Introduction
Over the last three decades, the number of breast
cancer cases has increased worldwide (1) to become the most likely cause of cancer
mortality and morbidity in females (2). Increased levels of aromatase expression
have been observed in breast lesions compared with normal breast
tissue (3,4) and alterations in aromatase expression
are associated with the pathogenesis of breast cancer (5,6).
Approximately two thirds of breast cancer cases
overexpress estrogen receptors (ER) and/or progesterone receptors
(PgR) (7,8). Consequently, endocrine therapy,
including tamoxifen or aromatase inhibitors (AIs), has become an
effective treatment for these patients. For decades, 5-year
tamoxifen administration was the gold standard for the adjuvant
endocrine treatment of breast cancer (9). More recently, postmenopausal patients
have also had the option of receiving AIs as an alternative to
tamoxifen, or following tamoxifen treatment (10). The presence and intensity of ER and/or
PgR are useful predictive markers for the response to hormone
therapy in clinical practice. Identification of more accurate
biomarkers for the most efficient patient selection to exclude
non-responsive patients remains crucial.
Aromatase, encoded by CYP19A1 gene, catalyzes
the final step of the conversion from androgens into estrogens
(3,11,12). In
premenopausal females, estrogen is predominantly produced by the
ovary, with a small amount generated by the aromatization of
adrenal and ovarian androgens in extragonadal tissue. Conversely,
in postmenopausal females, the aromatization of androgens from
extragonadal tissue becomes the prime origin of estrogen as the
ovary ceases to function (7,12,13).
It has previously been suggested that genetic
polymorphisms in CYP19A1 gene were associated with aromatase
activity as well as circulating steroid hormone levels in females
(3,11,12,14–16).
Therefore, it is biologically reasonable that CYP19A1 gene
polymorphism may be associated with the clinical outcome of hormone
therapy. Population-based studies of CYP19A1 gene
polymorphisms have revealed controversial results regarding their
potential association with the therapeutic efficacy of endocrine
treatment. Kuo et al (17)
revealed that the A allele of CYP19A1 rs4646 was
significantly in association with poorer distant disease-free
survival rate (P<0.05) and marginally associated with shorter
overall survival (OS; P=0.06) or disease-free survival (DFS;
P=0.07) in lymph node-negative, hormone receptor-positive patients
with endocrine therapy. In addition, a study conducted by
Garcia-Casado et al (18)
estimated that the same variant was significantly associated with
poorer progression-free survival (PFS) in patients with letrozole
neo-adjuvant therapy, and patients with genotypic variants of
rs4646 were more frequently represented in the non-responder cohort
(48 vs. 26%). However, Liu et al (19) suggested that A allele of rs4646 was
significantly associated with longer time to progression (TTP) and
OS when assessed in patients with metastatic breast cancer (MBC)
receiving anastrozole treatment, consistent with the study of
Colomer et al (20), which
indicated that TTP was significantly prolonged in patients with the
minor T allele of CYP19A1 rs4646 compared with those with
homozygous common allele (GG) from a cohort of postmenopausal MBC
with letrozole administration.
CYP19A1 gene is located at chromosomal locus
15q21.1 and has a complex structure with a regulatory region that
includes 10 tissue-specific non-coding upstream exons, with
separate promoters, which regulate transcription in different
tissues (21). Haplotype blocks 1 to
4 are located in this regulatory region. The rs1008805 polymorphism
(A/G) is located in block 3. The frequency of the minor allele (G)
is approximately 29.5% in Chinese females (22). A study conducted in Chinese population
demonstrated that single nucleotide polymorphisms (SNPs) in block 1
and 2 of CYP19A1 gene were associated with the plasma levels
of estrogen in postmenopausal females (23). Furthermore, it has been identified
that the G allele of the rs1008805 SNP was significantly associated
with the risk of breast cancer (24).
Consequently, hypotheses were formulated that rs1008805
polymorphism may be associated with response of hormone therapy,
which is still lacking supporting data.
Accordingly, a genetic analysis of CYP19A1
rs1008805 polymorphism was performed with a cohort of patients with
hormone receptor-positive early breast cancer in order to elucidate
whether rs1008805 variants were associated with the clinical
outcome of hormone therapy.
Patients and methods
Study cohort and data
A total of 287 Chinese females with hormone
receptor-positive stage I–II and operable stage III breast cancer,
according to the tumor-node-metastasis stage classification
(25), were enrolled in the present
study between 1 April 2004 and 31 July 2010 at Zhejiang Cancer
Hospital (Hangzhou, Zhejiang). All of the patients received hormone
therapy. In brief, 250 patients received tamoxifen therapy and 37
received third-generation aromatase inhibitors. A total of 274
(95.5%) of the patients received adjuvant chemotherapy, whereas 130
(45.3%) received radiotherapy. A total of 274 patients (95.5%)
received chemotherapy including cyclophosphamide, doxorubicin and
fluoracil or cyclophosphamide, epirubicin and fluoracil or
doxorubicin, cyclophosphamide (AC) or fluoracil, epirubicin and
cyclophosphamide followed by docetaxel or weekly paclitaxel
treatment or docetaxel, doxorubicin and cyclophosphamide,
cyclophosphamide and epirubicin or AC followed by docetaxel or
weekly paclitaxel, 8 (2.5%) with no chemotherapy and 6 (2.0%)
remained unknown. Human epidermal growth factor receptor-2 positive
females received Trastuzumab treatment. A 3-mlperipheral blood
sample was obtained and processed for DNA extraction in the
Department of Oncology. The pathologic review, blood samples and
genetic studies were approved by the institutional review board of
Zhejiang Cancer Hospital. All patients provided written informed
consent according to the guidelines of the Ethics Committee of
Zhejiang Cancer Hospital.
DNA extraction and genotyping
Genomic DNA was extracted from peripheral blood with
the AxyPrep Blood Genomic DNA Miniprep kit (Axygen; Corning Life
Sciences, Union City, CA, USA). Genotyping was conducted through
the Sequenom MassARRAY matrix-assisted laser
desorption/ionization-time of flight mass spectrometry platform
(Sequenom, San Diego, CA, USA) as previously described (26). Primers (5′-TCCTTACCGAATCACTACCC-3′ and
5′-CCTGCTATTACTTCCAACCC-3′) and single base extensions were
designed with Assay Designer software (version 3.0; Sequenom) and
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Multiplex PCR was performed in 5 µl volumes
containing 10 ng whole-genome-amplified genomic DNA, 2.5 pmol of
each PCR primer, 0.1 unit of HotStar Taq polymerase (Qiagen GmbH,
Hilden, Germany) and 2.5 µmol deoxynucleotides (dNTP; Qiagen GmbH).
Thermo cycling was performed at 94°C for 15 min followed by 45
cycles at 94°C for 20 sec, 56°C for 30 sec and 72°C for 1 min and a
final incubation at 72°C for 3 min. Unincorporated dNTPs were
deactivated with 0.3 units of shrimp alkaline phosphatase
(Sequenom) followed by primer extension using 5.4 pmol of each
primer extension probe, 50 µmol of the appropriate ddNTP
combination, and 0.5 units of iPLEX enzyme (Sequenom). The
extension reactions were performed at 94°C for 30 sec and then 94°C
for 5 sec, followed by 5 cycles at 52°C for 5 sec and 80°C for 5
sec for a total of 40 cycles, and then 72°C for 3 min. A cation
exchange resin was used to remove residual salt from the reactions.
Purified primer extension reaction products were spotted onto a
384-well spectro CHIP using the Mass ARRAY Nano dispenser and
determined by the mass spectrometer. Genotype analysis was
performed in real time using MassARRAY RT software (version
3.0.0.4) and analyzed using MassARRAY Typer software (version 3.4;
Sequenom).
Statistical analysis
The deviation from Hardy-Weinberg equilibrium (HWE)
was assessed with Pearson's χ2 test using the HWE
calculator described in Rodriguez et al (27).
Follow-up data as available on 31 December 2014,
were analyzed. DFS was defined as the date of the original surgery
for breast cancer to the date of recurrence or mortality from any
causes (28). DFS plots were produced
using the Kaplan-Meier estimator method. Differences in median DFS
were compared using the log-rank test.
Cox's regression analyses were conducted to estimate
the hazard ratio (HR) and corresponding 95% confidence interval
(CI) for each variable. The multivariate-adjusted HR for relapse
associated with the individual genotypes was examined for the
groups subsequent to adjusting for other variables (lymph node
positivity, tumor size >2 cm, negative hormone receptor status,
human epidermal growth factor receptor-2 positive status,
chemotherapy, hormone therapy, radiotherapy and body mass index
≥24). These analyses were performed using the SPSS statistical
software package (version 17.0; SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient features
A total of 287 females with hormone
receptor-positive early breast cancer were enrolled in the present
study, and the median age was 46 years (range, 20–73 years). As
presented in Table I, 217 patients
were premenopausal and 70 were postmenopausal. The
clinicopathological features details are also listed in Table I.
 | Table I.Association of CYP19A1
polymorphisms with clinical characteristics. |
Table I.
Association of CYP19A1
polymorphisms with clinical characteristics.
|
|
| Polymorphism type,
n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | AA | AG | GG | P-value |
|---|
| Total | 287 | 145 (50.5) | 124 (43.2) | 18 (6.3) |
|
| Menopausal
status |
|
|
|
| 0.902 |
|
Premenopausal | 217 | 111 (76.6) | 93 (75.0) | 13 (72.2) |
|
|
Postmenopausal | 70 | 34 (23.4) | 31 (25.0) | 5 (27.8) |
|
| Tumor size, cm |
|
|
|
| 0.672 |
| ≤2 | 102 | 52 (35.9) | 44 (35.5) | 6 (33.3) |
|
|
>2 | 168 | 82
(56.6) | 74 (59.7) | 12 (66.7) |
|
|
Unknown | 17 | 11 (7.6) | 6 (4.8) | 0 |
|
| Lymph node invasion
status |
|
|
|
| 0.853 |
|
Negative | 82 | 41 (28.3) | 37 (29.8) | 4 (22.2) |
|
|
Positive | 199 | 100 (69.0) | 85 (68.5) | 14 (77.8) |
|
|
Unknown |
6 | 4 (2.8) | 2 (1.6) | 0 |
|
| TNM stage |
|
|
|
| 0.266 |
|
I–II | 156 | 83
(57.2) | 65 (52.4) | 8 (44.4) |
|
|
III | 108 | 48 (33.1) | 50 (40.3) | 10 (55.6) |
|
|
Unknown | 23 | 14 (9.7) | 9 (7.3) | 0 |
|
| Estrogen receptor
status |
|
|
|
| 0.266 |
|
Negative | 36 | 16 (11.0) | 20 (16.1) | 0 |
|
|
Positive | 245 | 125 (86.2) | 102 (82.3) | 18 (100.0) |
|
|
Unknown |
6 | 4 (2.8) | 2 (1.6) | 0 |
|
| Progesterone
receptor status |
|
|
|
| 0.861 |
|
Negative | 65 | 33 (22.8) | 29 (23.4) | 3 (16.7) |
|
|
Positive | 216 | 108 (74.5) | 93 (75.0) | 15 (83.3) |
|
|
Unknown |
6 | 4 (2.8) | 2 (1.6) | 0 |
|
| Erb-B2 receptor
tyrosine |
|
|
|
| 0.095 |
|
kinase-2 status |
|
|
|
|
|
|
Negative | 174 | 79 (54.5) | 83 (66.9) | 12 (66.7) |
|
|
Positive | 70 | 37 (25.5) | 30 (24.2) | 3 (16.7) |
|
|
Unknown | 43 | 29 (20.0) | 11 (8.9) | 3 (16.7) |
|
| Body mass index,
kg/m2 |
|
|
|
| 0.671 |
|
<24 | 159 | 84 (57.9) | 64 (52.0) | 11 (61.1) |
|
|
≥24 | 126 | 61 (42.1) | 58 (47.2) | 7 (38.9) |
|
|
Unknown |
2 | 0 | 2 (0.8) | 0 |
|
In total, there were 145 patients with AA genotype,
124 with AG variant, and 18 with GG genotype. Genotype frequencies
observed in our patient cohort were consistent with Hardy-Weinberg
equilibrium (P>0.05, data not shown).
CYP19A1 rs1008805 polymorphism and DFS
in the whole cohort
Based on the analysis of all patients, no
significant differences were observed between rs1008805 genotypes
and DFS (AA vs. AG vs. GG, 58.3 vs. 57.7 vs. 42.7 months; P=0.638;
Table II). In addition, there was no
difference for DFS between patients with the minor allele, i.e., AG
or GG, and those carrying the homozygous common allele AA (AG or GG
vs. AA, 56.4 vs. 58.3 months; HR, 0.891; 95% CI, 0.675–1.177;
P=0.417; Table II). Furthermore,
when the population was split into two groups, one with GG variant
and the other with AG or AA genotypes, there was no association
between genotypes and DFS (GG vs. AG or AA, 42.7 vs. 58.0 months;
HR, 1.092; 95% CI, 0.593–2.010; P=0.777; Table II).
 | Table II.Association of CYP19A1
rs1008805 polymorphism with disease-free survival. |
Table II.
Association of CYP19A1
rs1008805 polymorphism with disease-free survival.
|
|
| Univariate |
Multivariatea |
|---|
|
|
|
|
|
|---|
| CYP19A1
polymorphism | Median DFS,
months | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| All patients |
|
|
|
|
|
| GG vs.
AG vs. AA | 42.7 vs. 57.7 | AG:AA,0.875 | 0.638 | AG:AA, 0.794 | 0.309 |
|
| vs. 58.3 | (0.657–1.167) |
| (0.586–1.075) |
|
|
|
| GG:AA,1.030 |
| GG:AA, 1.014 |
|
|
|
| (0.553–1.919) |
| (0.540–1.902) |
|
| GG vs.
AA/AG | 42.7 vs. 58.0 | 1.092
(0.593–2.010) | 0.777 | 1.121
(0.605–2.077) | 0.716 |
| AG/GG
vs. AA | 56.4 vs. 58.3 | 0.891
(0.675–1.177) | 0.417 | 0.817
(0.610–1.094) | 0.175 |
| Premenopausal
patients |
|
|
|
|
|
| GG vs.
AG vs. AA | 98.2 vs. 57.7 | AG:AA, 0.811 | 0.252 | AG:AA, 0.093 | 0.183 |
|
| vs. 56.2 | (0.587–1.120) |
| (0.7430–0.526) |
|
|
|
| GG:AA, 0.590 |
| GG:AA, 0.646 |
|
|
|
| (0.258–1.353) |
| (0.279–1.495) |
|
| GG vs.
AA/AG | 98.2 vs. 56.4 | 0.648
(0.286–1.468) | 0.294 | 0.737
(0.323–1.684) | 0.469 |
| AG/GG
vs. AA | 58.6 vs. 56.2 | 0.786
(0.574–1.076) | 0.132 | 0.783
(0.557–1.102) | 0.161 |
| Postmenopausal
patients |
|
|
|
|
|
| GG vs.
AG vs. AA | 58.2 vs. 89.2 | AG:AA, 3.750 | 0.019 | AG:AA, 1.015 | 0.116 |
|
|
| (1.371–10.256) |
| (0.476–2.165) |
|
|
| 32.7 vs. 89.2 | GG:AA, 1.086 |
| GG:AA, 3.468 |
|
|
|
| (0.572–2.062) |
| (1.160–10.369) |
|
| GG vs.
AA/AG | 32.7 vs. 70.6 | 3.613
(1.380–9.457) | 0.005 | 3.439
(1.251–9.456) | 0.017 |
| AG/GG
vs. AA | 52.4 vs. 89.2 | 1.288
(0.705–2.353) | 0.408 | 1.843
(0.875–3.883) | 0.108 |
CYP19A1 rs1008805 polymorphism and DFS
in premenopausal patients
In premenopausal females, there was no significant
association between rs1008805 genotypes and DFS (AA vs. AG vs. GG,
56.2 vs. 57.7 vs. 98.2 months; P=0.252; Table II). When the patients were clustered
into two groups, one with AA variant, and the other with AG or GG
genotypes, no significant differences in DFS were evident between
these subgroups (AG or GG vs. AA, 58.6 vs. 56.2 months; HR, 0.786;
95%CI, 0.574–1.076; P=0.132; Table
II). Besides these, there were also no differences for DFS
between patients with the homozygous minor allele (GG) and those
carrying the common allele (GG vs. AA or AG, 98.2 vs. 56.4 months;
HR, 0.648; 95%CI, 0.286–1.468; P=0.294; Table II).
CYP19A1 rs1008805 polymorphism and DFS
in postmenopausal patients
In postmenopausal patients, rs1008805 genotypes were
significantly associated with DFS (AA vs. AG vs. GG, 89.2 vs. 58.2
vs. 32.7 months; P=0.019; Table II;
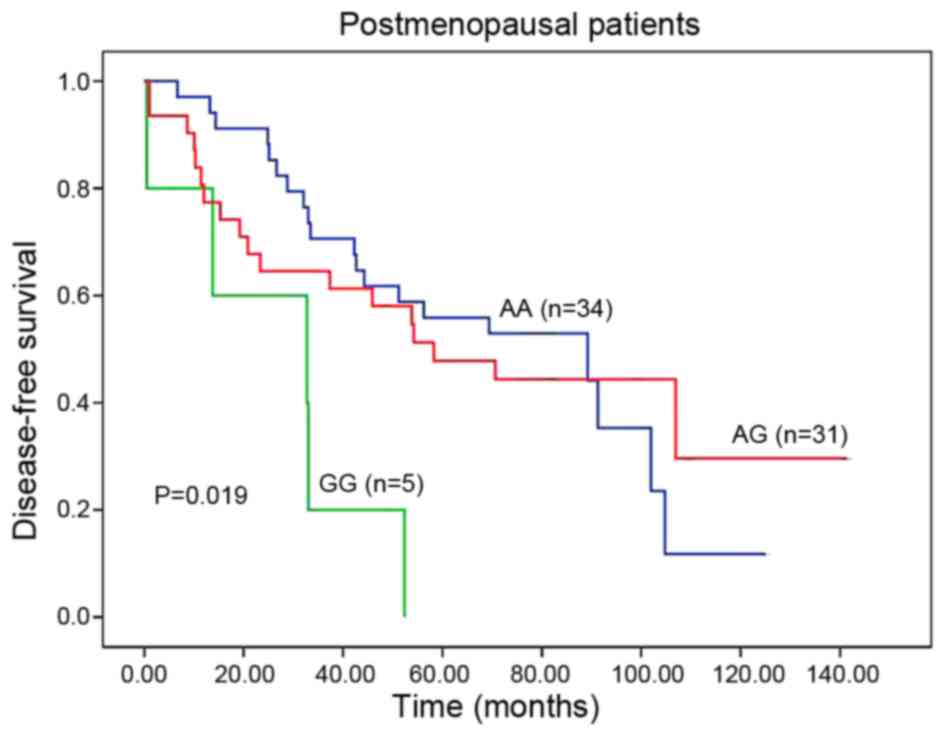
Fig. 1). When the population was
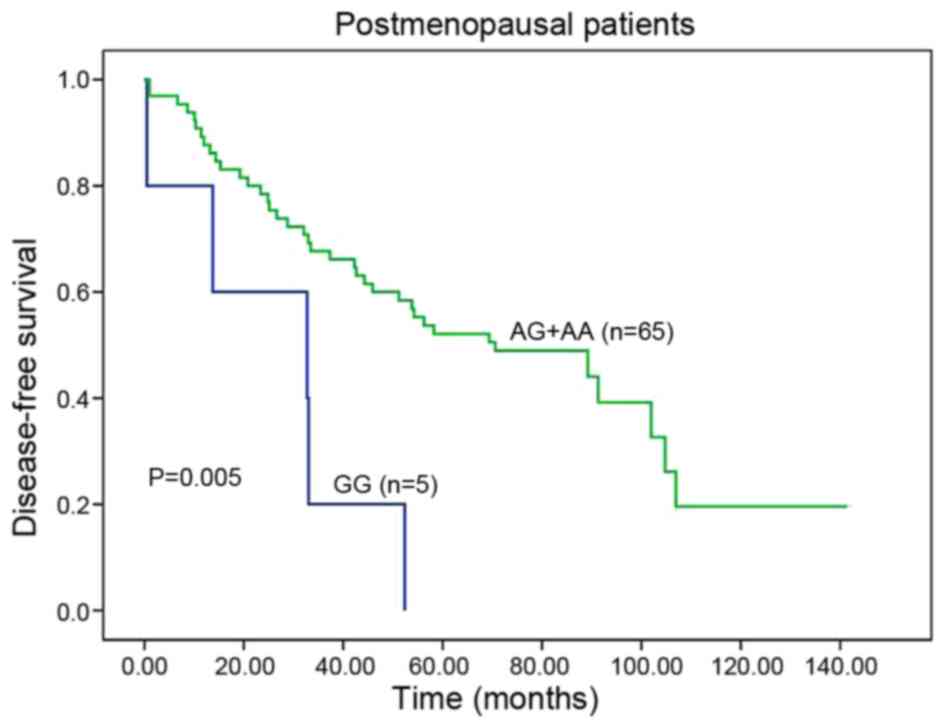
subdivided to two cohorts, the patients carrying GG variant had a
significantly poorer DFS than those harboring AA or AG genotypes
(GG vs. AA or AG, 32.7 vs. 70.6 months; HR, 3.613; 95% CI,
1.380–9.457; P=0.005; Table II;
Fig. 2). Furthermore, being adjusted
by patient features in multivariate analyses, GG genotype remained
an independent prognostic marker for DFS (HR, 3.439; 95% CI,
1.251–9.456; P=0.017; Table II).
However, there was no significant difference in DFS between females
harboring the minor allele and those with the homozygous common
allele in a univariate analysis (AG or GG vs. AA, 52.4 vs. 89.2
months; HR, 1.288; 95% CI, 0.705–2.353; P=0.408; Table II).
Discussion
The results of the present study describe an
association between rs1008805 variants of CYP19A1 gene and
the efficacy of hormone therapy in postmenopausal females with
early breast cancer. Previous studies attempting to identify the
association between the expression of aromatase mRNA or protein and
aromatase enzyme activity levels with therapeutic response have
been indefinite and contradictory (17–20). In
the present study, postmenopausal females with the homozygous GG
variant of CYP19A1 rs1008805 exhibit a poorer DFS when
compared with those carrying AG or AA genotypes. This difference
was further confirmed by multivariate analysis. These findings are
biologically reasonable when considering the essential role of
CYP19A1 gene in estrogen metabolism, its potential activity
in tumor growth and progression, as well as the potential
functional significance of CYP19A1 genetic
polymorphisms.
Several genotypic polymorphisms of CYP19A1
gene have generated inconsistent results with regard to their
potential association with clinical outcome. Liu et al
(19) demonstrated that TTP and OS
were significantly improved in patients with the variant alleles of
rs4646 (TT or TG) when compared with patients carrying the
wild-type allele (GG) in 272 females with MBC treated with
anastrozole. Besides, the data from Colomer et al (20) revealed that patients with the rare T
allele of rs4646 had a TTP that was three times that of those
harboring the homozygous common genotype (GG). However, the data
from 95 consecutive postmenopausal females with stage II–III
hormone receptor-positive breast cancer revealed that the T allele
of rs4646 was associated with poorer response to letrozole
neoadjuvant therapy, and the rare allele also appeared to be
associated with shorter PFS when compared with those carrying the
homozygous common allele, and this effect was particularly
significant among elderly patients with no operation following
letrozole induction (18). In
contrast, Ghimenti et al (29)
identified that rs6493497 and rs7176005 polymorphisms were not
associated with the efficacy of anastrozole neoadjuvant therapy,
aromatase mRNA basal expression level or expression alteration
caused by therapy.
Estrogen serves a key role in the growth and
progression of breast cancer through disrupting the processes of
cell differentiation and proliferation (30). Lønning et al (31) demonstrated that circulating estrogen
levels were significantly associated with poorer DFS in
postmenopausal patients. In a case-control cohort study, Rock et
al (32) indicated that total
estradiol, bioavailable estradiol and free estradiol circulating
concentrations were associated with the risk of recurrence.
Aromatase catalyzes the biosynthesis of estrogen in the adipose
tissues through the conversion of androgens (3,11,12). In addition, elevated levels of
aromatase expression have been detected in malignant breast lesions
compared with in normal breast tissue (4,33).
Besides, a number of previous studies have demonstrated that
CYP19A1 gene polymorphisms may be associated with increased
aromatase activity. Wang et al (34) and Gennari et al (35) indicated that rs6493497 and rs7176005
were associated with marked decrease in aromatase activity, and
Kristensen et al (13)
observed that longer TTTA repeats were associated with increased
aromatase activity. A population-based and in vitro study
revealed that a Thr364 mutation caused a sharp decrease in
aromatase protein levels and activity, whereas a Cys264 mutation
was associated with a slight decrease in allozyme activity.
Furthermore, the mechanism by which non-synonymous SNPs interfere
with the aromatase enzymatic activity was a consequence of an
alteration in the aromatase protein level (36). Of note, it has been suggested that
CYP19A1 polymorphisms were significantly associated with
hormone levels (14,15,37).
Previous studies have indicated that the rs4646 may be associated
with circulating hormone levels in postmenopausal breast cancer
(18,20). An analysis of five large prospective
cohort studies demonstrated that the A allele of rs727479 and
rs749292 were significantly associated with elevated levels of
estradiol and estrone (11). Haiman
et al (38) revealed that
females carrying 8-repeat allele of the TTTA polymorphism exhibited
increased estrogen levels compared with those harboring the
7-repeat allele. Cai et al (23) demonstrated that SNPs in block 1 and 2
of CYP19A1 gene were associated with plasma estrogen levels
in postmenopausal Chinese females. Analysis of a comprehensive
evaluation of majority variants in whole CYP19A1 gene
revealed that a two SNP haplotype (rs749292-rs727479 A-A) was
associated with a 15% high erestrogen levels in postmenopausal
females (39).
The presence of the G allele at rs1008805 has been
identified to be significantly associated with an increase in the
risk of breast cancer (40).
Additionally, Haiman et al (24) identified that the same genotype was
marginally significant associated with breast cancer risk. The
present study demonstrated that postmenopausal females with GG
variant of CYP19A1 rs1008805 have a poorer DFS for hormone
therapy compared with those carrying AG or AA genotypes. The
majority of SNPs are silent, and thus may not cause alterations to
the function or the expression of mRNA (41). However, CYP19A1 rs1008805 SNP
investigated in the present study appears to have an active effect.
This maybe linked with an advantage structural alteration to the
aromatase protein structure that causes it to be more active
(42). A number of other mechanisms
are also possible, including an alteration to a DNA-binding site
(43,44), mRNA stabilization, splicing or folding
alterations, and the modification of transcriptional and
post-translational regulation (45–47).
On the basis of these previous studies and the
results of the present study, it is hypothesized that
CYP19A1 rs1008805 G SNP may result in elevated aromatase
activity, higher protein levels, and thus an increase in
circulating estrogen concentrations in postmenopausal females. It
is a plausible hypothesis that the GG variant of rs1008805 may
cause diminished functional efficacy of hormone therapy when used
at the recommended dose, allowing estrogen synthesis to be
maintained in the subgroup of postmenopausal patients carrying the
GG genotype of rs1008805 during endocrine treatment. However, the
presumed difference in estrogen level would be decreased between
the premenopausal patients with the GG variant and those with AA or
AG, as estrogen is predominantly generated by the ovary in
premenopausal females, perhaps causing the effect of CYP19A1
variants on estrogen levels to be negligible for this group.
Therefore, the GG genotype was associated with decreased DFS in
postmenopausal patients with hormone therapy, whereas there was no
significant association between rs1008805 genotypes and DFS for
premenopausal females receiving endocrine treatment.
To conclude, it was demonstrated that the GG
genotype of rs1008805 SNP in the first exon of CYP19A1 gene
was significantly associated with inferior DFS in postmenopausal
females with hormone therapy. Testing for CYP19A1 gene
rs1008805 SNP as a predictive marker for the response to endocrine
therapy in hormone receptor-positive early breast cancer warrants a
larger independent prospective clinical evaluation.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Natural Science Foundation (grant nos. Y2101312, and
LY17H160041), the Zhejiang Provincial Medical Science and
Technology program (grant nos. 2010QNA006, 2015RCB005 and
2016KYA046), the Zhejiang Science and Technology program (grant no.
2016C33199), the Zhejiang Traditional Chinese Medicine Science and
Technology program (grant nos. 2013ZB022 and 2014ZB019) and the
Special fund of Wu Jieping Medical Foundation Clinical Research
(grant no. 320.670010007). This abstract was presented at the
GAP2016 Challenging Cancer Meeting, 24–28 April 2016, Barretos and
São Paulo, Brazil and was published as Abstract no. 666.
References
|
1
|
Pedraza AM, Pollan M, Pastor-Barriuso R
and Cabanes A: Disparities in breast cancer mortality trends in a
middle income country. Breast Cancer Res Treat. 134:1199–1207.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hortobagyi GN, de la Garza Salazar J,
Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC,
Martin M, Namer M, et al: The global breast cancer burden:
Variations in epidemiology and survival. Clinical Breast Cancer.
6:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bulun SE, Sebastian S, Takayama K, Suzuki
T, Sasano H and Shozu M: The human CYP19 (aromatase P450) gene:
Update on physiologic roles and genomic organization of promoters.
J Steroid Biochem Mol Biol. 86:219–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irahara N, Miyoshi Y, Taguchi T, Tamaki Y
and Noguchi S: Quantitative analysis of aromatase mRNA expression
derived from various promoters (I.4, I.3, PII and I.7) and its
association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in
human breast cancer. Int J Cancer. 118:1915–1921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Neill JS, Elton RA and Miller WR:
Aromatase activity in adipose tissue from breast quadrants: A link
with tumour site. Br Med J (Clin Res Ed). 296:741–743. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agarwal VR, Bulun SE, Leitch M, Rohrich R
and Simpson ER: Use of alternative promoters to express the
aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of
cancer-free and breast cancer patients. J Clin Endocrinol Metab.
81:3843–3849. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulun SE, Lin Z, Imir G, Amin S, Demura M,
Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al:
Regulation of aromatase expression in estrogen-responsive breast
and uterine disease: From bench to treatment. Pharmacol Rev.
57:359–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toniolo PG, Levitz M, Zeleniuch-Jacquotte
A, Banerjee S, Koenig KL, Shore RE, Strax P and Pasternack BS: A
prospective study of endogenous estrogens and breast cancer in
postmenopausal women. J Natl Cancer Inst. 87:190–197. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldhirsch A, Gelber RD and Coates AS:
What are the long-term effects of chemotherapy and hormonal therapy
for early breast cancer? Nat Clin Pract Oncol. 2:440–441. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burstein HJ, Prestrud AA, Seidenfeld J,
Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis
CA, Malin J, et al: American society of clinical oncology clinical
practice guideline: Update on adjuvant endocrine therapy for women
with hormone receptor-positive breast cancer. J Clin Oncol.
28:3784–3796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Mendelson CR and Simpson ER:
Characterization of the sequences of the human CYP19 (aromatase)
gene that mediate regulation by glucocorticoids in adipose stromal
cells and fetal hepatocytes. Mol Endocrinol. 9:340–349. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santen RJ, Brodie H, Simpson ER, Siiteri
PK and Brodie A: History of aromatase: Saga of an important
biological mediator and therapeutic target. Endocr Rev. 30:343–375.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristensen VN, Harada N, Yoshimura N,
Haraldsen E, Lonning PE, Erikstein B, Karesen R, Kristensen T and
Borresen-Dale AL: Genetic variants of CYP19 (aromatase) and breast
cancer risk. Oncogene. 19:1329–1333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haiman CA, Dossus L, Setiawan VW, Stram
DO, Dunning AM, Thomas G, Thun MJ, Albanes D, Altshuler D, Ardanaz
E, et al: Genetic variation at the CYP19A1 locus predicts
circulating estrogen levels but not breast cancer risk in
postmenopausal women. Cancer Res. 67:1893–1897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunning AM, Dowsett M, Healey CS, Tee L,
Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, et
al: Polymorphisms associated with circulating sex hormone levels in
postmenopausal women. J Natl Cancer Inst. 96:936–945. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitrunen K and Hirvonen A: Molecular
epidemiology of sporadic breast cancer. The role of polymorphic
genes involved in oestrogen biosynthesis and metabolism. Mutat Res.
544:9–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo SH, Yang SY, Lien HC, Lo C, Lin CH, Lu
YS, Cheng AL, Chang KJ and Huang CS: CYP19 genetic polymorphism
haplotype AASA is associated with a poor prognosis in premenopausal
women with lymph node-negative, hormone receptor-positive breast
cancer. Biomed Res Int. 2013:5621972013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Casado Z, Guerrero-Zotano A,
Llombart-Cussac A, Calatrava A, Fernandez-Serra A, Ruiz-Simon A,
Gavila J, Climent MA, Almenar S, Cervera-Deval J, et al: A
polymorphism at the 3′-UTR region of the aromatase gene defines a
subgroup of postmenopausal breast cancer patients with poor
response to neoadjuvant letrozole. BMC Cancer. 10:362010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Bai YX, Zhou JH, Sun XW, Sui H,
Zhang WJ, Yuan HH, Xie R, Wei XL, Zhang TT, et al: A polymorphism
at the 3′-UTR region of the aromatase gene is associated with the
efficacy of the aromatase inhibitor, anastrozole, in metastatic
breast carcinoma. Int J Mol Sci. 14:18973–18988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colomer R, Monzo M, Tusquets I, Rifa J,
Baena JM, Barnadas A, Calvo L, Carabantes F, Crespo C, Munoz M, et
al: A single-nucleotide polymorphism in the aromatase gene is
associated with the efficacy of the aromatase inhibitor letrozole
in advanced breast carcinoma. Clin Cancer Res. 14:811–816. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpson ER: Aromatase: Biologic relevance
of tissue-specific expression. Semin Reprod Med. 22:11–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long JR, Kataoka N, Shu XO, Wen W, Gao YT,
Cai Q and Zheng W: Genetic polymorphisms of the CYP19A1 gene and
breast cancer survival. Cancer Epidemiol Biomarkers Prev.
15:2115–2122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai H, Shu XO, Egan KM, Cai Q, Long JR,
Gao YT and Zheng W: Association of genetic polymorphisms in CYP19A1
and blood levels of sex hormones among postmenopausal Chinese
women. Pharmacogenet Genomics. 18:657–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haiman CA, Stram DO, Pike MC, Kolonel LN,
Burtt NP, Altshuler D, Hirschhorn J and Henderson BE: A
comprehensive haplotype analysis of CYP19 and breast cancer risk:
The multiethnic cohort. Hum Mol Genet. 12:2679–2692. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Theriault RL, Carlson RW, Allred C,
Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano
SH, Goldstein LJ, et al: Breast cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:753–761.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carbonnelle E, Mesquita C, Bille E, Day N,
Dauphin B, Beretti JL, Ferroni A, Gutmann L and Nassif X: MALDI-TOF
mass spectrometry tools for bacterial identification in clinical
microbiology laboratory. Clin Biochem. 44:104–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodriguez S, Gaunt TR and Day IN:
Hardy-Weinberg equilibrium testing of biological ascertainment for
Mendelian randomization studies. Am J Epidemiol. 169:505–514. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hudis CA, Barlow WE, Costantino JP, Gray
RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA,
Gelber RD, et al: Proposal for standardized definitions for
efficacy end points in adjuvant breast cancer trials: The STEEP
system. J Clin Oncol. 25:2127–2132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghimenti C, Mello-Grand M, Grosso E,
Scatolini M, Regolo L, Zambelli A and Chiorino G: Regulation of
aromatase expression in breast cancer treated with anastrozole
neoadjuvant therapy. Exp Ther Med. 5:902–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouchard MF, Taniguchi H and Viger RS:
Protein kinase A-dependent synergism between GATA factors and the
nuclear receptor, liver receptor homolog-1, regulates human
aromatase (CYP19) PII promoter activity in breast cancer cells.
Endocrinology. 146:4905–4916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lønning PE, Helle SI, Johannessen DC, Ekse
D and Adlercreutz H: Influence of plasma estrogen levels on the
length of the disease-free interval in postmenopausal women with
breast cancer. Breast Cancer Res Treat. 39:335–341. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rock CL, Flatt SW, Laughlin GA, Gold EB,
Thomson CA, Natarajan L, Jones LA, Caan BJ, Stefanick ML, Hajek RA,
et al: Reproductive steroid hormones and recurrence-free survival
in women with a history of breast cancer. Cancer Epidemiol
Biomarkers Prev. 17:614–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Jong PC, Blankenstein MA, van de Ven J,
Nortier JW, Blijham GH and Thijssen JH: Importance of local
aromatase activity in hormone-dependent breast cancer: A review.
Breast. 10:91–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Ellsworth KA, Moon I,
Pelleymounter LL, Eckloff BW, Martin YN, Fridley BL, Jenkins GD,
Batzler A, Suman VJ, et al: Functional genetic polymorphisms in the
aromatase gene CYP19 vary the response of breast cancer patients to
neoadjuvant therapy with aromatase inhibitors. Cancer Res.
70:319–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gennari L, Masi L, Merlotti D, Picariello
L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S,
Lucani B, et al: A polymorphic CYP19 TTTA repeat influences
aromatase activity and estrogen levels in elderly men: Effects on
bone metabolism. J Clin Endocrinol Metab. 89:2803–2810. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma CX, Adjei AA, Salavaggione OE, Coronel
J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei
AA, et al: Human aromatase: Gene resequencing and functional
genomics. Cancer Res. 65:11071–11082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tworoger SS, Chubak J, Aiello EJ, Ulrich
CM, Atkinson C, Potter JD, Yasui Y, Stapleton PL, Lampe JW, Farin
FM, et al: Association of CYP17, CYP19, CYP1B1, and COMT
polymorphisms with serum and urinary sex hormone concentrations in
postmenopausal women. Cancer Epidemiol Biomarkers Prev. 13:94–101.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haiman CA, Hankinson SE, Spiegelman D, De
Vivo I, Colditz GA, Willett WC, Speizer FE and Hunter DJ: A
tetranucleotide repeat polymorphism in CYP19 and breast cancer
risk. Int J Cancer. 87:204–210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haiman CA, Hsu C, de Bakker PI, Frasco M,
Sheng X, Van Den Berg D, Casagrande JT, Kolonel LN, Le Marchand L,
Hankinson SE, et al: Comprehensive association testing of common
genetic variation in DNA repair pathway genes in relationship with
breast cancer risk in multiple populations. Hum Mol Genet.
17:825–834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Talbott KE, Gammon MD, Kibriya MG, Chen Y,
Teitelbaum SL, Long CM, Gurvich I, Santella RM and Ahsan H: A CYP19
(aromatase) polymorphism is associated with increased premenopausal
breast cancer risk. Breast Cancer Res Treat. 111:481–487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Erichsen HC and Chanock SJ: SNPs in cancer
research and treatment. Br J Cancer. 90:747–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsigelny IF, Kotlovyi V and Wasserman L:
SNP analysis combined with protein structure prediction defines
structure-functional relationships in cancer related cytochrome
P450 estrogen metabolism. Curr Med Chem. 11:525–538. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al: A
single nucleotide polymorphism in the MDM2 promoter attenuates the
p53 tumor suppressor pathway and accelerates tumor formation in
humans. Cell. 119:591–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Skorupski P, Krol J, Starega J, Adamiak A,
Jankiewicz K and Rechberger T: An alpha-1 chain of type I collagen
Sp1-binding site polymorphism in women suffering from stress
urinary incontinence. Am J Obstet Gynecol. 194:346–350. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Truong TT, Weaver J, Bove BA,
Cattie K, Armstrong BA, Daly MB and Godwin AK: Intronic alterations
in BRCA1 and BRCA2: Effect on mRNA splicing fidelity and
expression. Hum Mutat. 27:427–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duan J, Wainwright MS, Comeron JM, Saitou
N, Sanders AR, Gelernter J and Gejman PV: Synonymous mutations in
the human dopamine receptor D2 (DRD2) affect mRNA stability and
synthesis of the receptor. Hum Mol Genet. 12:205–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin Y, Dietz HC, Montgomery RA, Bell WR,
McIntosh I, Coller B and Bray PF: Glanzmann thrombasthenia.
Cooperation between sequence variants in cis during splice site
selection. J Clin Invest. 98:1745–1754. 1996. View Article : Google Scholar : PubMed/NCBI
|