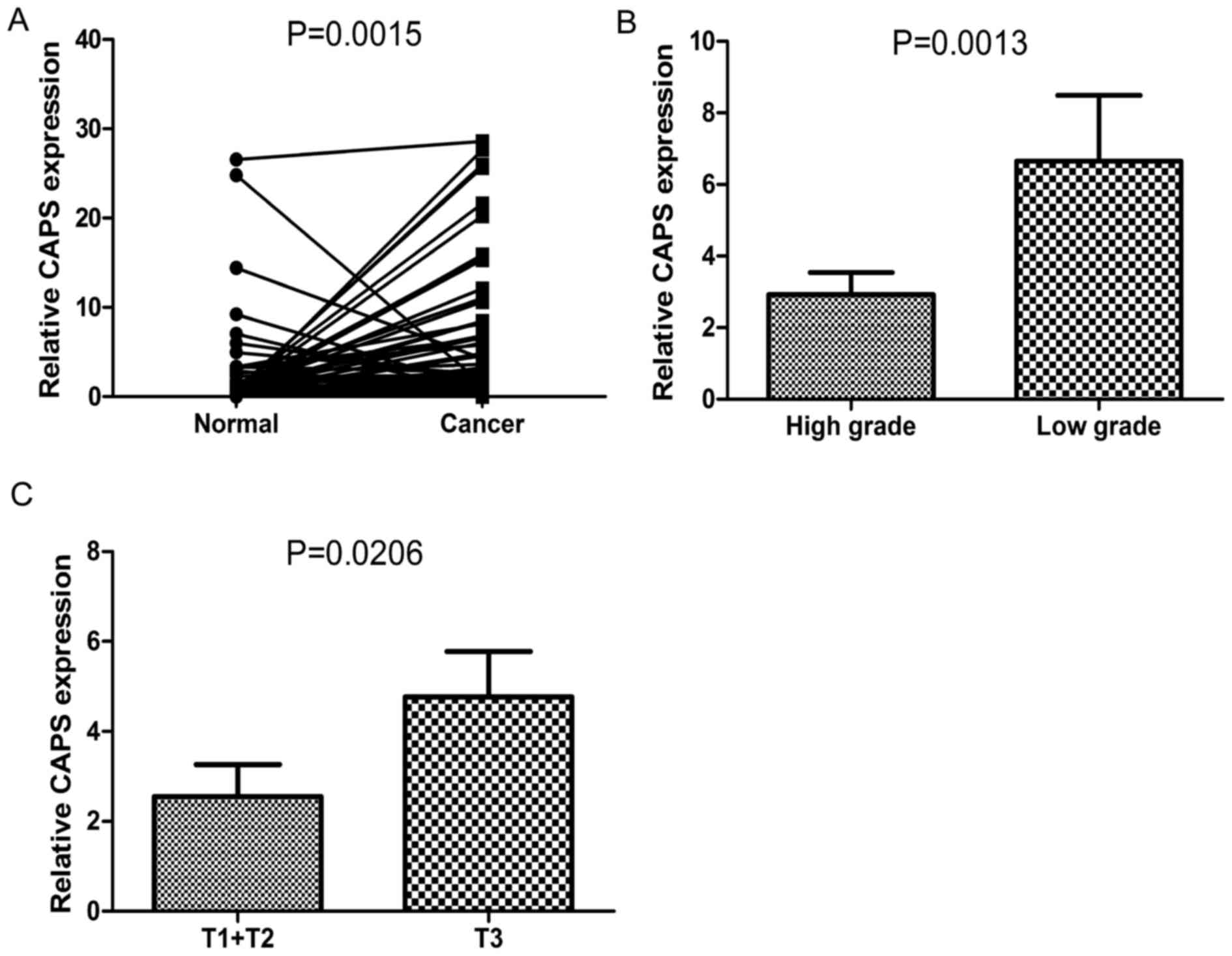
CAPS mRNA level is up regulated in
esophageal squamous cell cancer
The level of CAPS mRNA was upregulated in ESCC
tissues. The mRNA levels of CAPS in ESCC tissues were significantly
higher than those in adjacent non-cancerous tissues (P=0.0015;
Fig. 1A). In addition, CAPS mRNA
levels were significantly elevated in samples of low histological
grade compared with those of high histological grade (P=0.0013;
Fig. 1B). Similarly, a positive
association was also detected between CAPS mRNA levels and tumor
invasion (P=0.0206; Fig. 1C).
Association between CAPS mRNA levels
and clinicopathological parameters of ESCC patients
To evaluate the association between CAPS mRNA levels
and clinicopathological parameters, all patients were classified as
belonging to either high (cancer/normal ratio ≥2) or low
(cancer/normal ratio <2) CAPS expression groups according to the
ratio of cancer tissue expression to adjacent non-cancerous tissue
expression. The association between CAPS mRNA levels and patients'
clinicopathological parameters is shown in Table II. The results of this analysis
revealed that the level of CAPS mRNA in ESCC tissues was
significantly associated with tumor invasion depth (P=0.018) and
histological grade (P=0.017). However, no association was found
between the CAPS mRNA level and other clinicopathological
parameters, including gender, age, lymph node metastasis and TNM
stage.
 | Table II.Association of CAPS mRNA levels with
clinicopathological parameters. |
Table II.
Association of CAPS mRNA levels with
clinicopathological parameters.
|
|
| CAPS expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Total (n=104) | Low (n=51) | High (n=53) | P-value |
|---|
| Age, years |
|
|
| 0.665 |
|
<65 | 61 | 31 | 30 |
|
|
≥65 | 43 | 20 | 23 |
|
| Gender |
|
|
| 0.877 |
|
Male | 64 | 31 | 33 |
|
|
Female | 40 | 20 | 20 |
|
| Histological
grade |
|
|
| 0.017 |
|
Low | 20 | 5 | 15 |
|
|
High | 84 | 46 | 38 |
|
| Tumor invasion
depth |
|
|
| 0.018 |
|
T1/T2 | 53 | 32 | 21 |
|
|
T3/T4 | 51 | 19 | 32 |
|
| Lymph node
metastasis |
|
|
| 0.108 |
| No | 74 | 40 | 34 |
|
|
Yes | 30 | 11 | 19 |
|
| TNM stage |
|
|
| 0.180 |
|
I/II | 71 | 38 | 33 |
|
|
III | 33 | 13 | 20 |
|
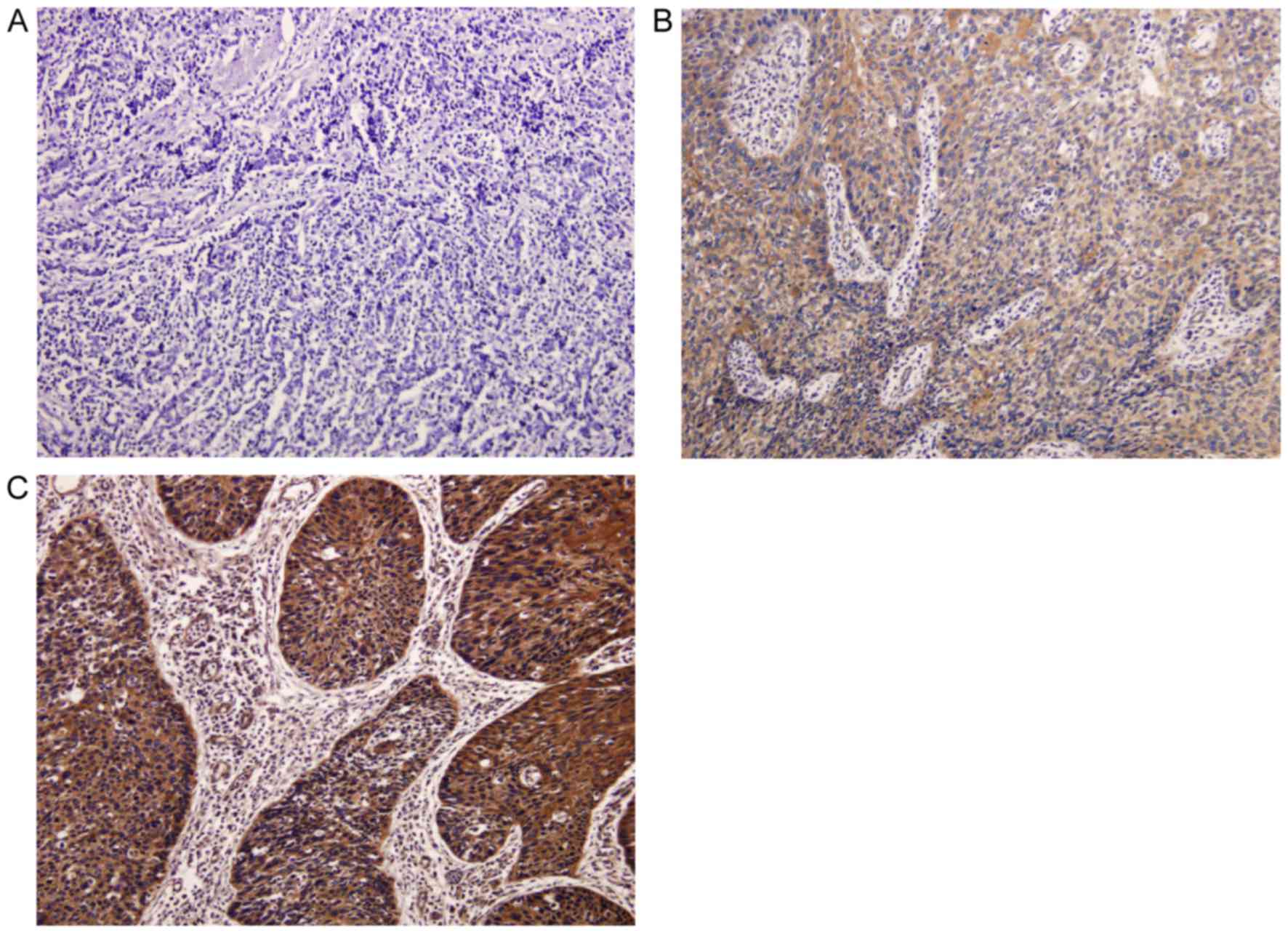
High CAPS protein expression in ESCC
tissue is associated with poor overall survival
CAPS protein expression was further analyzed in 64
ESCC tissues and 4 corresponding adjacent non-cancerous tissues.
The results of this analysis revealed that immunohistochemical
staining of CAPS was predominantly observed in the cytoplasm of
cancer tissues, whereas no or weak staining was found in adjacent
non-cancerous tissues (Fig. 2A). The
median score of tissue CAPS staining (2.5) was used as the cutoff
value to divide all patients into the low CAPS expression group
(n=29; Fig. 2B) and the high CAPS
expression group (n=35; Fig. 2C). The
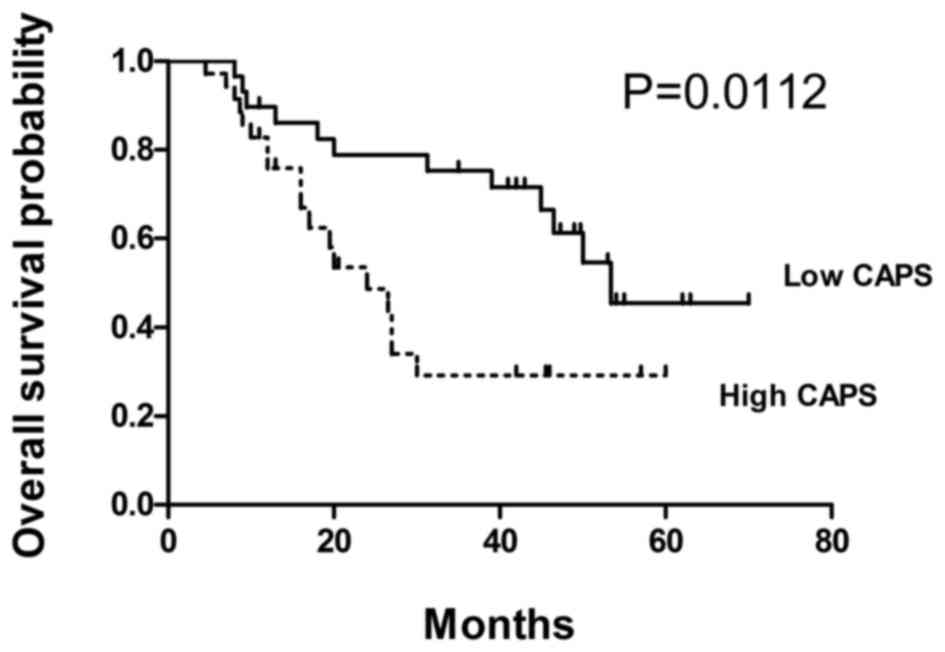
prognostic value of CAPS expression was assessed in patients with
ESCC using the Kaplan-Meier method and log-rank test. Results
demonstrated that high CAPS expression was significantly associated
with poorer overall survival (P=0.0112; Fig. 3).
Tissue CAPS is an independent
prognostic biomarker for ESCC
Univariate and multivariate analysis was conducted
using the Cox proportional hazards model to investigate whether
CAPS could serve as an independent survival predictor. In
univariate analysis, histological grade [hazard ratio (HR), 2.493;
95% confidence interval (CI), 1.117–5.564; P=0.026], tumor invasion
(HR, 2.483; 95% CI, 1.193–5.167; P=0.015), TNM stage (HR, 3.921;
95% CI, 1.737–8.851; P=0.001) and high CAPS expression (HR, 3.043;
95% CI, 1.441–6.423; P=0.004) were associated with poor survival of
ESCC patients (Table III).
Multivariate analysis revealed that TNM stage (adjusted HR, 2.748;
95% CI, 1.191–6.341; P=0.018) and high CAPS expression (adjusted
HR, 2.269; 95% CI, 1.030–4.998; P=0.042) remained independent
prognostic biomarkers (Table
III).
 | Table III.Cox proportional hazards regression
model analysis of prognostic factors. |
Table III.
Cox proportional hazards regression
model analysis of prognostic factors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, ≥65 vs. <65
years | 0.961
(0.466–1.980) | 0.914 |
|
|
| Gender, male vs.
female | 0.410
(0.156–1.073) | 0.069 |
|
|
| Histological grade,
3 vs. 1+2 | 2.493
(1.117–5.564) | 0.026 | 1.691
(0.718–3.979) | 0.229 |
| Tumor invasion
depth, T3+T4 vs. T1+T2 | 2.483
(1.193–5.167) | 0.015 | 1.448
(0.643–3.258) | 0.371 |
| LNM, positive vs.
negative | 1.331
(0.507–3.499) | 0.562 |
|
|
| TNM, III vs.
I+II | 3.921
(1.737–8.851) | 0.001 | 2.748
(1.191–6.341) | 0.018 |
| CAPS expression,
high vs. low | 3.043
(1.441–6.423) | 0.004 | 2.269
(1.030–4.998) | 0.042 |
Discussion
In the present study, RT-qPCR revealed that CAPS
mRNA expression appeared to be frequently upregulated in ESCC
tissues (Fig. 1A); the corresponding
CAPS protein overexpression was also confirmed by
immunohistochemical staining (Fig.
2C). To the best of our knowledge, this is the first study to
demonstrate the expression profile of CAPS in ESCC. CAPS was
initially identified in the canine thyroid cDNA library (3), followed by detection in certain mammals,
including humans (5), cows and
rabbits (6); however, it is absent
from mice and five other rodents (7).
A previous study revealed that the CAPS gene, consisting of 189
amino acids, is located at the p13.3 region of chromosome 19 in
humans (5). Recently, attention has
been directed to the association between CAPS and certain types of
carcinoma. Similar to the observations of the present study, CAPS
overexpressionhas been found in a range of cancer types, including
ovarian cancer (20), ependymoma
(14), endometrial cancer (15), lung cancer (16) and colorectal cancer (17). In a study concerning lung cancer and
chronic obstructive pulmonary disease (COPD), upregulated CAPS
expression was detected in lung cancer and lung cancer with COPD
groups when compared with the control group, indicating that CAPS
may serve as a biomarker fora lung cancer diagnosis (16).
Esophageal cancer is one of the most aggressive
cancer types worldwide owing to a lack of early typical symptoms
and effective non-invasive diagnostic methods (21). Despite the efforts to improve
diagnostic methods and therapeutic approaches, the quality of life
and overall survival time for patients with ESCC is far from
satisfactory. Therefore, the identification of novel biomarkers for
assisting the diagnosis and predicting the prognosis of patients
with ESCC is urgently required. In recent years, substantial
attention has been been paid to the identification of biomarker
targets, such as p53 (22) and heat
shock protein 70 (23). A previous
study showed that CAPS overexpression was significantly associated
with histological grade in endometrial cancer (24). Another study demonstrated that CAPS
could be a novel diagnostic biomarker for patients with colorectal
cancer. CAPS overexpression was positively associated with various
clinicopathological parameters, including histological grade, tumor
invasion, lymph node metastasis, TNM stage and distant metastasis
(17). As a result, we hypothesized
that the association between CAPS expression and
clinicopathological parameters is cancer type-dependent. The
present study examined CAPS mRNA levels in human ESCC tissues using
RT-qPCR, and to the best of our knowledge, for the first time,
demonstrated that CAPS mRNA expression was significantly associated
with tumor invasion and histological grade in ESCC (Fig. 1B and C; Table II). These results indicated that CAPS
may have a role in promoting ESCC progression.
Studies concerning ovarian cancer revealed that CAPS
was overexpressed in tumor tissues compared with healthy tissue
(20), and could be a predictive
marker for patients with favorable tumor biology and sensitivity to
treatment (13). Survival analysis
has indicated that CAPS is a potential survival predictor in breast
cancer patients receiving adjuvant tamoxifen (25). Previous studies also revealed that
CAPS was an independent prognostic factor for endometrial cancer
patients (15,24) and colorectal cancer patients (17). Therefore, we hypothesized that CAPS
might be a prognostic biomarker for patients with different types
of cancer. To verify this hypothesis in patients with ESCC, CAPS
protein expression was detected in ESCC tissues
viaimmunohistochemical staining. Results showed that 35 out of 64
tumors (54.69%) exhibited high CAPS expression, whereas 29 (45.31%)
exhibited low CAPS expression. Kaplan-Meier analysis and the
log-rank test found that ESCC patients with high CAPS expression
exhibited poorer overall survival rates compared with those
patients with low CAPS expression (Fig.
3). Univariate and multivariate analysis revealed that CAPS
expression was an independent survival predictor for ESCC (Table III).
CAPS is involved in several types of malignant
tumors. However, the molecular mechanism of CAPS function remains
elusive. As a Ca2+-binding protein, CAPS may mediate its
oncogenic effects through Ca2+ signaling, participating
in several cellular processes, such as cell proliferation and
apoptosis. Previous studies reported that alterations to
intracellular Ca2+homeostasis had a crucial role in
cancer development. In a study concerning prostate cancer, data
revealed that transient receptor potential cation channel subfamily
V member 6-dependent Ca2+ influx contributed to prostate
cancer development by enhancing proliferation of tumor cells and
protecting them from apoptosis (26).
Other studies demonstrated that higher plasma membrane channel
expression and Ca2+ influx were associated with
increased proliferation and tumor cell migration (27,28).
Therefore, we hypothesized that CAPS may promote tumorigenesis and
tumor progression by disturbing intracellular Ca2+
homeostasis. Aprevious study reported that CAPS was phosphorylated
and upregulated in response to thyrotropin and the cAMP cascade,
and down regulated by TPA and epidermal growth factor (9). CAPS may also contribute to tumorigenesis
and tumor progression through crosstalk between cAMP and EGF
signals. cAMP has a vital role in the proliferation of numerous
cell types (29,30). Compared with normal cells, lower cAMP
concentrations were found in certain tumor cells, which revealed
that cAM Phas a negative role in cell proliferation (30). Additionally, intracellular cAMP levels
were reported to be associated with the metastatic ability of tumor
cells (31,32). Epidermal growth factor signaling has
been well studied with regards to controlling cell proliferation,
differentiation and migration (33).
Although the aforementioned signaling pathways may contribute to
CAPS function in ESCC, further studies are required to clarify the
exact molecular mechanism.
To the best of our knowledge, the present study
demonstrated for the first time that CAPS mRNA and protein
expression levels were upregulated in human ESCC. High CAPS
expression was associated with histological grade and tumor
invasion depth. The overall survival time of patients with high
CAPS expression was significantly shorter than that of patients
with low CAPS expression. These results indicate that CAPS could be
a novel diagnostic indicator and an independent prognostic
biomarker in ESCC. Combining the pathological diagnosis with
assessment of CAPS expression levels may aid the diagnosis and
predict the prognosis of patients with ESCC.
Acknowledgements
The present study was supported by the Science and
Technology Innovation Team Support Plan in Universities of Henan
Province (grant no. 13IRTSTHN011). The authors would like to thank
Dr Xinfeng Chen (the First Affiliated Hospital of Zhengzhou
University, Zhengzhou, China) for providing assistance with the
experiments and Mr Jing He (Huashan Hospital Affiliated to Fudan
University, Shanghai, China) for partaking in valuable
discussions.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Yu X, Chen Q and Mao W: Neoadjuvant
versus adjuvant treatment: Which one is better for resectable
esophageal squamous cell carcinoma? World J Surg Oncol. 10:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lecocq R, Lamy F and Dumont JE: Pattern of
protein phosphorylation in intact stimulated cells: Thyrotropin and
dog thyroid. Eur J Biochem. 102:147–152. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamy F, Roger PP, Lecocq R and Dumont JE:
Differential protein synthesis in the induction of thyroid cell
proliferation by thyrotropin, epidermal growth factor or serum. Eur
J Biochem. 155:265–272. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El Housni H, Radulescu A, Lecocq R, Dumont
JE and Christophe D: Cloning and sequence analysis of human
calcyphosine complementary DNA. Biochim Biophys Acta. 1352:249–252.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nemoto Y, Ikeda J, Katoh K, Koshimoto H,
Yoshihara Y and Mori K: R2D5 antigen: A calcium-binding
phosphoprotein predominantly expressed in olfactory receptor
neurons. J Cell Biol. 123:963–976. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clément S, Dumont JE and Schurmans S: Loss
of calcyphosine gene expression in mouse and other rodents. Biochem
Biophys Res Commun. 232:407–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuasa HJ, Nakatomi A, Suzuki T and Yazawa
M: Genomic structure of the sponge, Halichondria okadai
calcyphosine gene. Gene. 298:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lecocq R, Lamy F and Dumont JE: Use of
two-dimensional gel electrophoresis and autoradiography as a tool
in cell biology: The example of the thyroid and the liver.
Electrophoresis. 11:200–212. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong H, Li X, Lou Z, Xu X, Su D, Zhou X,
Zhou W, Bartlam M and Rao Z: Crystal-structure and biochemical
characterization of recombinant human calcyphosine delineates a
novel EF-hand-containing protein family. J Mol Biol. 383:455–464.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lefort A, Lecocq R, Libert F, Lamy F,
Swillens S, Vassart G and Dumont JE: Cloning and sequencing of a
calcium-binding protein regulated by cyclic AMP in the thyroid.
EMBO J. 8:111–116. 1989.PubMed/NCBI
|
|
12
|
Mehrani H, Ghanei M, Aslani J and
Golmanesh L: Bronchoalveolar lavage fluid proteomic patterns of
sulfur mustard-exposed patients. Proteomics Clin Appl. 3:1191–1200.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Partheen K, Levan K, Osterberg L and
Horvath G: Expression analysis of stage III serous ovarian
adenocarcinoma distinguishes a sub-group of survivors. Eur J
Cancer. 42:2846–2854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Bont JM, den Boer ML, Kros JM, Passier
MM, Reddingius RE, Smitt PA, Luider TM and Pieters R:
Identification of novel biomarkers in pediatric primitive
neuroectodermal tumors and ependymomas by proteome-wide analysis. J
Neuropathol Exp Neurol. 66:505–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Min W, Huang C, Bai S, Tang M and
Zhao X: Proteomics-based approach identified differentially
expressed proteins with potential roles in endometrial carcinoma.
Int J Gynecol Cancer. 20:9–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pastor MD, Nogal A, Molina-Pinelo S,
Meléndez R, Salinas A, De la Peña González M, Martín-Juan J, Corral
J, García-Carbonero R, Carnero A and Paz-Ares L: Identification of
proteomic signatures associated with lung cancer and COPD. J
Proteomics. 89:227–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao W, Wang Q, Wang F, Jiang Y, Xu M and
Xu J: Abnormal expression of calcyphosine is associated with poor
prognosis and cell biology function in colorectal cancer. Onco
Targets Ther. 9:477–487. 2016.PubMed/NCBI
|
|
18
|
Rice TW, Blackstone EH and Rusch VW: 7th
edition of the AJCC cancer staging manual: Esophagus and
esophagogastric junction. Ann Surg Oncol. 17:1721–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skubitz AP, Pambuccian SE, Argenta PA and
Skubitz KM: Differential gene expression identifies subgroups of
ovarian carcinoma. Transl Res. 148:223–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tentzeris V, Lake B, Cherian T, Milligan J
and Sigurdsson A: Poor awareness of symptoms of oesophageal cancer.
Interact Cardiovasc Thorac Surg. 12:32–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujita Y, Nakanishi T, Miyamoto Y,
Hiramatsu M, Mabuchi H, Miyamoto A, Shimizu A, Takubo T and
Tanigawa N: Proteomics-based identification of autoantibody against
heat shock protein 70 as a diagnostic marker in esophageal squamous
cell carcinoma. Cancer Lett. 263:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Huang C, Bai S, Pan X, Zhou R, Wei Y
and Zhao X: Prognostic evaluation of epidermal fatty acid-binding
protein and calcyphosine, two proteins implicated in endometrial
cancer using a proteomic approach. Int J Cancer. 123:2377–2383.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johansson HJ, Sanchez BC, Forshed J, Stål
O, Fohlin H, Lewensohn R, Hall P, Bergh J, Lehtiö J and Linderholm
BK: Proteomics profiling identify CAPS as a potential predictive
marker of tamoxifen resistance in estrogen receptor positive breast
cancer. Clin Proteomics. 12:82015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raphaël M, Lehen'kyi V, Vandenberghe M,
Beck B, Khalimonchyk S, Abeele F Vanden, Farsetti L, Germain E,
Bokhobza A, Mihalache A, et al: TRPV6 calcium channel translocates
to the plasma membrane via Orai1-mediated mechanism and controls
cancer cell survival. Proc Natl Acad Sci USA. 111:E3870–E3879.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Déliot N and Constantin B: Plasma membrane
calcium channels in cancer: Alterations and consequences for cell
proliferation and migration. Biochim Biophys Acta. 1848:2512–2522.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Virgilio F: Purines, purinergic
receptors, and cancer. Cancer Res. 72:5441–5447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dumont JE, Jauniaux JC and Roger PP: The
cyclic AMP-mediated stimulation of cell proliferation. Trends
Biochem Sci. 14:67–71. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacManus JP, Whitfield JF, Boynton AL and
Rixon RH: Role of cyclic nucleotides and calcium in the positive
control of cell proliferation. Adv Cyclic Nucleotide Res.
5:719–734. 1975.PubMed/NCBI
|
|
31
|
Sheppard JR, Koestler TP, Corwin SP,
Buscarino C, Doll J, Lester B, Greig RG and Poste G: Experimental
metastasis correlates with cyclic AMP accumulation in B16 melanoma
clones. Nature. 308:544–547. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen TC, Hinton DR, Zidovetzki R and
Hofman FM: Up-regulation of the cAMP/PKA pathway inhibits
proliferation, induces differentiation, and leads to apoptosis in
malignant gliomas. Lab Invest. 78:165–174. 1998.PubMed/NCBI
|
|
33
|
Yarden Y and Pines G: The ERBB network: At
last, cancer therapy meets systems biology. Nat Rev Cancer.
12:553–563. 2012. View
Article : Google Scholar : PubMed/NCBI
|