Introduction
The prognosis of prostate cancer (PC) depends
greatly on tumor invasion and distant metastasis. In PC it has been
suggested that the tumor microenvironment plays an important role
in progression, acquisition of androgen independence, and distant
metastasis (1–3). We previously conducted a genome-wide
loss of heterozygosity/allelic imbalance (LOH/AI) scan of DNA from
the epithelium and stroma of 116 PCs and identified a total of 43
LOH/AI hot/cold spots, of which 17 were associated with both the
epithelium and stroma, 18 were unique to the epithelium, and eight
were unique to the stroma (4). We
also identified 15 LOH/AI markers that were correlated with Gleason
scores in that study. However, our understanding of the role of the
microenvironment in human PC remains limited.
Epithelial-to-mesenchymal transition (EMT) has
gained considerable attention as a conceptual paradigm to explain
invasive and metastatic behavior during cancer progression
(5). It has been proposed that EMT is
induced by cancer cells during metastatic dissemination from a
primary organ to secondary sites, but precisely how EMT occurs
during PC invasion and metastasis remains uncertain.
To clarify the mechanism of tumor progression and
metastasis in PC, and the role of the tumor microenvironment, we
investigated the molecular interaction of PC cells, prostatic
epithelium, and prostatic stroma through genome-wide gene
expression profiling. We hypothesized that PC cells might act on
stromal cells to induce their differentiation into
cancer-associated fibroblasts (CAFs), thus contributing to tumor
invasion and metastasis. Likewise, we hypothesized that CAFs could
act on surrounding normal epithelial cells to change their
characteristics into PC cells.
Materials and methods
Cell lines
The human PC cell lines LNCaP, PC-3, and 22Rv1 were
obtained from the American Type Culture Collection (Rockville, MD,
USA). The human normal prostate epithelial cell line PrEC and the
human normal prostate stromal cell line PrSC were purchased from
Lonza Group Ltd. (Basel, Switzerland). Cells were cultured as
monolayers in appropriate medium supplemented with 10% fetal bovine
serum (FBS), and maintained at 37°C in an atmosphere of humidified
air with 5% CO2.
Co-culture experiments
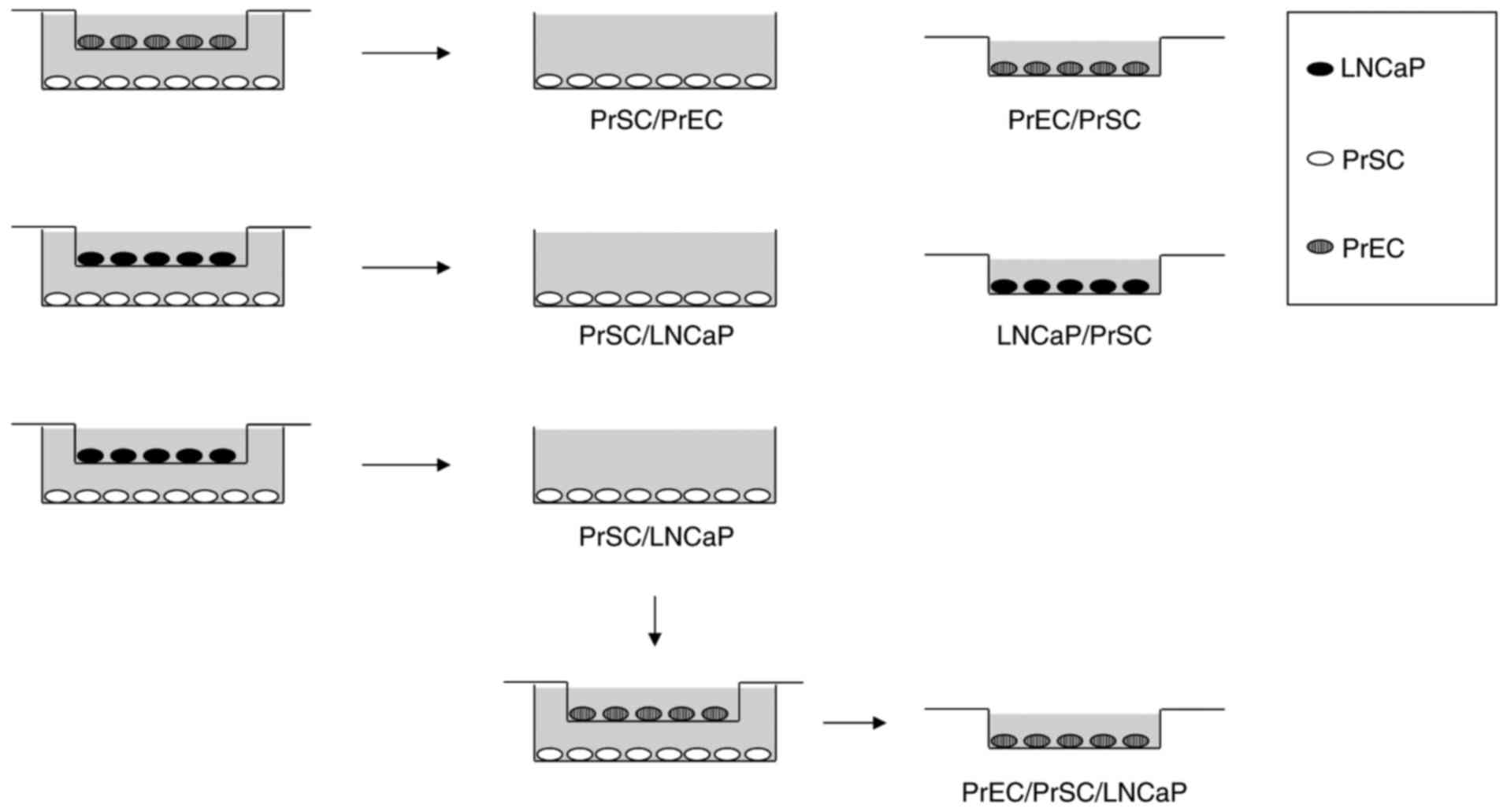
Co-culture experiments were performed as shown in
Fig. 1. LNCaP, PrEC, and PrSC cell
lines were cultured in 6-well plates or culture plate inserts (0.4
µm pore size; Corning Inc., Corning, NY, USA). On day 1, PrSC
(2×105 cells/well) were plated onto 6-well plates using
SCGM media (Lonza Group Ltd.) while PrEC (2×105
cells/well) and LNCaP (1×105 cells/well) were plated
onto culture plate inserts using PrEGM (Lonza Group Ltd.) or DMEM
plus 10% FBS media, respectively. On day two, culture media were
replaced with keratinocyte-SFM (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) plus 2% FBS and the inserts containing PrEC or
LNCaP were then transferred into 6-well plates containing PrSC
cells to initiate the experiment. On day five, total RNA was
isolated for microarray analysis. For PrEC/PrSC/LNCaP co-culture,
the inserts containing PrEC were transferred into 6-well plates
containing PrSC previously co-cultured with LNCaP (PrSC/LNCaP), and
cultured for three days.
cDNA microarray analysis and data
acquisition
Total RNA was extracted using the RNeasy Micro kit
(Qiagen GmbH, Hilden, Germany) after co-culture experiments,
according to the manufacturer's instructions. Briefly, total RNA
was reverse-transcribed to cDNA with T7-Oligo(dT) primer. The cDNA
synthesis product was used in an in vitro transcription
(IVT) reaction involving T7 RNA polymerase. An unlabeled
ribonucleotide mix was used in the first cycle of IVT
amplification. Unlabeled cRNA was then reverse-transcribed in the
first-strand cDNA synthesis step of the second cycle using random
primers. Subsequently, the T7-Oligo(dT) promoter primer was used
for the second-strand cDNA synthesis to generate a double-stranded
cDNA template containing T7 promoter sequences. The resultant
double-stranded cDNA was then amplified and labeled using a
biotinylated nucleotide analog/ribonucleotide mix in the second IVT
reaction. The labeled cRNA products were then fragmented, loaded
onto the GeneChip® Human Genome U133 Plus 2.0 array
(Affymetrix; Thermo Fisher Scientific, Inc.), and hybridized
according to the manufacturer's instructions. GeneChip®
array data were compared using the Kurabo custom analysis service
(Kurabo Industries Ltd., Osaka, Japan; Kurabo Industries Ltd. is an
authorized service provider for Affymetrix Japan K.K., Tokyo,
Japan). Differences in gene expression were assessed using the
following comparisons: PrSC/LNCaP vs. PrSC/PrEC, LNCaP/PrSC vs.
LNCaP, and PrEC/PrSC/LNCaP vs. PrEC/PrSC (Fig. 1; PrSC/LNCaP, PrSC co-cultured with
LNCaP; PrSC/PrEC, PrSC co-cultured with PrEC; LNCaP/PrSC, LNCaP
co-cultured with PrSC; PrEC/PrSC/LNCaP, PrEC co-cultured with
PrSC/LNCaP; PrEC/PrSC, PrEC co-cultured with PrSC). Raw intensity
data from the GeneChip® array were analyzed using the
GeneChip® Operating Software (Affymetrix; Thermo Fisher
Scientific, Inc.) and hierarchical clustering analyses were
conducted using Cluster and TreeView software (http://rana.lbl.gov/EisenSoftware.htm).
RNA isolation and semi-quantitative
RT-PCR
Total RNA was isolated using the RNeasy Micro kit
(Qiagen GmbH) from PC, PrEC, and PrSC cells according to the
manufacturer's instructions and reverse-transcribed using
Superscript II Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) with random primers, prior to performing PCR. PCR
primers were: 3-hydroxy-3-methylglutaryl-CoA synthase 1
(HMGCS1; forward, 5′-CTCCCTGACGTGGAATGTCT-3′; reverse,
5′-GAACTGTCTGCCCAGGTGAT-3′), 3-hydroxy-3-methylglutaryl-CoA
reductase (HMGCR; forward, 5′-CTTGCCGAGCCTAATGAAAG-3′;
reverse, 5′-TGACCCCCTGAGAAAGCTAA-3′), and glyceraldehyde
3-phosphate dehydrogenase (GAPDH; forward,
5′-CGGATTTGGTCGTATTGG-3′; reverse: 5′-TCCTGGAAGATGGTGATG-3′). PCR
conditions were as follows: initial denaturation at 94°C for 9 min,
followed by 28–30 cycles of denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec, and elongation at 72°C for 60 sec on
a C1000™ Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA).
Relative expression levels of mRNA were calculated in comparison to
those of GAPDH.
Small hairpin RNA-expressing
constructs and cell viability assay
We used SureSilencing short hairpin RNA (shRNA)
plasmids (Qiagen GmbH) for examining RNA interference effects on
the target genes. The target sequences of HMGCS1 and
HMGCR were 5′-GAAGGAACGTGGTACTTAGTT-3′ (shHMG CS1) and
5′-CAAGGAGCATGCAAAGATAAT-3′ (shHMG CR), respectively. The scrambled
sequence 5′-GGAATCTCATTCGATGCATAC-3′, which does not match any
human, mouse, or rat gene, was used as a negative control (shNC
vector). 22Rv1 cells that highly expressed both HMGCS1 and
HMGCR were plated onto 10 cm dishes (2×106
cells/dish) and transfected with 6 µg of each shRNA plasmids (shHMG
CS1, shHMG CR, or shNC) using FuGENE6 reagent (Promega Corporation,
Madison, WI, USA) according to the supplier's protocol. Cells were
selected in culture medium containing 1.0 mg/ml geneticin for 10
days, fixed with 100% methanol, and stained with 0.1% crystal
violet to evaluate colony formation. Cell viability was evaluated
by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay, with absorbance measured at 570 and 630 nm as a
reference using a microplate reader (THERMOmax; Molecular Devices,
LLC, Sunnyvale, CA, USA). Knockdown effects of these shRNA plasmids
on endogenous HMGCS1 or HMGCR expression were
validated 48 h following their transfection, by RT-PCR using the
primers described above.
Autocrine/paracrine cell proliferation
assay
Full-length human HMGCS1 and HMGCR
cDNA (accession nos. NM002130 and NM000859) were amplified and
cloned into the pcDNA3.1 (+) vector (Invitrogen; Thermo Fisher
Scientific, Inc.). To examine the autocrine effect of HMGCS1 and
HMGCR expression on PC cell growth, 22Rv1 cells were seeded into
6-well plates (5×105 cells/well) and transfected with
pcDNA3.1 (+) empty vector (mock), pcDNA3.1 (+)-HMGCS1, or pcDNA3.1
(+)-HMGCR expression vectors at a final concentration of 0.6 µg/ml
using FuGENE6 reagent (Promega), according to the manufacturer's
instructions. The proliferation of 22Rv1 cells overexpressing
HMGCS1 or HMGCR, or mock-transfected cells, was examined using a
cell counter (TC10™ Automated Cell Counter; Bio-Rad Laboratories)
during days 1–10. To examine the paracrine effect of HMGCS1 and
HMGCR on PC cell growth, PrSC cells (1×105 cells/well)
transfected with pcDNA3.1 (+) empty vector (mock), pcDNA3.1
(+)-HMGCS1, or pcDNA3.1 (+)-HMGCR expression vector at a final
concentration of 0.6 µg/ml and selected with 1.0 mg/ml geneticin,
were plated onto culture plate inserts, while 22Rv1 cells
(1×105 cells/well) were grown on 6-well plates. The
following day, culture media was replaced with keratinocyte-SFM
(Thermo Fisher Scientific, Inc.) plus 2% FBS and the inserts
containing transfected PrSC cells transferred into the 6-well
plates containing the 22Rv1 cells to initiate co-culture. Growth of
22Rv1 cells was calculated during days 1–14 using a cell
counter.
Tissue microarray samples and
immunohistochemical study
To further investigate HMGCS1 and HMGCR expression
in a larger number of tumor specimens, tissue microarray samples
containing 80 cases of PC and 16 normal prostate tissue, in
duplicate cores per case (PR1921; US Biomax, Inc., Rockville, MD,
USA) were obtained. The deparaffinized tissue sections were heated
in a microwave for 5 min for antigen retrieval. These sections were
incubated with a 1:200 diluted solution of a rabbit anti-HMGCS1
polyclonal antibody (ab87246; Abcam, Cambridge, UK) or a 1:50
diluted solution of a mouse anti-HMGCR monoclonal antibody (C-1,
sc-271595; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C and developed with peroxidase labeled-dextran
polymer followed by diaminobenzidine (Dako EnVision+ System; Dako,
Carpinteria, CA, USA). The sections were counterstained with
hematoxylin. For negative controls, primary antibody was omitted.
The stromal expression levels of HMGCS1 and HMGCR were determined
by calculating average intensities of positive stromal cells, using
a BZ-X Analyzer (Keyence Corporation, Osaka, Japan). Three random
fields were analyzed with a magnification of 400x. Correlations
between HMGCS1/HMGCR expression levels and clinicopathological
variables (tissue type, Gleason grade, tumor stage, tumor
classification, lymph node metastasis, distant metastasis, and
prostate-specific antigen (PSA) expression levels) were evaluated
using the Mann-Whitney U and Kruskal-Wallis tests. All
statistical analyses were performed using the software
JMP® (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant differences.
Results
Candidate genes identified by cDNA
microarray and cluster analysis
Before microarray analysis, we confirmed the
overexpression of α-smooth muscle actin (ACTA2) in
PrSC/LNCaP, which indicated the transition from normal stromal
cells to CAFs (data not shown). We identified 10 genes that were
upregulated in PrSC/LNCaP relative to PrSC/PrEC (signal log ratio
>2.0), which included insulin induced gene 1 (INSIG1),
HMGCS1, solute carrier family 14 member 1 (SLC14A1),
and noggin (NOG; Table I).
SLC14A1 has been reported to be regulated by androgens and
potentially involved in prostate carcinogenesis (6), while NOG is involved in EMT (7). INSIG1 mediates feedback control of
cholesterol synthesis by binding sterol regulatory element-binding
protein (SREBP) cleavage-activating protein (SCAP) and HMGCR. HMGCR
is the key enzyme of the mevalonate pathway and these facts
prompted us to investigate the mevalonate pathway enzymes HMGCS1
and HMGCR together, although the signal log ratio of HMGCR
was less than 2.0 (signal log ratio=1.4). We also identified 11
PC-specific genes that were upregulated in LNCaP/PrSC relative to
LNCaP (signal log ratio >3.0), which included fatty acid
synthase (FASN) and α-methylacyl-CoA racemase (AMACR;
Table II). We additionally
identified six genes that were upregulated in PrEC/PrSC/LNCaP
relative to PrEC/PrSC (signal log ratio >4.0), which included
the oncogene mitogen-activated protein kinase kinase kinase 8
(MAP3K8; Table III).
 | Table I.Upregulated genes in PrSC/LNCaP
compared with PrSC/PrEC. |
Table I.
Upregulated genes in PrSC/LNCaP
compared with PrSC/PrEC.
| Gene symbol | Gene title | Function |
|---|
| SCD | Stearoyl-CoA
desaturase (delta-9-desaturase) | Fatty acid
synthesis |
| INSIG1 | Insulin induced
gene 1 | Cholesterol
synthesis, steroid metabolism |
| HMGCS1 |
3-hydroxy-3-methylglutaryl-CoA synthase 1
(soluble) | Cholesterol
synthesis, lipid metabolism |
| HTR2B | 5-hydroxytryptamine
(serotonin) receptor 2B | Serotonin receptor
signaling pathway |
| OXTR | Oxytocin
receptor | G-protein coupled
receptor |
| PTHLH | Parathyroid
hormone-like hormone | Inhibitor of
osteoclastic bone resorption |
| IGFBP3 | Insulin-like growth
factor binding protein 3 | Cell growth
regulation |
| ALDH1A1 | Aldehyde
dehydrogenase 1 family, member A1 | Retinol
metabolism |
| SLC14A1 | Solute carrier
family 14 (urea transporter), member 1 (Kidd blood group) | Urea transport |
| NOG | Noggin | BMP signaling
pathway, EMT |
 | Table II.Upregulated genes in LNCaP/PrSC
compared with LNCaP. |
Table II.
Upregulated genes in LNCaP/PrSC
compared with LNCaP.
| Gene symbol | Gene title | Function |
|---|
| EGR1 | Early growth
response 1 | Transcriptional
regulation, BMP signaling pathway |
| SOX9 | SRY (sex
determining region Y)-box 9 | Skeletal
development, PKB signaling cascade |
| PLA2G2A | Phospholipase A2,
group IIA (platelets, synovial fluid) | phospholipid
metabolism |
| STC1 | Stanniocalcin
1 | Renal phosphate
reabsorption |
| DIO1 | Deiodinase,
iodothyronine, type I | Hormone
synthesis |
| FASN | Fatty acid
synthase | Fatty acid
synthesis |
| GINS2 | GINS complex
subunit 2 (Psf2 homolog) | DNA
replication |
| NFKBIZ | Nuclear factor of
kappa light polypeptide gene enhancer in B-cells inhibitor,
zeta | Regulation of
NF-kappa-B |
| UHRF1 | Ubiquitin-like with
PHD and ring finger domains 1 | DNA repair |
| AMACR | α-methylacyl-CoA
racemase | Lipid
metabolism |
| CLEC7A | C-type lectin
domain family 7, member A | Innate
immunity |
 | Table III.Upregulated genes in PrEC/PrSC/LNCaP
compared with PrEC/PrSC. |
Table III.
Upregulated genes in PrEC/PrSC/LNCaP
compared with PrEC/PrSC.
| Gene symbol | Gene title | Function |
|---|
| MAP3K8 | Mitogen-activated
protein kinase kinase kinase 8 | Oncogene |
| GABBR1 | γ-aminobutyric acid
(GABA) B receptor, 1 | G-protein coupled
receptor |
| TLR1 | Toll-like receptor
1 | Innate
immunity |
| SOD2 | Superoxide
dismutase 2, mitochondrial | Superoxide family
member, mutated in cancer |
| HERC5 | Hect domain and RLD
5 | Interferon-induced
E3 protein ligase |
| CMPK2 | Cytidine
monophosphate (UMP-CMP) kinase 2, mitochondrial | dUTP and dCTP
synthesis in mitochondria |
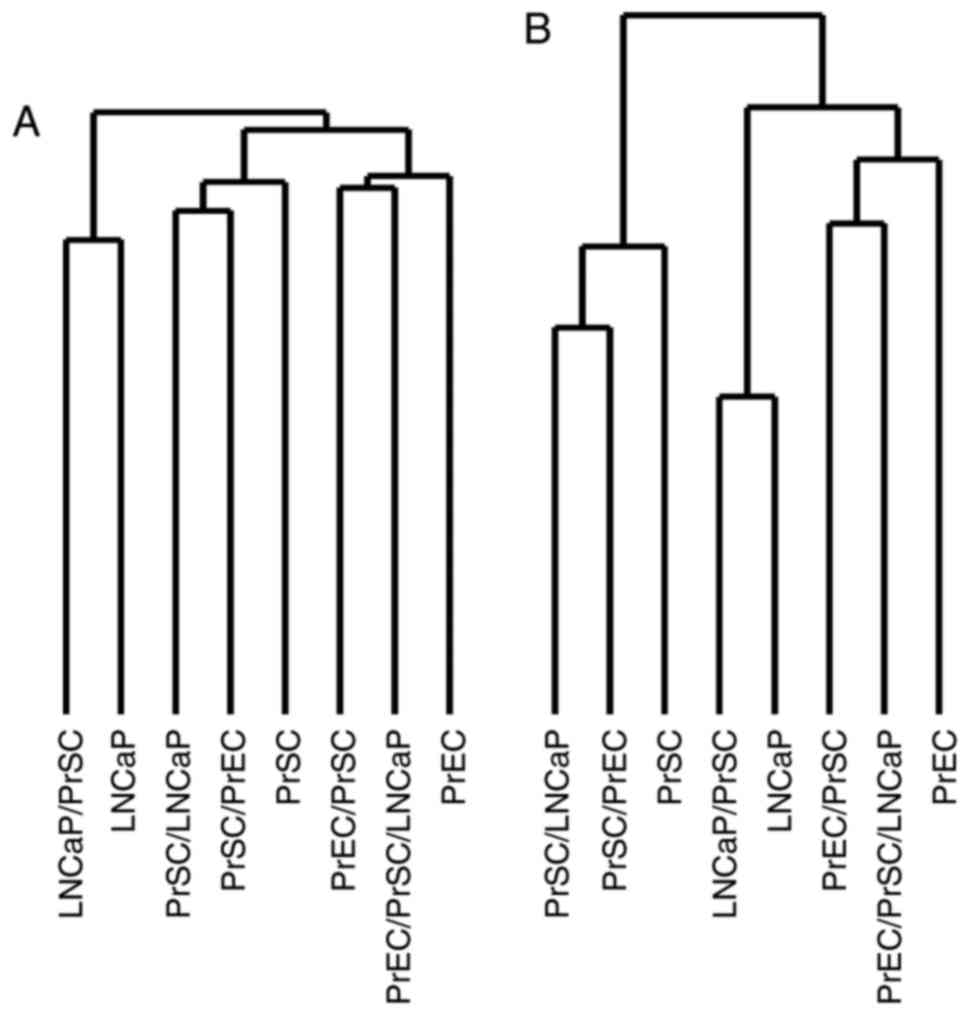
Unsupervised hierarchical clustering using full gene
expression profiles showed that the samples clustered into three
distinct groups related to LNCaPs, PrSCs and PrECs, respectively
(Fig. 2A). Interestingly, PrECs
clustered close to LNCaPs when clustering analysis was restricted
to the 767 genes most significantly upregulated or downregulated
(i.e., with a log ratio of >2.0 or <-2.0) in PrEC/PrSC/LNCaP,
that is PrEC co-cultured with CAFs (Fig.
2B).
Knockdown of HMGCS1 or HMGCR
expression by shRNA suppresses PC cell viability
To investigate the biological role of HMGCS1
and HMGCR in PC cells, we knocked down their endogenous
expression in 22Rv1 cells (Fig. 3A)
using vector-based RNA interference technology. Transfection of the
shRNA-expressing vectors shHMGCS1 or shHMGCR, clearly reduced
endogenous expression of HMGCS1 and HMGCR,
respectively (Fig. 3B), and resulted
in significant growth suppression as measured by both colony
formation assay and MTT assay (P<0.01; Fig. 3C and D, respectively). By contrast,
transfection of the negative control vector (shNC) had little or no
effect on HMGCS1 or HMGCR expression and did not
affect the viability of 22Rv1 cells (Fig.
3B-D).
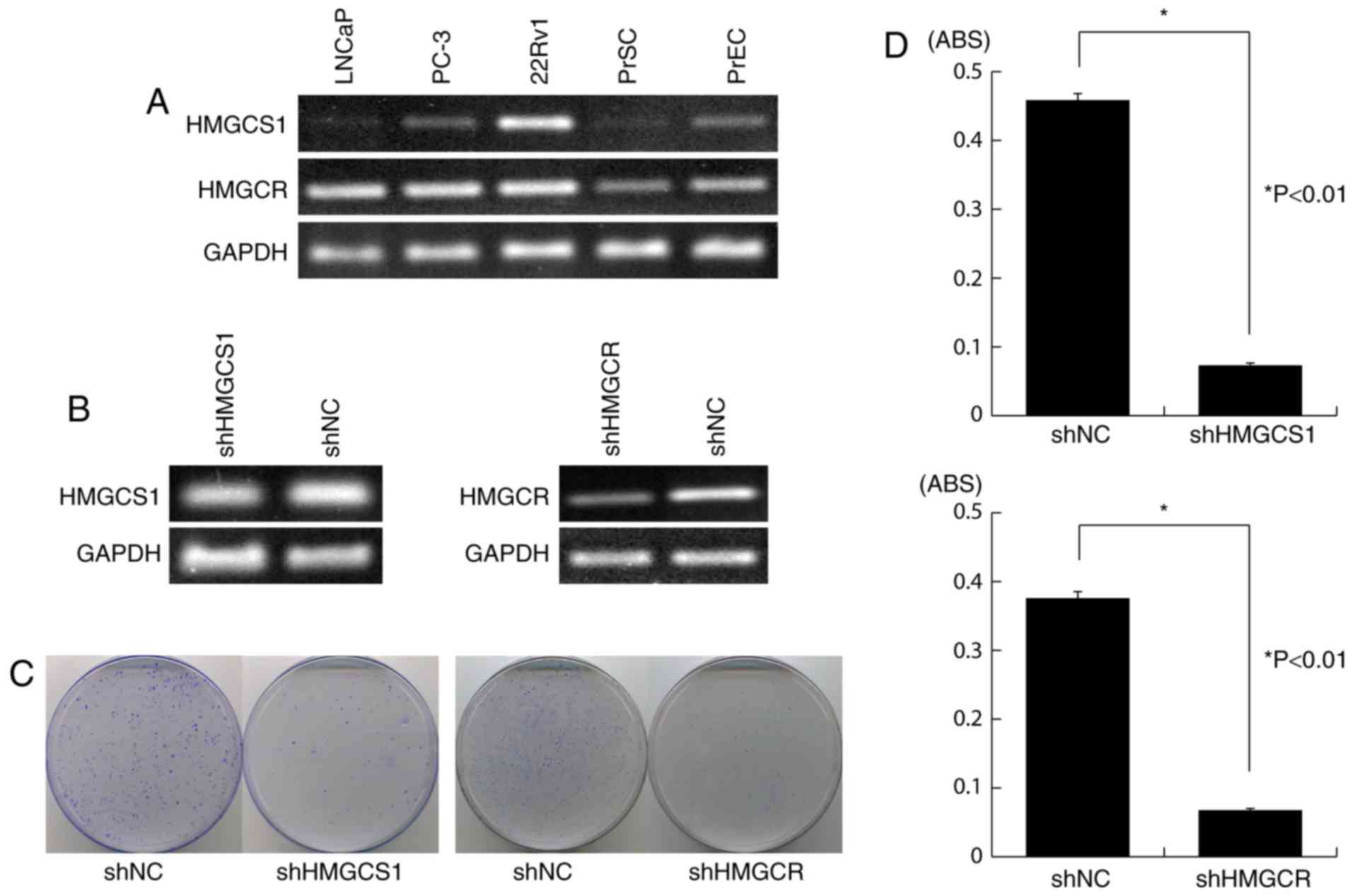 | Figure 3.Knockdown of HMGCS1 or
HMGCR expression by shRNA attenuates PC cell viability. (A)
Semi-quantitative RT-PCR showed that HMGCS1 and HMGCR
were overexpressed in 22Rv1 cells, compared with normal prostate
cells. GAPDH was used as a control for cDNA content; (B)
Semi-quantitative RT-PCR analysis of the knockdown effect on
endogenous HMGCS1 or HMGCR expression in 22Rv1 cells,
following transfection of shRNA-expressing vectors (shHMGCS1 or
shHMGCR) or the negative control vector, shNC; GAPDH was
used as a quantitative control; (C) Colony formation assay showing
decrease in the number of colonies in 22Rv1 cells transfected with
shHMGCS1 or shHMGCR vector, compared to control (shNC); (D) MTT
assay demonstrating significant suppression of PC cell viability
following transfection of shHMGCS1 or shHMGCR vector (mean ±
standard error). Experiments were carried out in triplicate, with
statistical analysis performed by Student's t-test (*P<0.01).
ABS, absorbance; PrEC, normal human prostate epithelial cells;
PrSC, normal human prostate stromal cells; sh, short hairpin; NC,
negative control; HMGCS1, 3-hydroxy-3-methylglutaryl-CoA synthase
1; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; RT-PCR, reverse
transcription-polymerase chain reaction; PC, prostate cancer. |
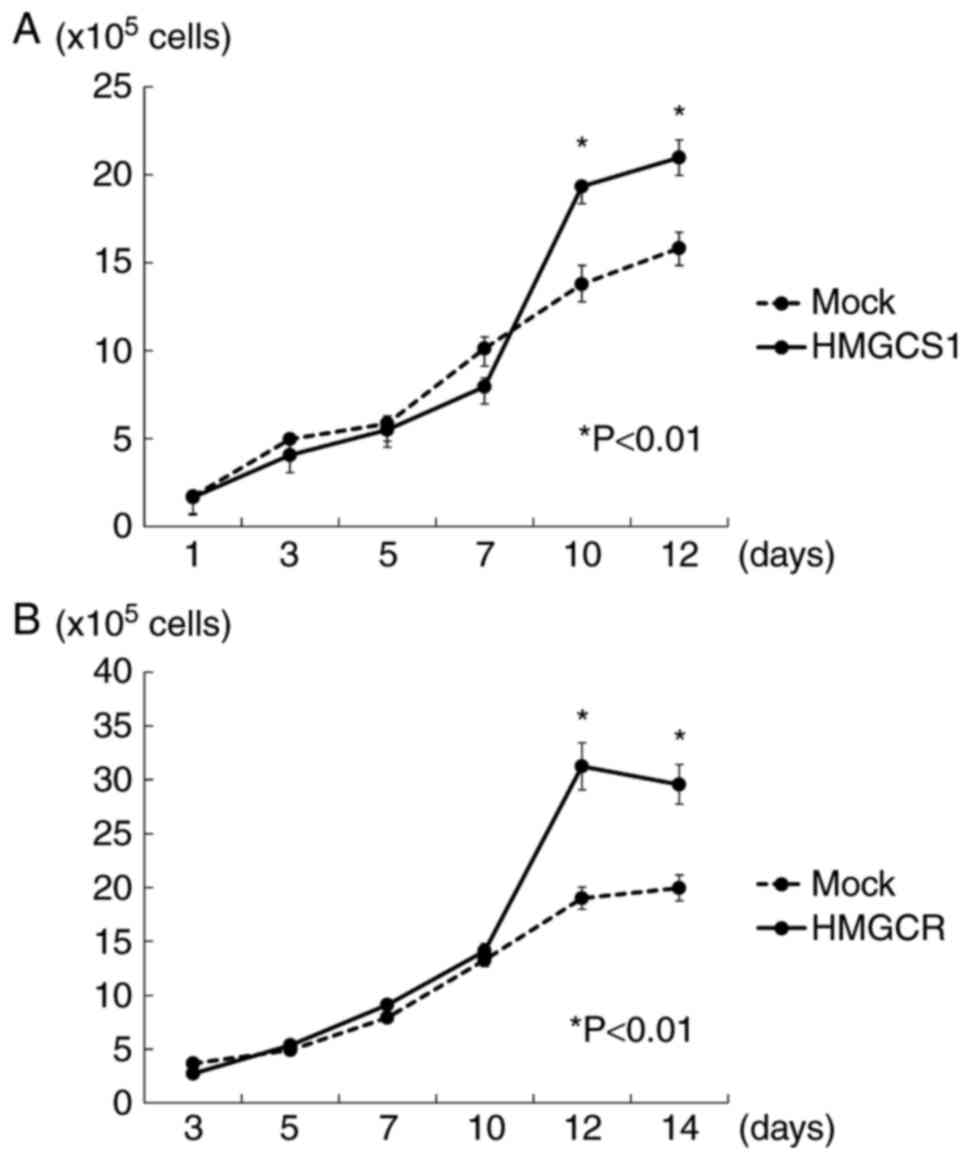
HMGCS1 or HMGCR overexpression
promotes PC cell proliferation through autocrine/paracrine
regulation
To further investigate the potential oncogenic
function of HMGCS1 and HMGCR, we examined the autocrine/paracrine
effects of these proteins on PC cell growth. The cell proliferation
assay revealed that 22Rv1 cells with exogenous overexpression of
HMGCR grew more rapidly than 22Rv1 mock-transfected cells,
indicating that HMGCR overexpression promotes PC cell proliferation
in an autocrine fashion. HMGCS1 overexpression however, did not
significantly stimulate PC cell growth (data not shown). We further
examined the influence of HMGCS1 and HMGCR overexpression in PC
stroma on PC cell growth using a co-culture experiment.
Overexpression of HMGCS1 or HMGCR in PC stromal cells was found to
induce a significantly higher growth rate of 22Rv1 cells than those
that were mock transfected (P<0.01; Fig. 4), indicating a paracrine effect.
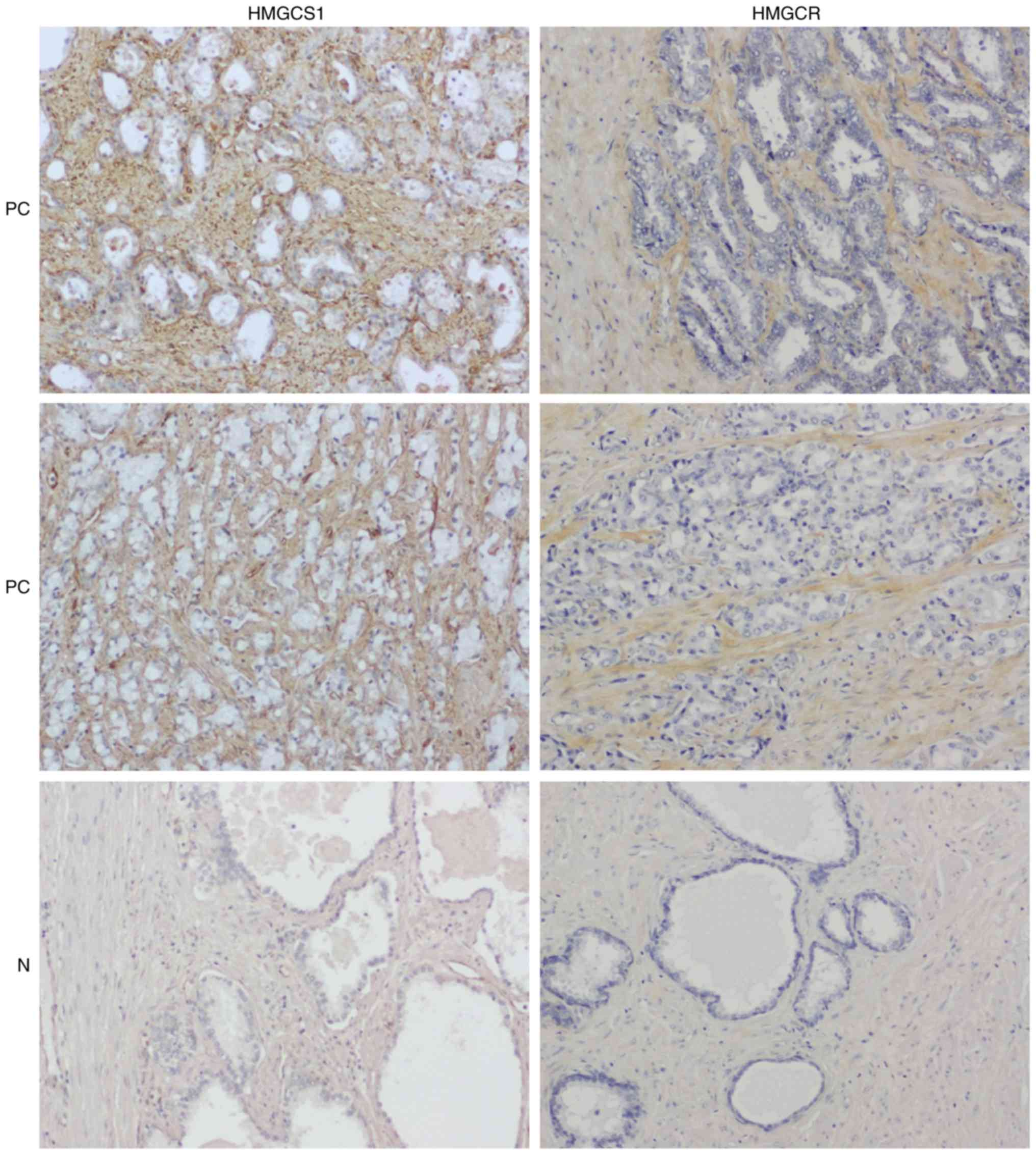
Immunohistochemical analysis of
primary PC specimens
We subsequently performed immunohistochemical
staining of 80 primary PC and 16 normal cases on tissue microarrays
(a total of 192 cores) with anti-HMGCS1 or anti-HMGCR antibodies.
Immunohistochemistry confirmed the overexpression of HMGCS1 and
HMGCR in PC stroma compared with normal prostatic stroma
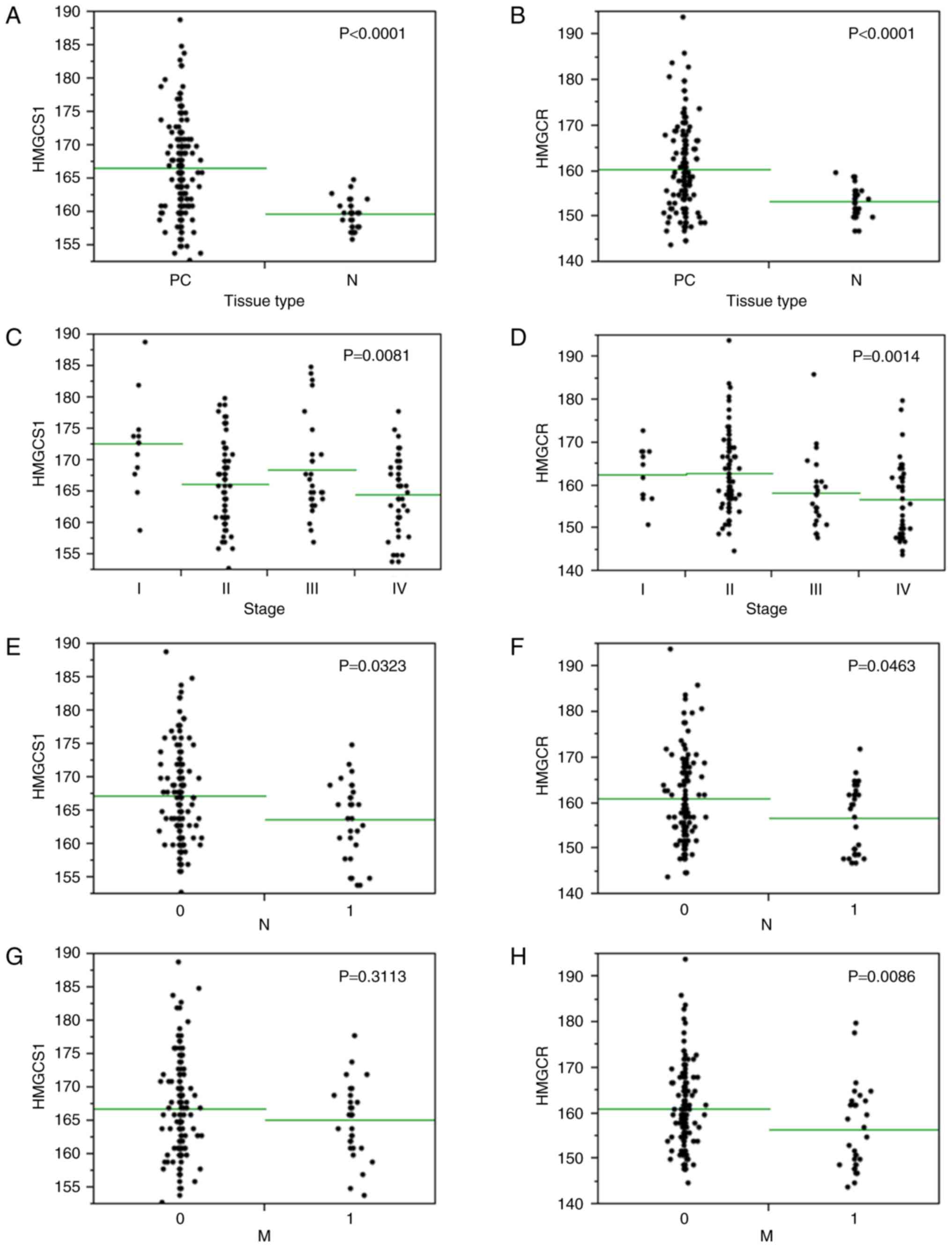
(P<0.0001; Figs. 5, 6A and B). To investigate the
clinicopathological significance of HMGCS1 and HMGCR stromal
expression in PC tissues, we next analyzed the relationship between
HMGCS1 or HMGCR stromal expression and the clinicopathological
variables of PC specimens, which is summarized in Table IV. This analysis revealed a
significant association between HMGCS1/HMGCR stromal expression and
several clinicopathological factors. Most notably, we observed that
HMGCS1 and HMGCR expression levels in PC stroma were inversely
associated with tumor stage (P<0.01; Fig. 6C and D), with higher stage PCs
associated with lower stromal expression levels of HMGCS1 and
HMGCR. Likewise, HMGCS1 and HMGCR stromal expression was
significantly lower in cases with lymph node metastasis (P<0.05;
Fig. 6E and F). HMGCR stromal
expression was also significantly lower in cases with distant
metastasis (P<0.01; Fig. 6G),
however HMGCS1 stromal expression levels had no correlation with
distant metastatic status (P=0.3113; Fig.
6H). There was no relationship between HMGCS1 or HMGCR stromal
expression and Gleason grade, tumor classification, or PSA
expression levels (data not shown).
 | Table IV.Clinicopathological variables of PC
cases. |
Table IV.
Clinicopathological variables of PC
cases.
| Number of cases
(cores) | 80 (160) |
|---|
| Age, years [median
(range)] | 69.5 (51–85) |
| Gleason grade, n,
per core |
|
| 3 | 16 |
| 4 | 74 |
| 5 | 60 |
| Tumor stage, n, per
case |
|
| I | 6 |
| II | 37 |
|
III | 14 |
| IV | 22 |
| Tumor
classification, n, per case |
|
| T1 | 4 |
| T2 | 47 |
| T3 | 22 |
| T4 | 6 |
| Lymph node
metastasis, n, per case |
|
|
Positive | 15 |
|
Negative | 64 |
| Distant metastasis,
n, per case |
|
|
Positive | 15 |
|
Negative | 64 |
| PSA expression
level (IHC), n, per core |
|
| − | 52 |
| + | 28 |
| ++ | 45 |
|
+++ | 32 |
Discussion
Our data suggest that stromal cells may influence PC
cell gene expression and contribute to their aggressiveness. In
keeping with this concept, AMACR, which is widely used as a
biomarker for PC, was included in the genes that were upregulated
in PC cells co-cultured with prostate stromal cells. The cluster
analyses support our hypothesis that CAFs might induce surrounding
normal epithelial cells to change their characteristics towards PC
cells. Interestingly, an oncogene, MAP3K8, was included
among the genes that were upregulated in prostate epithelial cells
when co-cultured with cancer-associated prostate stromal cells
(i.e., prostate stromal cells previously co-cultured with PC
cells). Among the genes identified in this study, we further
investigated the mevalonate pathway genes, HMGCS1 and
HMGCR. The mevalonate pathway is included in lipid
metabolism and best known as the target of statins. There is a
clear association between PCs and lipid metabolism or statins,
which prompted us to investigate the mevalonate pathway genes,
HMGCS1 and HMGCR. Notably, we investigated for the
first time the roles of HMGCS1 in human PC cells and tissues.
Tables I, II, and III
are derived from normal prostate stromal cells, PC cells, and
normal prostate epithelial cells, respectively, and these tables
indicate that their gene expression could change by co-culture with
the different type of cells. Because HMGCS1 and HMGCR
were the upregulated genes in stroma, those were not among the
genes in Tables II and III, which were derived from PC and
epithelium.
HMGCS1 condenses acetyl-CoA with acetoacetyl-CoA to
form HMG-CoA, which is the substrate for HMGCR. An association of
HMGCS1 with PC has not been previously reported, although links
with other cancers have been described (8–14). Lee
et al suggested HMGCS1 as one of the candidates involved in
tumor stem-like breast cancer cells (8), while Pandyra et al reported that
dipyridamole acts as a potentiator of statin anticancer activity in
multiple myeloma and acute myelogenous leukemia by attenuating the
feedback response that upregulates HMGCS1 and HMGCR after statin
treatment (10). In particular, these
authors showed that direct targeting of multiple levels of the
mevalonate pathway, including blockade the sterol-feedback loop
initiated by statin treatment, is an effective and targetable
anti-tumor strategy. HMGCS1 has also been linked to drug response
or resistance in other studies (14,15). Such
observations suggested the possibility that HMGCS1 and HMGCR may
similarly represent molecular targets for the treatment of PC.
HMGCR is the rate-limiting enzyme for cholesterol
synthesis and the pharmacological target for statins. The
association of HMGCR with PC has been previously described in
several studies (16–21). It has been reported that PC cells show
high expression of HMGCR and that the inhibition of HMGCR with the
use of statins lowers the viability of castration-resistant PC
cells (17). Li et al
identified two miRNAs, miR-185 and miR-342, that control
lipogenesis and cholesterogenesis in PC cells by inhibiting
SREBP-1 and SREBP-2 expression and downregulating
their targeted genes, which includes HMGCR (18). miR-185 and 342 inhibit tumorigenicity,
cell growth, migration, and invasion, and induce apoptosis through
blockade of the SREBP metabolic pathway in PC cells, representing a
novel targeting mechanism for PC therapy.
In the present study, overexpression of HMGCS1 or
HMGCR in PC stroma promoted PC cell growth (Fig. 4). However, the stromal expression of
HMGCS1 or HMGCR was associated with less aggressive PC (Fig. 6C-H). HMGCS1 and HMGCR might be needed
for PC growth until PC progresses to aggressive disease, and then
downregulated once PC invasion or metastasis has been developed.
Several studies that support our findings have been reported on
HMGCR (22–26), although there are no studies available
on HMGCS1. Previous studies indicated that high HMGCR expression
was associated with less aggressive tumor characteristics and HMGCR
expression was a good prognostic marker in breast cancer (23–26).
Associations of positive HMGCR expression with more favorable tumor
characteristics and a prolonged survival were also shown in other
type of cancer such as colorectal cancer (22). We analyzed and provided the stromal
expression data of HMGCS1 and HMGCR using clinical PC specimens in
this study, since PC specimens showed apparent stromal expression
of HMGCS1 and HMGCR (Fig. 5).
Unfortunately, there were no follow up data available for the
tissue microarray, and we then need to find if HMGCS1 and HMGCR
have an influence on survival or progression of PC patients in the
future study.
Our findings suggest that overexpression of the
mevalonate pathway genes, HMGCS1 and HMGCR is likely
to be involved in PC cell growth through an autocrine/paracrine
mechanism, supporting their potential of these proteins as
molecular targets for PC therapy. Furthermore, immunohistochemical
studies indicate that HMGCS1 and HMGCR stromal overexpression might
be key factor in regulating the transition from organ-confined to
metastatic disease in PC. We used 22Rv1 cell line for further
experiments because only 22Rv1 overexpressed both HMHCS1 and
HMGCR and should be relevant to these genes, while we
confirmed the knockdown effect of shRNA on HMHCS1 and
HMGCR by the mRNA expression results (not protein
expression), as seen in the many studies published previously
(27–32). The HMGCS1 and HMGCR
mRNAs were overexpressed in 22Rv1 cells (Fig. 3A) and their knockdown effect by shRNA
was validated on mRNA level (Fig.
3B). The use of a single cell line to verify the hypothesis and
the absence of data regarding the knockdown effect on protein
expression are two limitations of the present study. Further
functional analysis of the role of HMGCS1 and HMGCR in regulating
the interaction between PC and PC stroma are required, in order to
fully elucidate their potential as molecular targets in the
treatment of PC.
References
|
1
|
Chung LW, Baseman A, Assikis V and Zhau
HE: Molecular insights into prostate cancer progression: The
missing link of tumor microenvironment. J Urol. 173:10–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung SY, Hsieh CL, Law A, Zhau HE, Pathak
S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, et al:
Coevolution of prostate cancer and bone stroma in three-dimensional
coculture: Implications for cancer growth and metastasis. Cancer
Res. 68:9996–10003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuxhorn JA, Ayala GE and Rowley DR:
Reactive stroma in prostate cancer progression. J Urol.
166:2472–2483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashida S, Orloff MS, Bebek G, Zhang L,
Zheng P, Peehl DM and Eng C: Integrated analysis reveals critical
genomic regions in prostate tumor microenvironment associated with
clinicopathologic phenotypes. Clin Cancer Res. 18:1578–1587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: Paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaarala MH, Hirvikoski P, Kauppila S and
Paavonen TK: Identification of androgen-regulated genes in human
prostate. Mol Med Rep. 6:466–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kestens C, Siersema PD, Offerhaus GJ and
van Baal JW: BMP4 signaling is able to induce an
epithelial-mesenchymal transition-like phenotype in barrett's
esophagus and esophageal adenocarcinoma through induction of
SNAIL2. PLoS One. 11:e01557542016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee WJ, Kim SC, Yoon JH, Yoon SJ, Lim J,
Kim YS, Kwon SW and Park JH: Meta-analysis of tumor stem-like
breast cancer cells using gene set and network analysis. PLoS One.
11:e01488182016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meerzaman DM, Yan C, Chen QR, Edmonson MN,
Schaefer CF, Clifford RJ, Dunn BK, Dong L, Finney RP, Cultraro CM,
et al: Genome-wide transcriptional sequencing identifies novel
mutations in metabolic genes in human hepatocellular carcinoma.
Cancer Genomics Proteomics. 11:1–12. 2014.PubMed/NCBI
|
|
10
|
Pandyra A, Mullen PJ, Kalkat M, Yu R, Pong
JT, Li Z, Trudel S, Lang KS, Minden MD, Schimmer AD and Penn LZ:
Immediate utility of two approved agents to target both the
metabolic mevalonate pathway and its restorative feedback loop.
Cancer Res. 74:4772–4782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pandyra AA, Mullen PJ, Goard CA, Ericson
E, Sharma P, Kalkat M, Yu R, Pong JT, Brown KR, Hart T, et al:
Genome-wide RNAi analysis reveals that simultaneous inhibition of
specific mevalonate pathway genes potentiates tumor cell death.
Oncotarget. 6:26909–26921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van der Meer DL, Degenhardt T, Väisänen S,
de Groot PJ, Heinäniemi M, de Vries SC, Müller M, Carlberg C and
Kersten S: Profiling of promoter occupancy by PPARalpha in human
hepatoma cells via ChIP-chip analysis. Nucleic Acids Res.
38:2839–2850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wali VB, Haskins JW, Gilmore-Hebert M,
Platt JT, Liu Z and Stern DF: Convergent and divergent cellular
responses by ErbB4 isoforms in mammary epithelial cells. Mol Cancer
Res. 12:1140–1155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao M, Li H, Bu X, Lei C, Fang Q and Hu
Z: Quantitative proteomic analysis of cellular resistance to the
nanoparticle abraxane. ACS Nano. 9:10099–10112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rokosz LL, Boulton DA, Butkiewicz EA,
Sanyal G, Cueto MA, Lachance PA and Hermes JD: Human cytoplasmic
3-hydroxy-3-methylglutaryl coenzyme A synthase: Expression,
purification, and characterization of recombinant wild-type and
Cys129 mutant enzymes. Arch Biochem Biophys. 312:1–13. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bull CJ, Bonilla C, Holly JM, Perks CM,
Davies N, Haycock P, Yu OH, Richards JB, Eeles R, Easton D, et al:
Blood lipids and prostate cancer: A Mendelian randomization
analysis. Cancer Med. 5:1125–1136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Cox ME and Wasan KM: Effect of
simvastatin on castration-resistant prostate cancer cells. Lipids
Health Dis. 13:562014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Chen YT, Josson S, Mukhopadhyay NK,
Kim J, Freeman MR and Huang WC: MicroRNA-185 and 342 inhibit
tumorigenicity and induce apoptosis through blockade of the SREBP
metabolic pathway in prostate cancer cells. PLoS One. 8:e709872013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menter DG, Ramsauer VP, Harirforoosh S,
Chakraborty K, Yang P, Hsi L, Newman RA and Krishnan K:
Differential effects of pravastatin and simvastatin on the growth
of tumor cells from different organ sites. PLoS One. 6:e288132011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murtola TJ, Syvälä H, Pennanen P, Bläuer
M, Solakivi T, Ylikomi T and Tammela TL: The importance of LDL and
cholesterol metabolism for prostate epithelial cell growth. PLoS
One. 7:e394452012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai M, Martinez-Arguelles DB, Aprikian
AG, Magliocco AM and Papadopoulos V: De novo steroid biosynthesis
in human prostate cell lines and biopsies. Prostate. 76:575–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bengtsson E, Nerjovaj P, Wangefjord S,
Nodin B, Eberhard J, Uhlén M, Borgquist S and Jirström K: HMG-CoA
reductase expression in primary colorectal cancer correlates with
favourable clinicopathological characteristics and an improved
clinical outcome. Diagn Pathol. 9:782014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borgquist S, Djerbi S, Pontén F,
Anagnostaki L, Goldman M, Gaber A, Manjer J, Landberg G and
Jirström K: HMG-CoA reductase expression in breast cancer is
associated with a less aggressive phenotype and influenced by
anthropometric factors. Int J Cancer. 123:1146–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borgquist S, Jögi A, Pontén F, Rydén L,
Brennan DJ and Jirström K: Prognostic impact of tumour-specific
HMG-CoA reductase expression in primary breast cancer. Breast
Cancer Res. 10:R792008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brennan DJ, Laursen H, O'Connor DP,
Borgquist S, Uhlen M, Gallagher WM, Pontén F, Millikan RC, Rydén L
and Jirström K: Tumor-specific HMG-CoA reductase expression in
primary premenopausal breast cancer predicts response to tamoxifen.
Breast Cancer Res. 13:R122011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gustbée E, Tryggvadottir H, Markkula A,
Simonsson M, Nodin B, Jirström K, Rose C, Ingvar C, Borgquist S and
Jernström H: Tumor-specific expression of HMG-CoA reductase in a
population-based cohort of breast cancer patients. BMC Clin Pathol.
15:82015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anazawa Y, Nakagawa H, Furihara M, Ashida
S, Tamura K, Yoshioka H, Shuin T, Fujioka T, Katagiri T and
Nakamura Y: PCOTH, a novel gene overexpressed in prostate cancers,
promotes prostate cancer cell growth through phosphorylation of
oncoprotein TAF-Ibeta/SET. Cancer Res. 65:4578–4586. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anchi T, Tamura K, Furihata M, Satake H,
Sakoda H, Kawada C, Kamei M, Shimamoto T, Fukuhara H, Fukata S, et
al: SNRPE is involved in cell proliferation and progression of
high-grade prostate cancer through the regulation of androgen
receptor expression. Oncol Lett. 3:264–268. 2012.PubMed/NCBI
|
|
29
|
Ashida S, Furihata M, Katagiri T, Tamura
K, Anazawa Y, Yoshioka H, Miki T, Fujioka T, Shuin T, Nakamura Y
and Nakagawa H: Expression of novel molecules, MICAL2-PV (MICAL2
prostate cancer variants), increases with high Gleason score and
prostate cancer progression. Clin Cancer Res. 12:2767–2773. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Satake H, Tamura K, Furihata M, Anchi T,
Sakoda H, Kawada C, Iiyama T, Ashida S and Shuin T: The
ubiquitin-like molecule interferon-stimulated gene 15 is
overexpressed in human prostate cancer. Oncol Rep. 23:11–16.
2010.PubMed/NCBI
|
|
31
|
Tamura K, Makino A, Hullin-Matsuda F,
Kobayashi T, Furihata M, Chung S, Ashida S, Miki T, Fujioka T,
Shuin T, et al: Novel lipogenic enzyme ELOVL7 is involved in
prostate cancer growth through saturated long-chain fatty acid
metabolism. Cancer Res. 69:8133–8140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Togashi A, Katagiri T, Ashida S, Fujioka
T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T
and Nakamura Y: Hypoxia-inducible protein 2 (HIG2), a novel
diagnostic marker for renal cell carcinoma and potential target for
molecular therapy. Cancer Res. 65:4817–4826. 2005. View Article : Google Scholar : PubMed/NCBI
|