Introduction
As one of the most common types of cancer of the
male reproductive system, the incidence of prostate cancer is
ranked second of all malignant tumors of males. At present, the
incidence of prostate cancer, in the USA, has surpassed lung cancer
as the highest incidence, threatening the health of males (1,2) According
to the latest data issued by The National Cancer Center of China,
prostate cancer, since 2008, is ranked first in terms of the
incidence of tumors in urinary system (3). Conventional treatment for prostate
cancer includes hormonotherapy, radiotherapy and chemotherapy as
well as surgery. Nevertheless, there remain great difficulties in
treatment of castrate-resistant prostate cancer (CRPC) due to its
high post-surgery recurrence rate or no response to the endocrine
therapies, leaving few treatment options. Radiotherapy, as one of
the major therapies for prostate cancer, has been widely applied in
treatment of prostate cancer in all stages, in which the
radiosensitivity is a key factor intimately affecting the efficacy
of radiotherapy. Thus, how to increase the radiosensitivity of
tumors is a study hotspot in the domain of tumor therapeutics. In
recent years, as the basic study of tumors has acquired continuous
development, especially the development in molecular biology, how
to improve the efficacy of radiotherapy and chemotherapy by
application of targeted therapy with gene drugs has become a new
development trend (4).
Replication-selective oncolytic adenovirus (RSOAds) represents a
method of targeted gene therapy of tumors, while the construction
of RSOAds dual-targeted and dual-regulated prostate specific
antigen (PSA) and human telomerase reverse transcriptase (hTERT)
could further enhance the targeting effect of therapeutic gene to
the prostatic tumor tissues, which is expected to generate better
efficacy in clinical treatment (5).
In the present study, the aim was set to construct the
125I-RSOAds-hTERT/PSA nuclide-oncolytic virus marker by
labelling the hTERT/PSA double-regulation replicative oncolytic
adenovirus with 125I, results of which would serve as
experiment basis for later studies of therapies for CRPC by
125I-RSOAds-hTERT/PSA markers to explore new, efficient
and safe methods for treatment of CRPC.
Materials and methods
Agent and instruments
Major agents
PBS (Zhongshan Goldenbridge, Beijing, China),
N-bromosuccinimide (NBS) (Sigma, St. Louis, MO, USA),
Na··4125I (Chengdu Zhonghe Gaotong Isotope Co., Ltd.,
Chengdu, China), acetone (ChengDu KeLong Chemical Co., Ltd.,
Chengdu, China), human serum albumin (HSA; Hualan Biological
Engineering, Inc., Henan, China), SephadexG-10 gel (Nanjing
SenBeiJia Biological Technology Co., Ltd., Nanjing, China).
Instruments
γ immunity meter (PerkinElmer, Inc., Waltham, MA,
USA), whirlpool-like mixers (Qiliner Instruments of Haimen Co.,
Ltd., Haimen, China), automatic sampling instrument (Shanghai
Qingpu Luxi Instrument Plant, Shanghai, China), and chromatography
column (1×40 cm) (Shanghai Wuxiang Chemical Co., Ltd., Shanghai,
China).
Identification of the best conditions for
labelling
Effect of dosage of 125I on the labelling
rate was carried out. Briefly, 100 µl of 8×108 VP/ml
125I-RSOAds-hTERT/PSA virus solution and 0.1 µl of
Na125I (~0.04 m Ci) were added in 1.5 ml centrifuge tube
and mixed uniformly. Then 100 µl of NBS solution at the
concentration of 0.5 mg/ml and 20 µl of PBS (0.05 m, pH=7.6) were
added and the tube was sealed and placed into whirlpool-like mixer
for uniform mixing, after 3 min of reaction, 10 µl of 2% HAS was
added to stop the reaction, the dosages of NBS solution and
125I-RSOAds-hTERT/PSA virus solution as well as other
conditions were kept unchanged, and we assayed the influence of
dosage of 125I (setting dosages: 0.1, 0.5, 1, 2, 3, 4
and 5 µl) on the labelling rate of 125I-RSOAds-hTERT/PSA
nuclide-oncolytic adenovirus markers with the same methods and
measurements of the labelling rate by paper chromatography: Paper
chromatography conditions were set as follows: filter paper (size,
3; Xinhua Co., Hangzhou, China) as stationary phase; acetone as
developers; normal saline 1:1 (v/v).
A marker was made by a pencil every 1 cm on the
filter paper with a total length of 15 cm. The above labelled
reaction solution (0.2 µl) was added at the start point of filter
paper, then spread the paper in the developers (~300 µl). The
fully-spread filter paper was taken out and dried until the front
edge of solvent moved 2 cm from the edge of paper. Then, the paper
was cut into pieces by the markers, and the counting of
radioactivity (cpm) of each piece was measured by γ-counter. The
following formula was used for labelling rate calculation:
Labelling rate(%)=125I-RSOAds-hTERT/PSA
peak counting (cpm)125I-RSOAds-hTERT/PSA peak counting
(cpm)+free125I peak counting (cpm)×100
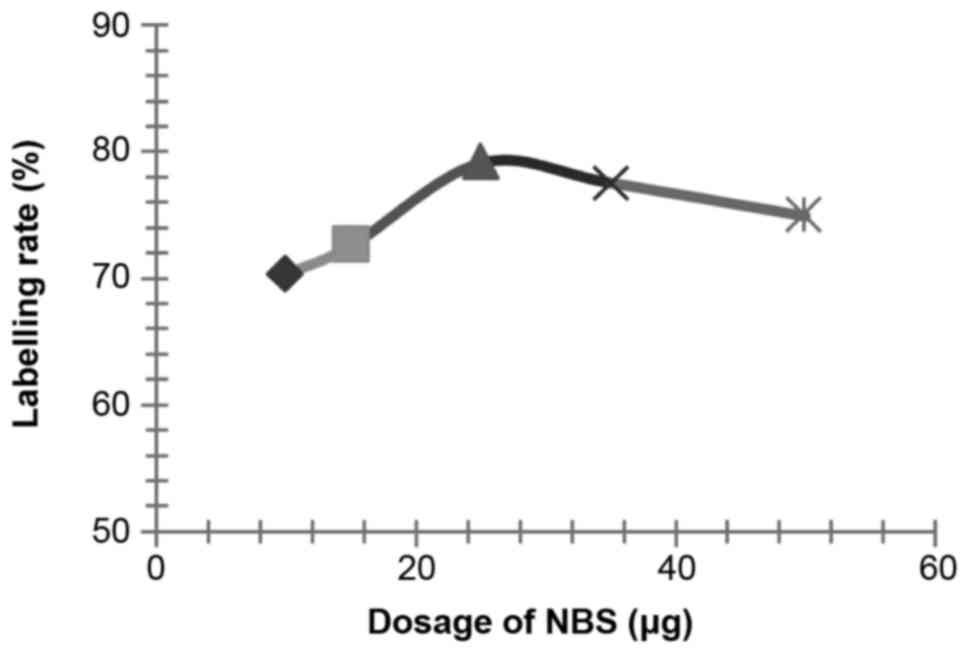
Influences of NBS dosage on the labelling
rate
According to the methods described above, influences
of various dosages of NBS (setting dosage: 30, 50, 70 and 100 µl)
on the labelling rate of 125I-RSOAds-hTERT/PSA
nuclide-oncolytic adenovirus markers were respectively assayed.
Measurements of the labelling rate where performed by paper
chromatography.
Influence of virus solution volume on the
labelling rate
According to the methods previously described,
influence of various volumes of 125I-RSOAds-hTERT/PSA
virus solution (setting volume: 25, 50, 75, 100, 130 and 150 µl) on
the labelling rate of 125I-RSOAds-hTERT/PSA
nuclide-oncolytic adenovirus markers were respectively assayed.
Measurements of the labelling rate were performed by paper
chromatography.
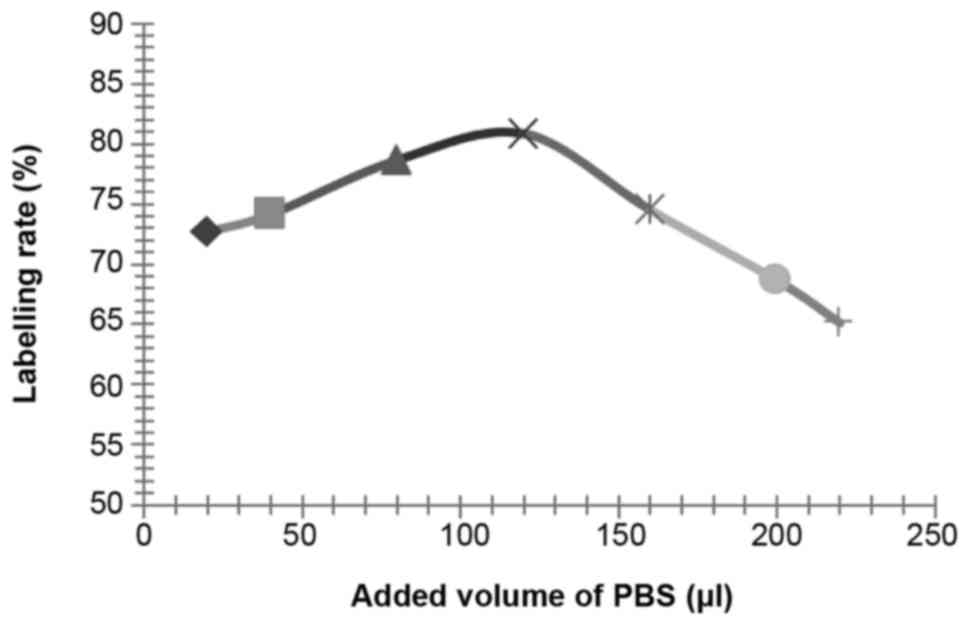
Influence of other reaction conditions on the
labelling rate
According to the above described methods, the
influence of reaction duration (setting value: 1, 2, 3, 4, 5, 6, 7
and 8 min), pH values (setting value: 6.5, 7.0, 7.5, 8.0 and 8.5)
and PBS reaction volumes (setting value: 20, 30, 40, 50, 60, 80,
100, 150 and 200 µl, the effect of PBS was to adjust the pH value)
on the labelling rate of 125I-RSOAds-hTERT/PSA
nuclide-oncolytic adenovirus markers were respectively assayed.
Isolation and purification of makers
Gel-filtration chromatography was applied in
isolation and purification of 125I-RSOAds-hTERT/PSA.
After labelling reactions, gel-filtration chromatography was
performed with SephadexG-10 chromatography columns (1×40 cm) in the
mixture of the above reactions. A certain volume of HSA was added
in the columns for saturation to inhibit the non-specific
absorption of RSOAds-hTERT/PSA to the columns before loading the
sample. Then the sample was loaded and diluted with PBS (pH=7.6) to
0.5 ml. Thereafter, eluent was collected continuously into 50 tubes
by automatic sampling instruments, each tube containing 1 ml of
eluent (~15 drops). 10 µl of eluent was extracted from each tube to
measure the radioactive counts (min−1) and to identify
the peak positions of markers and free 125I. The sample
with the first peak (the 125I-RSOAds-hTERT/PSA) was
selected to be preserved at 4°C for use after filtration and
sterilization by microporous membrane (0.22 µm).
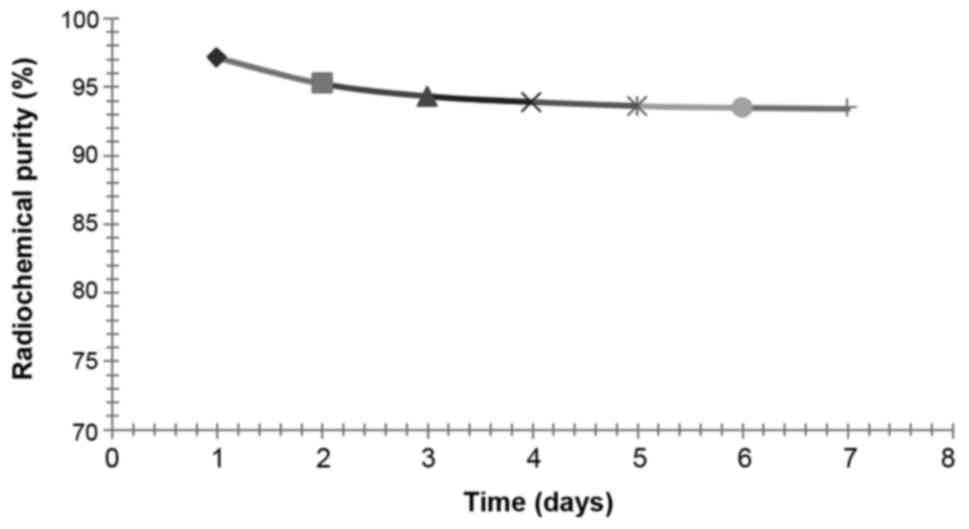
Measurements of radiochemical purity
The markers acquired from the experiment 2 were
preserved at 4°C, and radiochemical purity was determined on days
1, 2, 3, 4, 5, 6 and 7 after labelling by paper chromatography.
Radiochemical purity of the sample with the first peak of
radioactivity was assayed by paper chromatography, and the method
was the same as the assay of labelling rate.
Radiochemical purity
(%)(%)=125I-RSOAds-hTERT/PSA peak counting
(cpm)125I-RSOAds-hTERT/PSA peak counting (cpm)+free125I peak
counting (cpm)×100
Results
Identification of the best conditions
for labelling
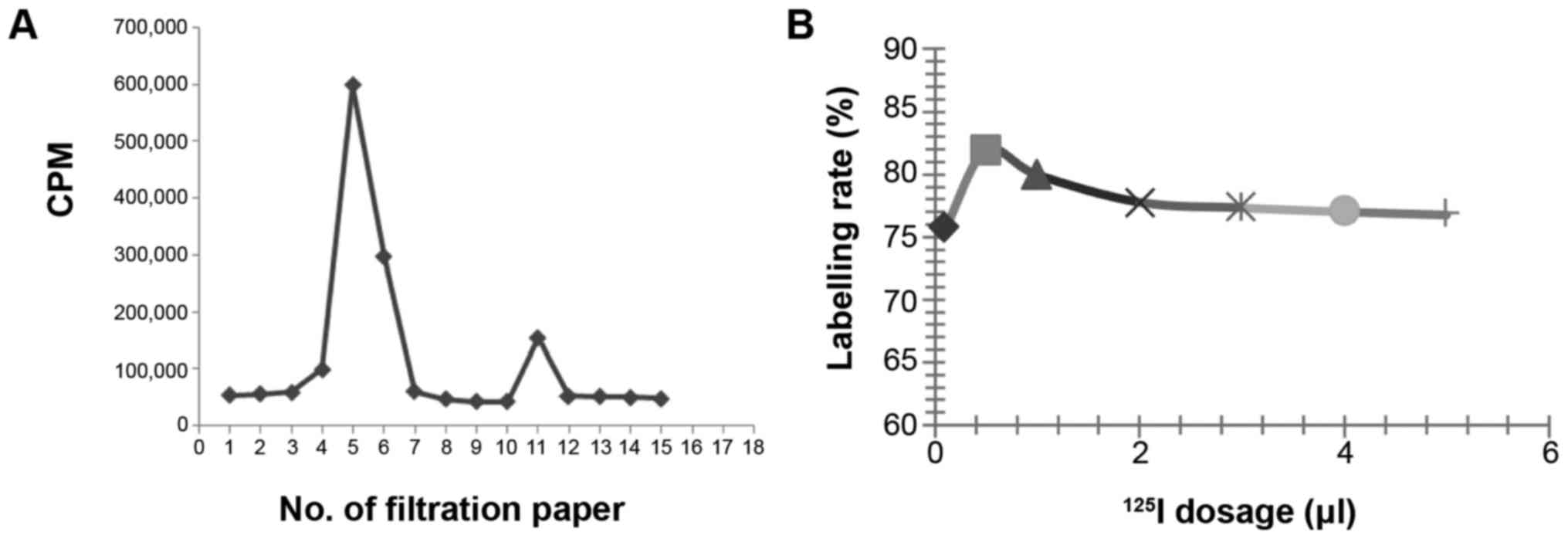
Influence of 125I dosage on the labelling
rate. Labelling rate of 125I-RSOAds-hTERT/PSA was
assayed by paper chromatography according to the differences in
diffusion coefficients between 125I-RSOAds-hTERT/PSA
markers and free 125I, i.e., the latter larger than the
former and two peaks appeared on the paper, peak I and peak II:
Peak I represents the peak of 125I-RSOAds-hTERT/PSA and
peak II represents the peak of free 125I.
It was identified (Fig.
1) that the labelling rate was relatively high when the dosage
of 125I was 0.5 µl (~0.2 m Ci, 7.4 MBq).
Influence of other factors on the
labelling rate
The influence of NBS, dosage of virus solution,
reaction duration, pH and PBS volume on the labelling rate is shown
in Figs. 2–6.
According to the results of the above experiments,
the best conditions were identified as: 0.5 µl of 125I
(~0.2 m Ci, 7.4 MBq), 25 µg of NBS, 100 µl of 8×109
VP/ml 125I-RSOAds-hTERT/PSA virus solutions, 3-min
reaction duration, pH 7.5 and 120 µl of PBS.
Measurements of radiochemical
purity
After isolation and purification of
125I-RSOAds-hTERT/PSA by gel-filtration chromatography,
the radiochemical purity of markers, assayed by the paper
chromatography, was >95%. The results of radiochemical purity at
difference time points are shown in Fig.
7. After 7 days of preservation in the refrigerator at 4°C, the
radiochemical purity of 125I-RSOAds-hTERT/PSA was stable
at approximately 93–94%.
Discussion
As a newly emerging targeted gene therapy of tumors,
RSOAds therapy is used to induce the virus to selectively replicate
and exert the oncolytic effect inside tumor cells by genetic
transformation of the virus. Selective oncolytic effect, one of the
common features of all RSOAds, did not affect normal cells
(6). Various viruses could be
transformed into oncolytic forms, including adenovirus, retrovirus,
herpes simplex virus, vaccinia virus and measles virus. The
oncolytic adenovirus, transformed from the adenovirus, has been the
research hotspot due to a better understanding and various research
of the adenovirus, including its genomic structure, function,
mobidity and safety (7). Studies have
shown that the deletion of E1A region of virus would not impair the
virus infection ability and its intracellular selective replication
ability. Under the conditions of deletion of E1B-55 kDa, virus
could retain its intracellular selective replicative ability in
cells with p53 mutation, but its survival time would be affected,
thus reducing the oncolytic activity. The expression of E3-11.6 kDa
and E4 would enhance the viral cytotoxic effect. Depletions of E3
10.4/14.5/14.7 regions would enhance the sensitivity to cytokine
and antitumor immunity (8). Satoh
et al found obvious suppression effect of AxdAdB-3, the
oncolytic adenovirus dual-regulated by E1A mutation and E1B
depletion, on the prostate cancer cells DU145, PC3 and LNCaP, in
which the results of animal experiments also indicated its
significant anti-prostate cancer effect (9). Cherubini et al also confirmed
that oncolytic adenovirus AdΔΔ with the depletions of E1A-CR2 and
E1B-19K was a very effective targeted-gene carrier in treatment of
prostate cancer and pancreatic cancer (10). In our studies, it was confirmed that
dual-regulated oncolytic adenovirus could be constructed with the
E1A and E1B genes of virus being regulated by hTERT promoter and
HIF-1 promoter, which would serve as a carrier in treatment of
bladder cancer by targeted super-antigen SEA, and better targeted
effect and killing tumor cell effect were indicated in in
vitro experiments (5).
125I, a kind of synthetic radioactive
nuclides, could emit gamma-rays with low energy and a short
distance of only 1.7 mm after transplantation into the body. Low
dosage exposure of 125I inhibited mitosis of tumor cells
for the integrity of DNA of tumor cells in mitotic phase which is
impaired by the gamma-ray, thus leading to the death of tumor
cells, instead of continuous mitosis. Moreover, gamma-ray could
also re-oxidize the hypoxic cells to increase the sensitivity of
tumor cells to gamma-ray (11). Zhu
et al found that 125I, the radioactive particle,
through significantly lowering the rate of positive cells in
vascular endothelial growth factor and microvascular density in
tumor tissues, could effectively destroy the micro-vessel of
tumors, limit the vessel formation of the tumor, reduce the blood
supply to tumors, thereby killing the tumor cells and lessening the
chance of tumor metastasis (12).
Since the short-distance radiotherapy could easily realize a
high-dosage exposure to local part of tumor tissues with less
influence on surrounding normal tissues but significantly improved
clinical efficacy and fewer incidences of complications,
125I is one of the major methods in treatment of
prostate cancer in many clinical practices. However, for cases with
surrounding fascia or seminal vesicle infiltrated by tumor cells,
or metastasis in other major organs or pelvic lymph nodes, or even
uncontrollable osseous metastasis, simple application of
125I could scarcely achieve radical cure, but should be
complemented or combined with other effective treatments (13).
We successfully incorporated the 125I
into the 125I- RSOAds-hTERT/PSA markers to produce the
targeted effect on the prostate cancer cells, which could be
expected to kill the tumor cells specifically and increase the
clinical efficacy of CRPC. Twigger et al found that not only
could reovirus exert the synergistic effect with radiation exposure
in in vitro experiments to promote the apoptosis of tumor
cells, but also its effect was not affected by the sequence of
radiation (14). Animal experiments
also confirmed that the control effect of combination of reovirus
and radiation exposure was significantly higher than that of simple
application of virus or radiation exposure. Chen et al found
that combined treatment by prostate-specific adenovirus (CV706) and
radiotherapy could exert the synergistic suppression effect on the
tumor growth after 7–42 days of combined therapy for the
heterotopic model of prostate cancer. Besides, after 6 weeks of
combined therapy, the prostate-specific antibody level was
significantly lower (~1% of the value in control group), showing
more significant effect than simple application of virus (~86% of
the control group) or radiotherapy (~139% of the control group)
(15). Dilley et al (8) confirmed that radiotherapy combined with
another kind of prostate-specific adenovirus (CV787) could
significantly lessen the average volume of tumor (~34% of the
volume in control group); complete regression of tumor in mice was
found to increase to 80% after at least 8 weeks of therapy.
Moreover, an obvious decrease was found in serum level of
prostate-specific antibody in subjects who accepted combined
therapy compared to those who accepted simple adenovirus or
radiotherapy.
At present, various shortages remain in treatment of
tumors by simple oncolytic adenovirus or radioactive nuclide, thus
we need to take some measures to enhance the antitumor ability of
virus and nuclide. Labelling the oncolytic adenovirus with
radioactive nuclide to exert the oncolytic and radiation effect, by
which the cancer cells would be destroyed, thus improving the
efficacy of treatment, could be an effective option to enhance the
antitumor effect of oncolytic adenovirus, and contribute to an
accurate distribution of radioactive nuclide around the tumor
cells, which could lessen the dosage of radioactive nuclide and
relieve its influence on the normal tissues. Attempts have already
been made to treat tumors with an enhanced synergistic effect
produced by oncolytic adenovirus labelled by radioactive nuclide
(16).
For prostate cancer tissues, hTERT has been
currently reported as the cancer biomarker with the widest
spectrum, while the PSA is the widely-accepted molecular marker for
prostate cancer. If the dynamic integration between these two
markers were realized, they would be the best combined tumor
markers for prostate cancer. In recent 5 years, we have been
working on the RSOAds targeted gene therapy for tumors of urinary
system. According to the pathologic and immunological
characteristics, we have successfully constructed the dual-targeted
and dual-regulated tumor-specific oncolytic adenovirus carrier by
PSA and hTERT to enhance the target effect of the therapeutic gene
on the tumor tissues of prostate, which is expected to acquire
better efficacy. This technique has applied for the European Patent
(European Patent Application no. 10153380.0, February 11,
2010).
The oncolytic adenovirus labelled by 125I
and dual-regulated by hTERT-PSA promoter possesses high target
effect and the carried 125I could be administered by
local injection or intravenous injection. After the tumor cells are
infected by the virus, the nuclide, with the replication of virus,
would be separated from the dual-regulated oncolytic adenovirus and
concentrated in the tumor cells, thus realizing an accurate
distribution of 125I in the tumor cells and overcoming
the deficiency that the nuclide could hardly be applied in the
targeted therapy in clinical practices. 125I, in
addition to the continuous killing effect on the tumor cells, it
could also consecutively emit the short-distance ionizing radiation
to the oncolytic adenovirus, making the tumor stem cells which are
resistant to radiotherapy or could not be killed by oncolytic
adenovirus more susceptible, and jointly exerting a better killing
effect on the prostate tumor cells. For the dosage of oncolytic
adenovirus and radioactive nuclide, it might be possible to reduce
the dosage in theory to alleviate or avoid the toxic side effect of
radioactive nuclide.
Thus, we infer that the combination of oncolytic
adenovirus and radioactive nuclide, in addition to the direct
killing effect on tumor cells, could exert the targeted-therapeutic
effect on the tumor microenvironment, which could remedy the
deficiency when they were applied individually: The targeted
infection of tumor cells by oncolytic adenovirus could increase the
sensitivity of tumor tissues to the radiotherapy, and the radiation
could also enhance the oncolytic ability of oncolytic adenovirus.
The synergistic effect of which could effectively inhibit the
growth of tumor stem cells which were resistant to the radiotherapy
and were difficult to kill by the oncolytic adenovirus to achieve
the radical treatment of the tumor. Besides, a series of influences
would be exerted on the tumor growth microenvironment by the
suppression effect of radiation on the tumor micro-vessels and a
certain degree of antitumor immunity induced by oncolytic virus to
realize the objective of targeted-therapy for tumor
microenvironment.
So far, there are few reports on the application of
in vitro construction of 125I-RSOAds-hTERT/PSA
marker for targeted treatment of CRPC. The results of the present
study indicated that the radiochemical purity of
125I-RSOAds-hTERT/PSA reached >95% and its stability
could be maintained at 4°C for 7 days, at approximately 93–94%.
These results serve as the experimental basis for later antitumor
experiments with markers, and are expected to provide new options
for the treatment of prostate cancer.
Acknowledgements
The present study was supported by the Research and
Development of Key Programs of Jiangsu (no. BE2015623), the Nation
Science Foundation of China (no. 81272557), the Natural Science
Foundation of Jiangsu (no. BK2012647), the Peak Projects for 6
kinds of Genius of Jiangsu (no. WSW-185), the International S&T
Cooperation Program of China (no. 2014DFA31480), the Scientific and
Technological Programs of Xuzhou (no. XM13B079), the Jiangsu
Province Medical Young Talent (QNRC2016386) and the Medical
Innovation Team of Jiangsu Province (CXTD-2016-48).
References
|
1
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Djenaba JA, Soman A, Rim SH and
Master VA: Recent trends in prostate cancer incidence by age,
cancer stage, and grade, the United States, 2001–2007. Prostate
Cancer. 2012:6913802012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottolino-Perry K, Diallo JS, Lichty BD,
Bell JC and McCart JA: Intelligent design: Combination therapy with
oncolytic viruses. Mol Ther. 18:251–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu J, Xuan X, Han C, Hao L, Zhang P, Chen
M, He H, Fan T and Dong B: Anti-tumor function of double-promoter
regulated adenovirus carrying SEA gene, in the treatment of bladder
cancer. Cell Biochem Biophys. 62:353–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gil Z, Rein A, Brader P, Li S, Shah JP,
Fong Y and Wong RJ: Nerve-sparing therapy with oncolytic herpes
virus for cancers with neural invasion. Clin Cancer Res.
13:6479–6485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto M and Curiel DT: Current issues
and future directions of oncolytic adenoviruses. Mol Ther.
18:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dilley J, Reddy S, Ko D, Nguyen N, Rojas
G, Working P and Yu DC: Oncolytic adenovirus CG7870 in combination
with radiation demonstrates synergistic enhancements of antitumor
efficacy without loss of specificity. Cancer Gene Ther. 12:715–722.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoh M, Wang H, Ishidoya S, Abe H, Moriya
T, Hamada H and Arai Y: Oncolytic virotherapy for prostate cancer
by E1A, E1B mutant adenovirus. Urology. 70:1243–1248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cherubini G, Kallin C, Mozetic A,
Hammaren-Busch K, Müller H, Lemoine NR and Halldén G: The oncolytic
adenovirus AdΔΔ enhances selective cancer cell killing in
combination with DNA-damaging drugs in pancreatic cancer models.
Gene Ther. 18:1157–1165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bolla M, van Poppel H, Collette L, van
Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset
JF, van Velthoven R, et al: European Organization for Research and
Treatment of Cancer: Postoperative radiotherapy after radical
prostatectomy: A randomised controlled trial (EORTC trial 22911).
Lancet. 366:572–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu X, Palmer MR, Makrigiorgos GM and
Kassis AI: Solid-tumor radionuclide therapy dosimetry: New
paradigms in view of tumor microenvironment and angiogenesis. Med
Phys. 37:2974–2984. 2010. View Article : Google Scholar
|
|
13
|
Lagerlöf JH, Kindblom J and Bernhardt P:
3D modeling of effects of increased oxygenation and activity
concentration in tumors treated with radionuclides and
antiangiogenic drugs. Med Phys. 38:4888–4893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Twigger K, Vidal L, White CL, De Bono JS,
Bhide S, Coffey M, Thompson B, Vile RG, Heinemann L, Pandha HS, et
al: Enhanced in vitro and in vivo cytotoxicity of combined reovirus
and radiotherapy. Clin Cancer Res. 14:912–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, DeWeese T, Dilley J, Zhang Y, Li
Y, Ramesh N, Lee J, Pennathur-Das R, Radzyminski J, Wypych J, et
al: CV706, a prostate cancer-specific adenovirus variant, in
combination with radiotherapy produces synergistic antitumor
efficacy without increasing toxicity. Cancer Res. 61:5453–5460.
2001.PubMed/NCBI
|
|
16
|
Mi YX, Li YC and Long YH: Imaging of
radioiodine-labeled KH901, a tumor-specific oncolytic recombinant
adenovirus, in nude mice with human hepatocellular carcinoma. Nucl
Med Commun. 31:405–410. 2010.PubMed/NCBI
|