Introduction
Jun activation domain-binding protein 1 (JAB1) was
originally identified as a co-activator of c-Jun that stabilizes
its DNA-binding through protein-protein interaction (1). Subsequently, JAB1 was found to interact
with numerous other proteins, affecting protein stability and
transcriptional activity of its interacting partner proteins, which
are involved in the regulation of the cell cycle, signal
transduction and DNA repair (2). JAB1
has an important role in tumorigenesis, inactivating tumor
suppressor proteins and activating oncogenic transcription factors.
JAB1 facilitates the translocation of tumor suppressor proteins,
including p53 (3,4), Smad7 (5),
Runx3 (6) and the cyclin-dependent
kinase inhibitor p27Kip1 (7,8), from the
nucleus to the cytoplasm, where they are subsequently degraded in
the proteasome. JAB1 is also a transcriptional co-activator of
c-Jun (1), hypoxia-inducible
factor-1α (9,10) and signal transducer and activator of
transcription 3 (STAT3) (11). JAB1
is positively regulated by oncogenic transcription factors STAT3
and β-catenin/TCF-4 (12,13). STAT3 positively regulates JAB1
expression through its binding to the JAB1 promoter
(12), and HER2 increases JAB1
expression through the binding of β-catenin/TCF-4 to the
JAB1 promoter in human breast cancer cells (13). These findings suggest that JAB1
is a target gene of STAT3 and β-catenin/TCF-4. Overall, with our
recent findings that JAB1 positively regulates STAT3 DNA-binding
activity in human colon cancer cells (11), the results of these studies suggest
that the JAB1-STAT3 activation loop exists in human colorectal
cancer cells. Furthermore, high JAB1 expression has been reported
to be associated with poor prognosis in numerous malignant
carcinomas, including ovarian cancer (14,15), oral
squamous cell carcinoma (16),
laryngeal squamous cell carcinoma (17), hepatocellular carcinoma (18), glioma (19), soft-tissue sarcoma (20), pancreatic cancer (21), esophageal squamous cell carcinoma
(22), lung cancer (23) and non-Hodgkin's lymphoma (24). However, the association between JAB1
expression and prognosis in colorectal cancer remains largely
unknown. The objectives of the present study were therefore to
elucidate the associations between JAB1 and STAT3 expression and
recurrence in colorectal cancer.
In the present study, it was found that high
JAB1 expression in primary colorectal cancer tissues is an
independent predictor of recurrence following 5-fluorouracil
(5-FU)-based adjuvant chemotherapy in colorectal cancer patients,
and high expression of both JAB1 and STAT3 in primary
colorectal cancer tissues is associated with a lower
recurrence-free survival rate compared to high expression of only
JAB1 or STAT3.
Materials and methods
Patients and clinical samples
A total of 57 patients with colorectal cancer who
underwent surgical treatment at Yamaguchi University Hospital (Ube,
Yamaguchi, Japan), Yamaguchi Saiseikai Shimonoseki General Hospital
(Shimonoseki, Yamaguchi, Japan) and Yamaguchi Rosai Hospital
(Sanyo-Onoda, Yamaguchi, Japan) between April 2012 and December
2013 were enrolled in the present study. All patients had stage II
or III colorectal cancer and were treated with FOLFOX, UFT/UZEL or
Xeloda following curative surgical operation. Primary colorectal
cancer tissues from 50 patients (age range, 48–84 years; 31 males
and 19 females) were immediately taken from resected colorectal
tissues and kept at −80°C until total RNA extraction, followed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). Additionally, 7 paired samples of primary colorectal
cancer tissue and liver metastasis from 7 patients (age range,
63–81 years; 6 males and 1 female) who had undergone mainly
5-FU-based chemotherapy were formalin-fixed and paraffin-embedded
immunohistochemical staining. Written informed consent was obtained
from all patients, and approval was provided by Institutional
Review Board of Yamaguchi University Hospital and the affiliated
hospitals. The samples were used in accordance with the Declaration
of Helsinki.
Regimens of adjuvant chemotherapy
The choice of adjuvant chemotherapy was made by the
patient in consultation with the surgeon. UFT/LV was given in
5-week cycle consisting of 4 weeks of treatment and 1 week of rest.
The cycle was repeated at least 5 times. The UFT dose was 300
mg/m2/day and LV dose 75 mg/day. Xeloda was administered
at a dose of 1,250 mg/m2 twice a day for 14 days,
followed by 7 days of rest. Standard care included a total of 8
cycles. Modified FOLFOX6 [a modified folinic acid, leucovorin (LV),
5-FU, and oxaliplatin (OX)] regimen was as follows; OX 85
mg/m2, LV 200 mg/m2, 5-FU bolus 400
mg/m2, 5-FU infusion 2,400 mg/m2 over 46 h.
The treatment was repeated every 2 weeks. Standard care included a
total of 12 cycles.
Immunohistochemical staining
Tissue specimens were fixed with 20% formalin for
3–5 days at room temperature and paraffin-embedded. Sections (3-µm
thick) from the tissue specimens were deparaffinized with xylene at
room temperature and rehydrated with graded ethanol. The tissue
sections were then incubated with 3% hydrogen peroxide
(H2O2) in methanol for 30 min to block
endogenous peroxidase activity, followed by antigen retrieval at
95°C for 20 min in Dako Target Retrieval solution (Agilent
Technologies, Inc., Santa Clara, CA, USA). Tissue sections were
subsequently incubated in Dako Protein Block Serum-Free
Ready-to-Use (Agilent Technologies, Inc.) for 30 min at room
temperature to prevent non-specific binding, and were then
incubated with anti-rabbit polyclonal JAB1 (FL-334) primary
antibody (catalog no. sc-9074; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) diluted at 1:100 overnight at 4°C. After washing
several times with phosphate-buffered saline, the sections were
incubated with anti-rabbit immunoglobulin conjugated to horseradish
peroxidase (EnVision+ HRP-labeled polymer anti-rabbit system;
catalog no. K4002; Dako North America, Inc., Carpinteria, CA, USA)
as a secondary antibody for 30 min at room temperature. The
sections were treated with 0.2 mg/ml diaminobenzidine for 15 sec
and counterstained in Mayer's hematoxylin for 30 sec. Images were
captured from at least 10 randomly selected fields per sample using
an All-in-One fluorescence microscope (magnification, ×40; KEYENCE,
Osaka, Japan). Immunoreactivity was independently evaluated by
two.
RT-qPCR
Resected primary colorectal cancer tissues were
disrupted in RLT buffer (Qiagen, Valencia, CA, USA) and homogenized
by shaking with stainless steel beads using a Mixer Mill MM300
(both from Qiagen). Total RNA was isolated using an RNeasy Mini kit
(Qiagen), according to the manufacturer's protocol. Reverse
transcription was performed using PrimeScript RT Master Mix
(Perfect Real-Time; Takara Bio, Inc., Otsu, Japan). The template
cDNAs were amplified using a QuantiTect SYBR-Green PCR kit (Qiagen)
with the specific primers. The sequences of the primers were as
follows: JAB1 forward, 5′-GCAGTGGTGATTGATCCAAC-3′ and
reverse, 5′-GTCTGGTACTCAGAAGGTCC-3′; STAT3 forward,
5′-CACTACTAAAGTCAGGTTGCTGGTC-3′ and reverse,
5′-AACGTCCCCAGAGTCTTTGTC-3′; MCL1 forward,
5′-CACAGACGTTCTCGTAAGGAC-3′ and reverse,
5′-GATGCCACCTTCTAGGTCCTC-3′; cyclin D1 forward,
5′-CGAGAAGCTGTGCATCTACACC-3′ and reverse,
5′-TTCCACTTGAGCTTGTTCACC-3′; and GAPDH forward,
5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTTGGCAGGTT−3′. The PCR thermocycling conditions were
95°C for 15 min, followed by 50 cycles of 95°C for 10 sec and 60°C
for 30 sec. JAB1 and STAT3 expression was normalized
to GAPDH expression. RT-qPCR was performed using LightCycler
software version 3.5 (Roche Applied Science, Penzberg, Germany),
and data were evaluated using the 2−ΔΔCq method
(25).
Statistical analysis
Statistical analyses were performed using SPSS
Statistics 20 for Windows (SPSS, Inc., Chicago, IL, USA).
Differences between groups were analyzed using the paired t-test,
Mann-Whitney U test or χ2 test, as appropriate. The
association between mRNA expression levels was assessed using
Pearson's correlation coefficient. Survival curves were generated
using the Kaplan-Meier method and compared using the log-rank test.
Receiver operating characteristic (ROC) curve analysis was used to
determine the optimum cut-off values for predicting outcome. To
determine the cut-off values for JAB1 and STAT3
expression, ROC curves were constructed by plotting all possible
sensitivity/1-specificity pairs in the training set. The Cox
proportional hazards regression model was used to identify the
variables associated with recurrence-free survival. P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between JAB1 and STAT3
expression, and clinicopathological parameters in primary
colorectal cancer tissues
To investigate JAB1 and STAT3
expression level in 50 primary colorectal cancer tissues, RT-qPCR
was performed. ROC curve analysis was used to obtain the optimal
cut-off values of JAB1 and STAT3 expression, and this
was used to classify 50 primary colorectal cancer tissues into high
or low expression group of JAB1 or STAT3. The
association between JAB1 and STAT3 expression, and
clinicopathological parameters was investigated. As shown in
Table I, high JAB1 expression
was not associated with any of the investigated clinicopathological
parameters, including age, sex, tumor location, histological grade,
invasion depth, lymphatic metastasis, lymphatic invasion, venous
invasion and tumor-node-metastasis (TNM) stage. However, high
STAT3 expression was significantly associated with advanced
TNM stage (P=0.04).
 | Table I.The association between JAB1
and STAT3 expression, and clinicopathological variables in
primary colorectal cancer tissues. |
Table I.
The association between JAB1
and STAT3 expression, and clinicopathological variables in
primary colorectal cancer tissues.
|
| JAB1
expression |
| STAT3
expression |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
variables | Low | High | P-value | Low | High | P-value |
|---|
| Total | 33 | 17 |
| 33 | 17 |
|
| Gender |
|
| 0.23 |
|
| 0.23 |
|
Male | 20 | 11 |
| 20 | 11 |
|
|
Female | 13 | 6 |
| 13 | 6 |
|
| Age (years) | 70.0±10.6 | 72.1±6.6 | 0.48 | 70.5±9.8 | 71.2±8.9 | 0.80 |
| Location |
|
| 0.84 |
|
| 0.84 |
|
Right | 13 | 8 |
| 13 | 8 |
|
|
Left | 10 | 4 |
| 10 | 4 |
|
|
Rectum | 10 | 5 |
| 10 | 5 |
|
| Histological
grade |
|
| 0.77 |
|
| 0.77 |
|
Well | 3 | 2 |
| 4 | 1 |
|
|
Moderate | 26 | 14 |
| 26 | 14 |
|
|
Poor | 4 | 1 |
| 3 | 2 |
|
| Invasion depth |
|
| 0.40 |
|
| 0.40 |
| T2 | 1 | 1 |
| 1 | 1 |
|
| T3 | 22 | 8 |
| 22 | 8 |
|
| T4 | 10 | 8 |
| 10 | 8 |
|
| Lymphatic
metastasis |
|
| 0.87 |
|
| 0.05 |
| + | 21 | 11 |
| 18 | 14 |
|
| − | 12 | 6 |
| 15 | 3 |
|
| Lymphatic
invasion |
|
| 0.96 |
|
| 0.96 |
| + | 30 | 16 |
| 30 | 16 |
|
| − | 3 | 1 |
| 3 | 1 |
|
| Venous
invasion |
|
| 0.20 |
|
| 0.49 |
| + | 15 | 11 |
| 16 | 10 |
|
| − | 18 | 6 |
| 17 | 7 |
|
| Stage |
|
| 0.13 |
|
| 0.04 |
| 2a | 9 | 1 |
| 10 | 0 |
|
| 2b | 3 | 5 |
| 5 | 3 |
|
| 3a | 1 | 0 |
| 1 | 0 |
|
| 3b | 13 | 5 |
| 12 | 6 |
|
| 3c | 7 | 6 |
| 5 | 8 |
|
Association between JAB1 and STAT3
expression in primary colorectal cancer tissues
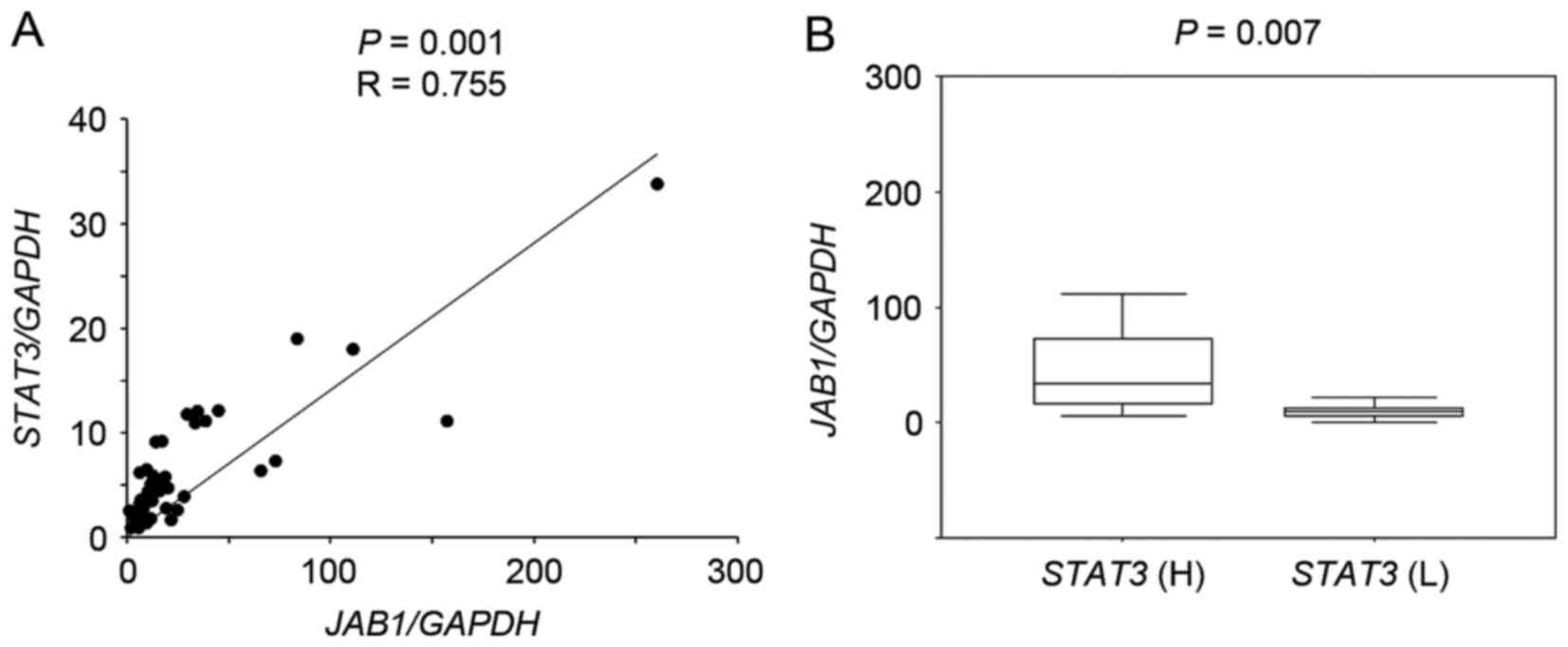
RT-qPCR followed by scatter plot analysis showed
that JAB1 expression significantly correlated with
STAT3 expression in primary colorectal cancer tissues
(Fig. 1A, P=0.001, r=0.755), and
JAB1 expression in tumors with high STAT3 expression
was significantly increased compared with that in tumors with low
STAT3 expression (Fig. 1B,
P=0.007).
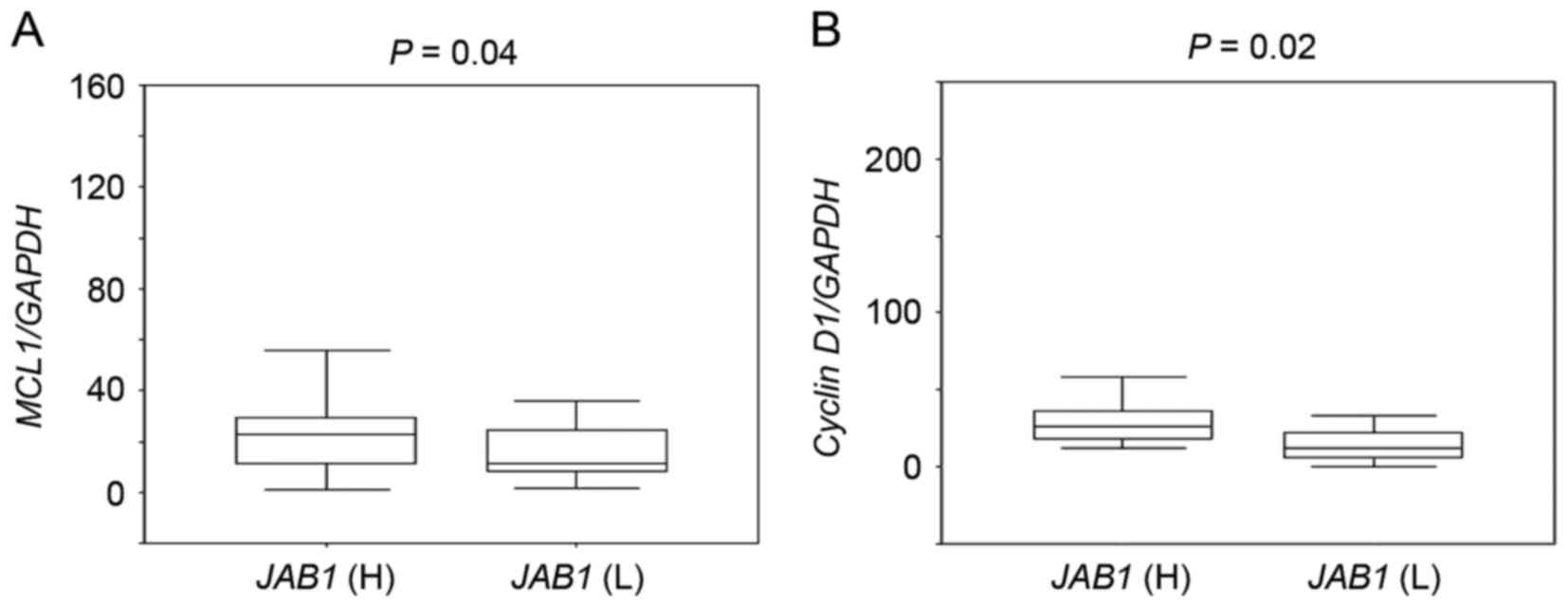
Expression of the STAT3 target genes
MCL1 and cyclin D1 is associated with JAB1 expression
The association between JAB1 expression and
the expression of the STAT3 target genes MCL1 and cyclin D1
was then investigated. MCL1 and cyclin D1 expression in
tumors with high JAB1 expression was significantly higher
than that in tumors with low JAB1 expression (Fig. 2, P=0.04 and P=0.02, respectively).
High JAB1 expression in primary
colorectal cancer tissues is associated with a lower
recurrence-free survival rate following 5-FU-based adjuvant
chemotherapy
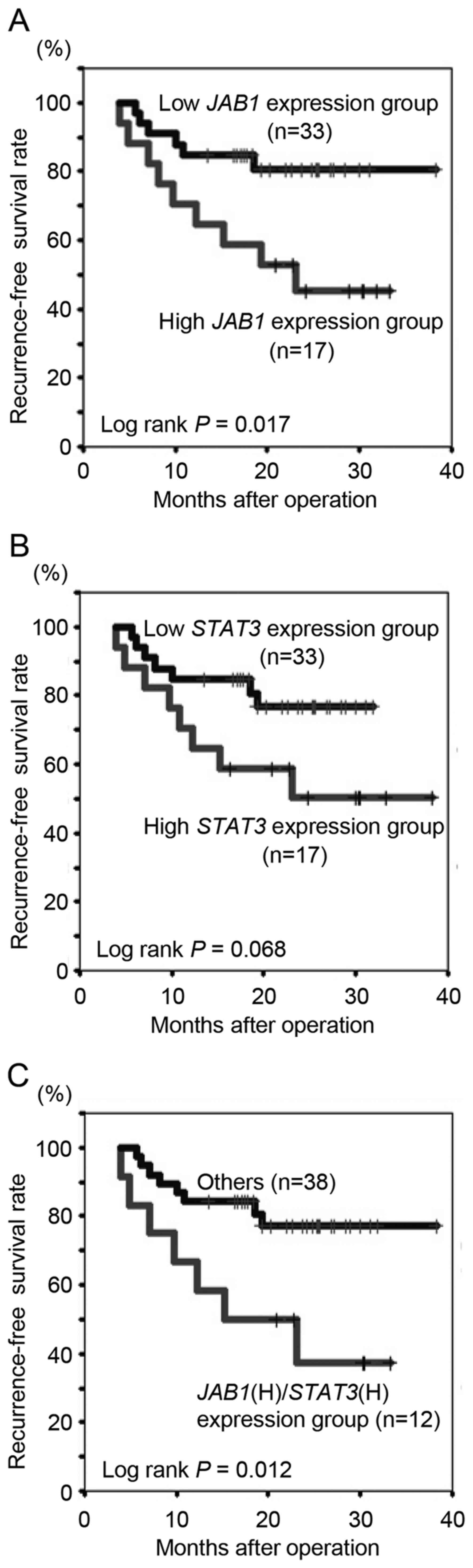
The present study determined whether
clinicopathological variables, including JAB1 and
STAT3 expression, in primary colorectal cancer tissues were
associated with recurrence following 5-FU-based adjuvant
chemotherapy. The median follow-up period for all patients was 21.4
months (range, 5.6–38.3 months). Histological grade (poor),
invasion depth (T4) and JAB1 expression (high) were
significantly associated with recurrence (Table II, P=0.03, 0.01 and 0.01,
respectively). To further examine the associations between
JAB1 and STAT3 expression and recurrence-free
survival rate following 5-FU-based adjuvant chemotherapy,
Kaplan-Meier analyses were performed. Patients with high
JAB1 expression had a significantly decreased
recurrence-free survival rate following 5-FU-based adjuvant
chemotherapy compared to those with low JAB1 expression
(Fig. 3A, P=0.017), whilst there was
no significant difference in survival with respect to STAT3
expression (Fig. 3B, P=0.068).
Patients with high expression of both JAB1 and STAT3
had a significantly decreased recurrence-free survival rate
following 5-FU-based adjuvant chemotherapy compared to all other
patients (Fig. 3C, P=0.012), and
compared to patients with high expression of only JAB1 or
STAT3.
 | Table II.Association between recurrence
following fluorouracil-based adjuvant chemotherapy and
clinicopathological variables, including JAB1 and
STAT3 expression, in primary colorectal cancer tissues. |
Table II.
Association between recurrence
following fluorouracil-based adjuvant chemotherapy and
clinicopathological variables, including JAB1 and
STAT3 expression, in primary colorectal cancer tissues.
| Clinicopathological
variables | No recurrence | Recurrence | P-value |
|---|
| Total | 35 | 15 |
|
| Gender |
|
| 0.98 |
|
Male | 23 | 8 |
|
|
Female | 12 | 7 |
|
| Age (years) | 71.2±8.3 | 69.6±11.7 | 0.60 |
| Location |
|
| 0.29 |
|
Right | 14 | 7 |
|
|
Left | 12 | 2 |
|
|
Rectum | 9 | 6 |
|
| Histological
grade |
|
| 0.03 |
|
Well | 3 | 2 |
|
|
Moderate | 31 | 9 |
|
|
Poor | 1 | 4 |
|
| Invasion depth |
|
| 0.01 |
| T2 | 2 | 0 |
|
| T3 | 26 | 4 |
|
| T4 | 7 | 11 |
|
| Lymphatic |
|
| 0.73 |
| metastasis |
| + | 21 | 11 |
|
| − | 14 | 4 |
|
| Lymphatic |
|
| 0.45 |
| invasion |
| + | 33 | 13 |
|
| − | 2 | 2 |
|
| Venous |
|
| 0.16 |
| invasion |
| + | 18 | 8 |
|
| − | 17 | 7 |
|
| Stage |
|
| 0.10 |
| 2a | 10 | 0 |
|
| 2b | 4 | 4 |
|
| 3a | 1 | 0 |
|
| 3b | 13 | 5 |
|
| 3c | 7 | 6 |
|
| Oxaliplatin |
|
| 1.00 |
| + | 7 | 3 |
|
| − | 28 | 12 |
|
| JAB1 |
|
| 0.01 |
|
High | 8 | 9 |
|
|
Low | 27 | 6 |
|
| STAT3 |
|
| 0.06 |
|
High | 9 | 8 |
|
|
Low | 26 | 7 |
|
Cox proportional hazard regression analysis was then
performed to identify independent prognostic factors. The
recurrence-free survival rate following 5-FU-based adjuvant
chemotherapy was strongly associated with histological grade
(poor), invasion depth (T3) and JAB1 expression (high) on
univariate analysis (Table III,
P=0.02, 0.01 and 0.03, respectively). In multivariate analysis,
high JAB1 expression was the only independent predictor of
recurrence-free survival following 5-FU-based adjuvant chemotherapy
(Table III, P=0.04).
 | Table III.Univariate analysis and multivariate
analysis for clinicopathological variables affecting the
recurrence-free survival rate following fluorouracil-based
chemotherapy. |
Table III.
Univariate analysis and multivariate
analysis for clinicopathological variables affecting the
recurrence-free survival rate following fluorouracil-based
chemotherapy.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender (male vs.
female) | 1.55
(0.56–4.28) | 0.40 |
|
|
| Age (years) | 0.99
(0.94–1.04) | 0.63 |
|
|
| Location
(rectum) | 1.83
(0.65–5.15) | 0.25 |
|
|
| Histological grade
(poor) | 4.07
(1.29–12.9) | 0.02 | 3.10
(0.90–10.65) | 0.07 |
| Invasion depth
(≥T3) | 4.10
(1.40–12.0) | 0.01 | 2.80
(0.89–8.87) | 0.08 |
| Lymphatic
metastasis (+) | 1.66
(0.53–5.20) | 0.39 |
|
|
| Lymphatic invasion
(+) | 0.45
(0.10–2.02) | 0.30 |
|
|
| Venous invasion
(+) | 0.96
(0.35–2.65) | 0.94 |
|
|
| Stage (≥3a) | 1.85
(0.59–5.80) | 0.29 |
|
|
| JAB1 (high) | 3.27
(1.16–9.20) | 0.03 | 2.80
(1.07–8.78) | 0.04 |
| STAT3 (high) | 2.45
(0.90–6.86) | 0.08 |
|
|
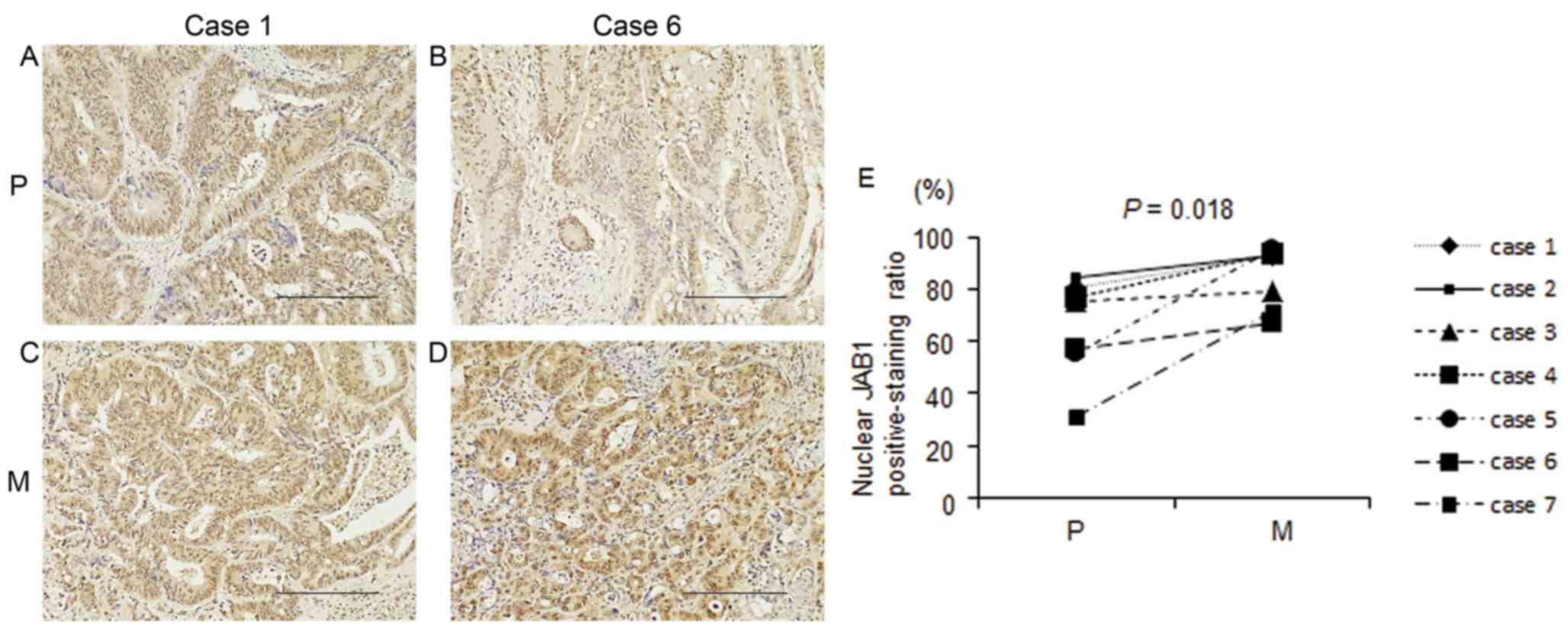
JAB1 protein expression in primary
colorectal cancer tissues and liver metastases following mainly
5-FU-based chemotherapy
Seven paired samples of primary colorectal cancer
tissue and liver metastasis from 7 patients who had undergone
mainly 5-FU-based chemotherapy were prepared. Clinical variables,
including the chemotherapy regimen and treatment effects, are shown
in Table IV. Nuclear and cytoplasmic
JAB1 protein expressions were detected in both primary colorectal
cancer tissues and liver metastases. Nuclear and cytoplasmic JAB1
protein expressions in liver metastases appeared to be increased
compared with those in primary colorectal cancer tissues (Fig. 4A-D), and the proportion of cells with
positive nuclear JAB1 expression in liver metastases was
significantly increased compared with that in primary colorectal
cancer tissues (Fig. 4E,
P=0.018).
 | Table IV.Clinicopathological characteristics
of colorectal patients with liver metastasis. |
Table IV.
Clinicopathological characteristics
of colorectal patients with liver metastasis.
| Case no. | Regimen | Cycle | Effect | Invasion depth | Histological
grade | Lymph invasion | Venous
invasion |
|---|
| 1 | Pmab + FOLFOX | 6 | PR | SE | mod | 1 | 1 |
| 2 | Bmab + Xelox | 11 | PD | SS | mod | 1 | 0 |
| 3 | Bmab + FOLFIRI | 6 | SD | SS | mod | 1 | 1 |
| 4 | Pmab + FOLFOX | 6 | PR | SS | pap | 1 | 1 |
| 5 | Bmab + FOLFOX | 10 | SD | SS | mod | 2 | 2 |
| 6 | Bmab + Xeloda | 4 | PR | SS | mod | 0 | 0 |
| 7 | Bmab + FOLFIRI | 20 | SD | SS | mod | 1 | 2 |
Discussion
It has been reported that JAB1 expression is
transcriptionally regulated through STAT3 binding to the
JAB1 promoter in human breast cancer cells (12). Furthermore, our previous study
revealed that JAB1 positively regulates STAT3 DNA-binding activity
in human colorectal cancer cells (11). Consistent with these findings, in the
present study, it was found that JAB1 expression was
increased in tumors with high STAT3 expression compared with
that in tumors with low STAT3 expression, and that the STAT3
target genes MCL1 and cyclin D1 had increased expressed in
tumors with high JAB1 expression compared to those with low
JAB1 expression. These findings suggest that JAB1 cooperates
with STAT3, and that the JAB1-STAT3 activation loop is present in
human colorectal cancer cells. Notably, patients with tumors that
highly expressed both JAB1 and STAT3 had a lower
recurrence-free survival rate following 5-FU-based adjuvant
chemotherapy than those patients with tumors that highly expressed
only JAB1 or STAT3, suggesting that the JAB1-STAT3
activation loop has an important role in recurrence following
5-FU-based adjuvant chemotherapy. Furthermore, the present findings
indicate that high JAB1 expression in primary colorectal
cancer is a significant predictor of recurrence following this
treatment. It was also found that the proportion of tumor cells
with positive nuclear JAB1 expression was significantly increased
in liver metastases following mainly 5-FU-based chemotherapy
compared with that in primary colorectal cancer tissues. Notably, a
recent study found that nuclear JAB1 expression was increased in
recurrent nasopharyngeal carcinoma following radiotherapy compared
with primary nasopharyngeal carcinoma (26). Together with the present findings,
this suggests that nuclear JAB1 has a role in recurrence.
Additional studies are required to elucidate the association
between nuclear JAB1 and recurrence.
Overall, the present findings suggest that the
JAB1-STAT3 activation loop can confer resistance to 5-FU-based
adjuvant chemotherapy and is thus a potential therapeutic target in
recurrent colorectal cancer following 5-FU-based adjuvant
chemotherapy.
Acknowledgements
This study was supported by the Oncochishin Project
from Yamaguchi University. The authors thank Dr K. Ueki (Yamaguchi
Saiseikai Shimonoseki General Hospital, Shimonoseki, Yamaguchi,
Japan) and Dr C. Kato (Yamaguchi Rosai Hospital, Sanyo-Onoda,
Yamaguchi, Japan) for their assistance in acquiring samples and
collecting information for colorectal cancer patients. The authors
also thank Yukari Hironaka (Department of Surgery and Clinical
Science, Yamaguchi University Graduate School of Medicine, Ube,
Yamaguchi, Japan) for providing technical assistance.
References
|
1
|
Claret FX, Hibi M, Dhut S, Toda T and
Karin M: A new group of conserved coactivators that increase the
specificity of AP-1 transcription factors. Nature. 383:453–457.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shackleford TJ and Claret FX: JAB1/CSN5: A
new player in cell cycle control and cancer. Cell Div. 5:262010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh W, Lee EW, Sung YH, Yang MR, Ghim J,
Lee HW and Song J: Jab1 induces the cytoplasmic localization and
degradation of p53 in coordination with Hdm2. J Biol Chem.
281:17457–17465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XC, Chen J, Su CH, Yang HY and Lee
MH: Roles for CSN5 in control of p53/MDM2 activities. J Cell
Biochem. 103:1219–1230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim BC, Lee HJ, Park SH, Lee SR, Karpova
TS, McNally JG, Felici A, Lee DK and Kim SJ: JAB1/CSN5, a component
of the COP9 signalosome, regulates transforming growth factor beta
signaling by binding to Smad7 and promoting its degradation. Mol
Cell Biol. 24:2251–2262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Choi JK, Cinghu S, Jang JW, Lee
YS, Li YH, Goh YM, Chi XZ, Lee KS, Wee H and Bae SC: JAB1/CSN5
induces the cytoplasmic localization and degradation of RUNX3. J
Cell Biochem. 107:557–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomoda K, Kubota Y and Kato J: Degradation
of the cyclin-dependentkinase inhibitor p27Kip1 is
instigated by Jab1. Nature. 398:160–165. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomoda K, Kubota Y, Arata Y, Mori S, Maeda
M, Tanaka T, Yoshida M, Yoneda-Kato N and Kato JY: The cytoplasmic
shuttling and subsequent degradation of p27Kip1 mediated
by JAB1/CSN5 and the COP9 signalosome complex. J Biol Chem.
277:2302–2310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM,
Bae SK, Park JW, Kim KR and Kim KW: Jab1 interacts directly with
HIF-1alpha and regulates its stability. J Biol Chem. 277:9–12.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bemis L, Chan DA, Finkielstein CV, Qi L,
Sutphin PD, Chen X, Stenmark K, Giaccia AJ and Zundel W: Distinct
aerobic and hypoxic mechanisms of HIF-alpha regulation by CSN5.
Genes Dev. 18:739–744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishimoto A, Kugimiya N, Hosoyama T, Enoki
T, Li TS and Hamano K: JAB1 regulates unphosphorylated STAT3
DNA-binding activity through protein-protein interaction in human
colon cancer cells. Biochem Biophys Res Commun. 438:513–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shackleford TJ, Zhang Q, Tian L, Vu TT,
Korapati AL, Baumgartner AM, Le XF, Liao WS and Claret FX: Stat3
and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate
Jab1/CSN5 expression in mammary carcinoma cells. Breast Cancer Res.
13:R652011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu MC, Chang HC and Hung WC: HER-2/neu
transcriptionally activates Jab1 expression via the
AKT/beta-catenin pathway in breast cancer cells. Endocr Relat
Cancer. 14:655–667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui L, Dong Y, Watanabe Y, Yamaguchi F,
Sugimoto K and Tokuda M: Clinical significance of Skp2 expression,
alone and combined with Jab1 and p27 in epithelial ovarian tumors.
Oncol Rep. 15:765–771. 2006.PubMed/NCBI
|
|
15
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K, Tai Y and Tokuda M: Jab1 expression is associated with
inverse expression of p27 (kip1) and poor prognosis in epithelial
ovarian tumors. Clin Cancer Res. 7:4130–4135. 2001.PubMed/NCBI
|
|
16
|
Harada K, Kawashima Y, Yoshida H and Sato
M: High expression of Jun activation domain-binding protein 1
(Jab1) is a strong prognostic marker in oral squamous cell
carcinoma patients treated by UFT in combination with radiation.
Anticancer Res. 26:1615–1619. 2006.PubMed/NCBI
|
|
17
|
Dong Y, Sui L, Watanabe Y, Yamaguchi F,
Hatano N and Tokuda M: Prognostic significance of Jab1 expression
in laryngeal squamous cell carcinomas. Clin Cancer Res. 11:259–266.
2005.PubMed/NCBI
|
|
18
|
Wang Y, Yu YN, Song S, Li TJ, Xiang JY,
Zhang H, Lu MD, Ji F and Hu LQ: JAB1 and phospho-Ser10 p27
expression profile determine human hepatocellular carcinoma
prognosis. J Cancer Res Clin Oncol. 140:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He SM, Zhao ZW, Wang Y, Zhao JP, Wang L,
Hou F and Gao GD: Potential role of Jun activation domain-binding
protein 1 and phosphorylated p27 expression in prognosis of glioma.
Brain Tumor Pathol. 29:3–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorbye SW, Kilvaer TK, Valkov A, Donnem T,
Smeland E, Al-Shibli K, Bremnes RM and Busund LT: Prognostic impact
of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLoS
One. 7:e470682012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukumoto A, Ikeda N, Sho M, Tomoda K,
Kanehiro H, Hisanaga M, Tsurui Y, Tsutsumi M, Kato JY and Nakajima
Y: Prognostic significance of localized p27Kip1 and
potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep.
11:277–284. 2004.PubMed/NCBI
|
|
22
|
Wang F, Wang Y, Yu X, Yang D, Wang Z, Lu
C, Yuan Z, Xiao M and Shen A: Significance of Jab1 expression in
human esophageal squamous cell carcinoma. J Clin Gastroenterol.
43:520–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osoegawa A, Yoshino I, Kometani T,
Yamaguchi M, Kameyama T, Yohena T and Maehara Y: Overexpression of
Jun activation domain-binding protein 1 in nonsmall cell lung
cancer and its significance in p27 expression and clinical
features. Cancer. 107:154–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Fei M, Cheng C, Zhang D, Lu J, He
S, Zhao Y, Wang Y and Shen A: Jun activation domain-binding protein
1 negatively regulate p27 kip1 in non-Hodgkin's lymphomas. Cancer
Biol Ther. 7:460–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu T, Su B, Wang C, Wang S, Huang H, Pan
Y, Wang D, Wei W, Claret FX and Yang H: Molecular markers to assess
short-term disease local recurrence in nasopharyngeal carcinoma.
Oncol Rep. 33:1418–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|