Introduction
Esophageal cancer is the eighth-most common type of
cancer globally, and due to the poor prognosis associated with
esophageal cancer, it is the sixth most common cause of
cancer-associated mortality (1). An
estimate indicates that, in 2011, there were 3,372,175 incidences
of cancer and 2,113,048 cancer mortalities (2). Histologically, esophageal cancer can be
divided into squamous cell carcinoma (SCC) and adenocarcinoma. At
present, potential curative treatment options include surgical
resection, chemotherapy and chemoradiation. However, as esophageal
cancer exhibits few characteristic clinical manifestations, the
majority of patients are diagnosed at the advanced stage of the
disease (3). In the advanced stages
of the disease, the tumor will have already metastasized, and the
most important types of treatment are chemotherapy or radiotherapy.
Currently, chemotherapy usually comprises of cisplatin and
5-fluorouracil-based therapy, but the increase in survival rate is
limited and the 5-year survival rate of esophageal cancer remains
low (4). Due to high prevalence rate
of esophageal cancer, novel approaches are required to prevent and
treat the disease. As a result, attention is being paid at present
to numerous phytochemicals that are being explored as potential
chemopreventive agents that may reverse or suppress esophageal
cancer progression.
Apoptosis is the active process of programmed cell
death. Apoptosis depends on an intrinsic apoptotic pathway, which
occurs in the mitochondria, or on an extrinsic apoptotic pathway,
which involves Fas death receptors (5). The B-cell lymphoma (Bcl) protein family
regulates the mitochondrial apoptosis pathway by controlling the
permeability of the outer mitochondrial membrane. When the
expression of the pro-apoptosis protein Bcl-2-associated X protein
(Bax) is increased, the pro-survival protein Bcl-2 cannot bind to
every Bax protein, and thus apoptosis is triggered (6). Apoptosis stimuli increase the level of
expression of p53, due to an increase in the permeability of the
outer mitochondrial membrane and the release of the apoptotic
protease activating factor (APAF-1) and the protein of the direct
inhibitor of apoptosis (IAP) binding protein with low PI
(Smac/DIABLO) from the mitochondria (7). Subsequent to the binding of cytochrome
c and dATP, APAF-1 forms an oligomeric apoptosome. This
apoptosome may stimulate the subsequent caspase cascade that
commits the cell to apoptosis (8). In
addition to this, the Smac/DIABLO protein complex inhibits the
binding of X-linked inhibitor of apoptosis protein (XIAP) in order
to promote apoptosis (9).
2,4,6-trihydroxy-3-(4-hydroxyphenyl)-propiophenone
(phloretin), which can be found in apple tree leaves and the
Manchurian apricot, is known to exhibit antioxidative,
antimicrobial, anti-inflammatory and antitumor properties (10). Phloretin has been shown to exert
antitumor activity through the inhibition of protein kinase C (PKC)
activity and the induction of apoptosis (11). The proliferation of the colon cancer
HT-29 cells, Fischer bladder cell carcinoma cell lines and
Lymphatic tumors have been found to be inhibited by phloretin
(12). The effects and anticancer
mechanisms of phloretin on esophageal cancer remains unclear. The
present study therefore investigated the potential molecular
mechanism of phloretin-induced tumor cell apoptosis in EC-109
cells.
Materials and methods
Cell culture
The esophageal cancer EC-109 cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cell lines were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 100 mg/ml penicillin and streptomycin
(Invitrogen, Thermo Fisher Scientific, Inc.) in 5% CO2
at 37.5°C.
Reagents
Phloretin, purity >99%, was purchased from Xi'an
Plants of Grass Technology Co. Ltd. (Xi'an, China).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) was purchased from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany). Benzyloxycarbony (Cbz)-l-Val-Ala-Asp
(OMe)-fluoromethylketone (Z-VAD-FMK), electrochemiluminescence
(ECL), protease inhibitors (phenylmethanesulfonyl fluoride) and
phosphatase inhibitors (PhosSTOP) were purchased from Beyotime
Institute of Biotechnology (Beijing, China). Antibodies against BAX
(#50599-2-Ig; dilution, 1:500), Bcl-2 (#12789-1-AP; dilution,
1:600), APAF-1 (#21710-1-AP; dilution, 1:200), DIABLO (#10434-1-AP;
dilution, 1:1,000), XIAP (#10037-1-Ig; dilution, 1:400) and p53
(#10442-1-AP; dilution, 1:700) were purchased from ProteinTech
Group, Inc. (Wuhan, China).
Cellular proliferation assay
Cell viability was measured using an MTT assay. A
total of 1×104 cells were cultured in a 96-well plate
and treated with 60–100 µg/ml phloretin for 6, 12, 24 or 48 h. In
total, 1 mg/ml of MTT solution (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was then added to the medium, and the cells
were incubated for an additional 4 h at 37.5°C. The medium was then
removed, and 100 µl dimethyl sulfoxide was added to dissolve the
solid residue. The absorbance of each well was read at 540 nm using
a micro-ELISA reader. Percent cell survival was defined as the
relative absorbance of treated vs. untreated cells.
Cellular apoptosis assay
An Annexin V:PE apoptosis detection kit (Beyotime
Institute of Biotechnology) was used to measure the number of
apoptotic cells subsequent to the cells being treated with
phloretin at different concentrations of 60, 70 and 80 µg/ml for 12
h. The cells were trypsinized and washed twice with cold PBS, and
re-suspended in 1% bovine serum albumin solution with 5 µl Annexin
V:PE and 5 µl l 7-AAD at a concentration of 1×105
ml/cells in a total volume of 100 µl. The cells were gently mixed,
and incubated in the dark for 15 min at room temperature. A quota
of 1 µl for Annexin V:PE apoptosis detection kit (#C1062; Beyotime
Institute of Biotechnology) was subsequently added to each test
tube and the number of apoptotic cells was quantified by flow
cytometry using the BD LSR II Analyzer (BD Biosciences, Franklin
Lakes, NJ, USA) within 1 h.
Western blot analysis
The expression levels of the cellular proteins were
determined using western blotting assays. The EC-109 cells were
treated with 60 µg/ml phloretin for 6, 12 and 24 h. The EC-109
cells were washed with PBS at 4°C subsequent to treatment with
phloretin, and lysed with 200 µl radioimmunoprecipitation assay
buffer (50 mmol 4-(2-hydroxyethyl)-1-piperazineethanesulphoric acid
at pH 7.5, 150 mmol NaCl, 10% glycerol, 1.5 mmol MgCl2, 1% Triton-X
100, 1 mmol EDTA at pH 8.0, 10 mmol sodium pyrophosphate and 10
mmol sodium fluoride) containing a mixture of protease inhibitors
(phenylmethanesulfonyl fluoride; 1:100) and a mixture of
phosphatase inhibitors (PhosSTOP; 1:40; all, Beyotime Institute of
Biotechnology). The present study used the Pierce BCA protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) to measure protein
concentrations. Equal amounts of protein samples were
electrophoresed on 8–12% SDS-PAGE mini-gel subsequent to thermal
denaturation for 10 min at 100°C. The proteins were then
transferred onto a polyvinylidene fluoride membrane at 200 mA for 2
h at 4°C. The membranes were probed with the previously indicated
antibodies with the previously indicated concentrations overnight
at 4°C, then blotted with a horseradish peroxidase-conjugated cow
anti-rabbit secondary antibody (ProteinTech Group, Inc.).
Visualization of the membranes was performed using the Bio-Rad
ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with ECL (Beyotime Institute of Biotechnology). The western blot
was repeated three times, and the blots were quantitatively
analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All the presented data were confirmed in a minimum
of 3 independent experiments, and are expressed as the mean ±
standard deviation. Statistical comparisons were made by unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Phloretin causes dose-dependent and
time-dependent growth inhibition in EC-109 cells
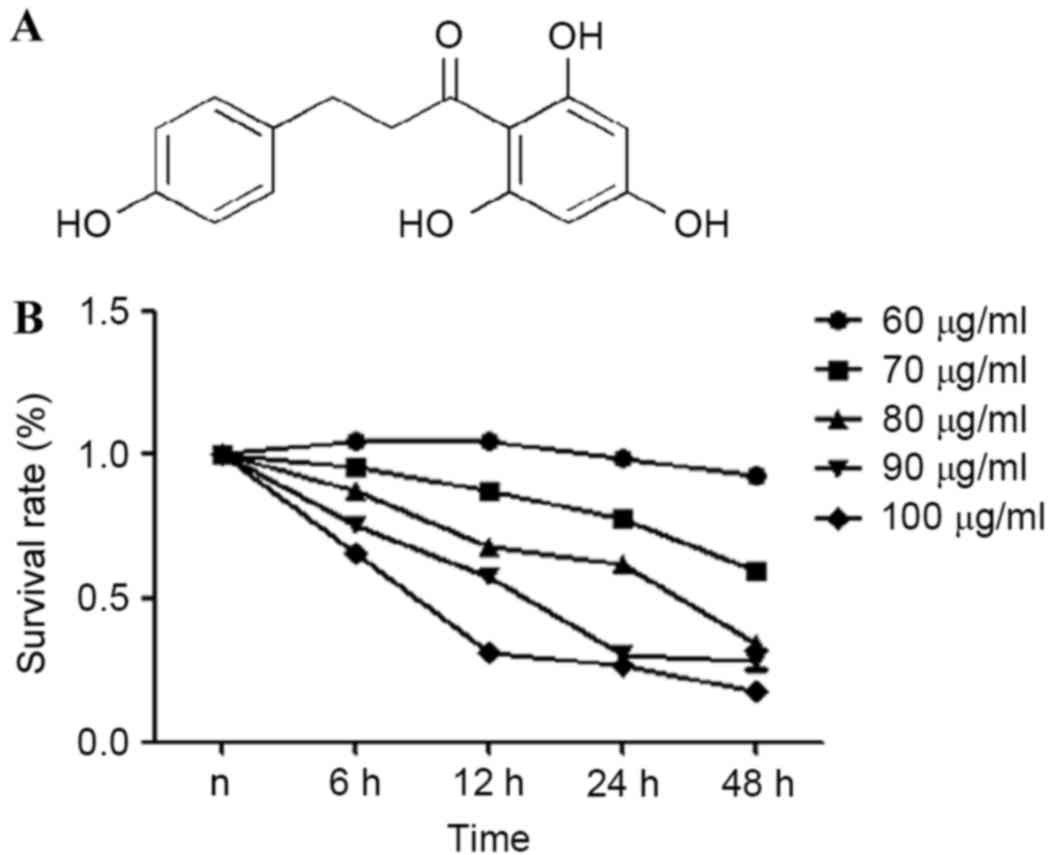
Chemical structure of phloretin is presented in
Fig. 1A. An MTT assay was used to
estimate the effects of phloretin on the viability of the EC-109
cells. From the results obtained, it was demonstrated that the
survival rate of the EC-109 cells treated with 60–100 µg/ml
phloretin for 6, 12, 24 and 48 h significantly reduced in a dose-
and time-dependent manner, as illustrated in Fig. 1B.
Phloretin induces apoptosis in EC-109
cells
Subsequent to the confirmation that the treatment of
human esophageal cancer with phloretin resulted in a reduction in
cell viability, the present study then investigated whether the
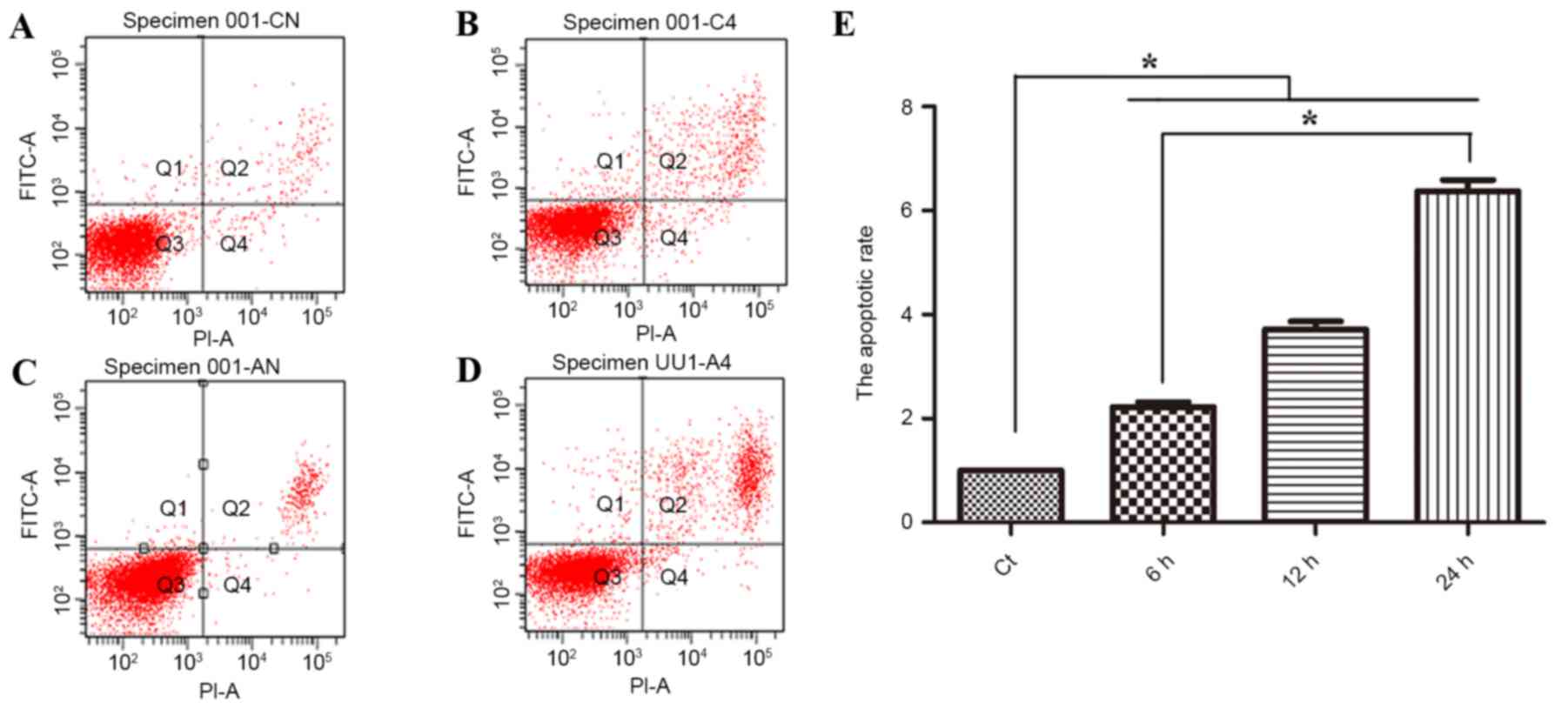
effect of phloretin is associated with apoptosis. As revealed in
Fig. 2A, flow cytometric analysis
suggested that subsequent to treatment with 60, 70 and 80 µg/ml
phloretin for 12 h, the apoptotic index of the EC-109 cells
increased to 225.6±16.0, 375±24.7 and 634±44.6%, respectively
(*P<0.05, compared with the standard control group).
Phloretin induces changes in the
expression levels of the Bcl protein family
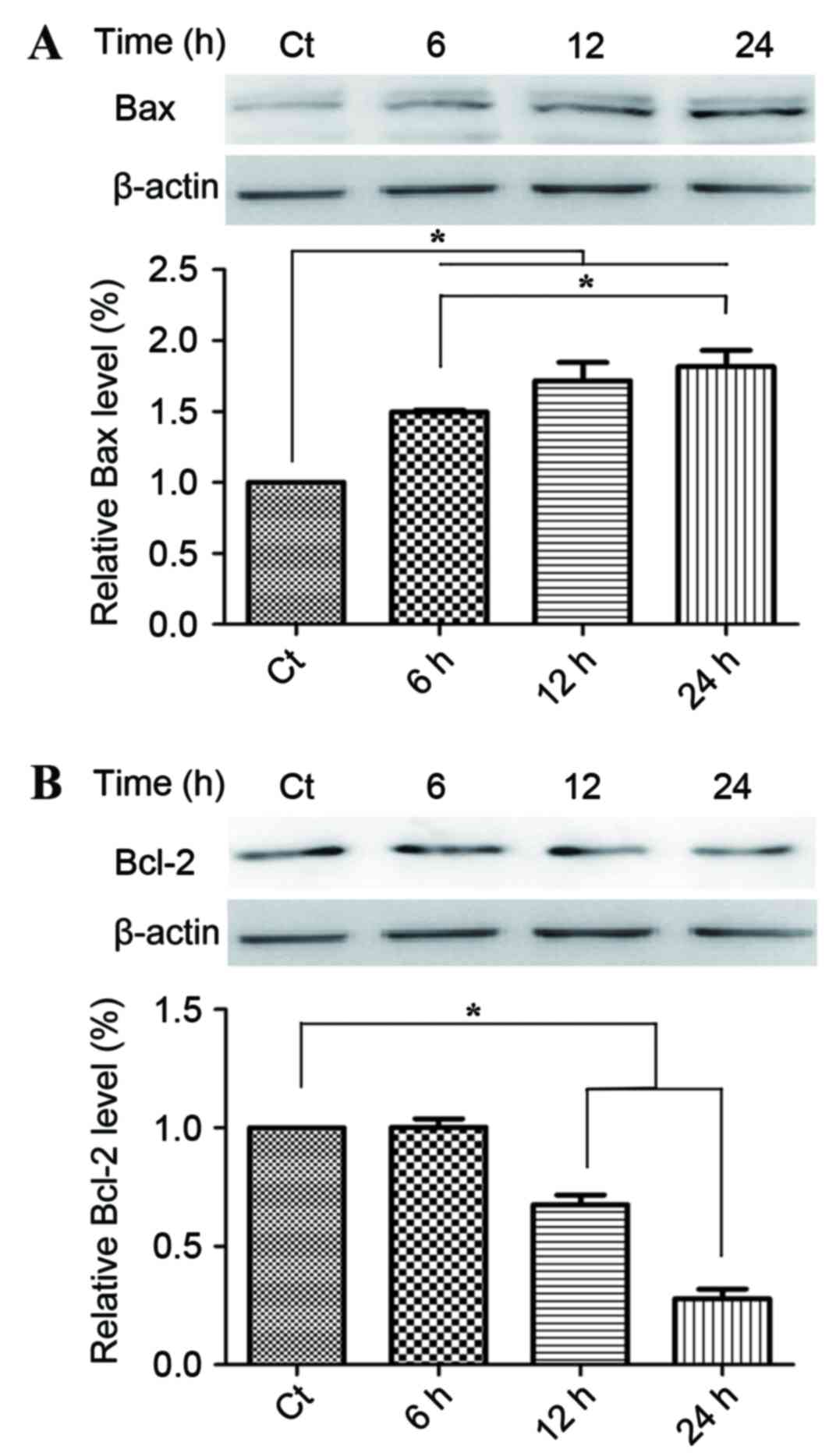
Following on from the observation of the regulatory
effect of phloretin on the apoptosis in the EC-109 cells, the
present study then examined the effect of phloterin on the
expression of the key apoptotic regulators Bax and Bcl-2. Fig. 3A provides statistical calculations of
the Bcl protein family expression ratios, demonstrating an increase
in the level of Bax, whilst Fig. 3B
demonstrates a decline in Bcl-2 levels that consequently led to the
increase of the relative rate of Bax/Bcl-2 which evidently occurred
in a time-dependent manner.
Phloretin causes an increase in the
expression levels of p53
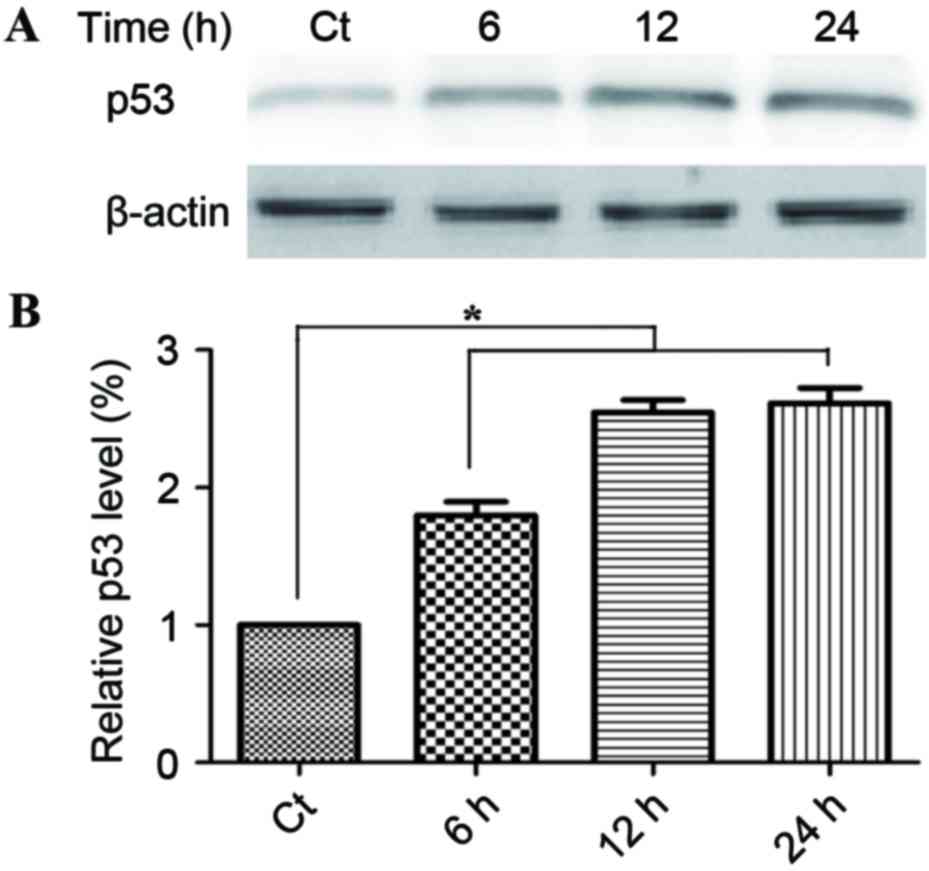
The induction of apoptosis is a key aspect of the
tumor-suppressive activity of p53. Fig.
4A demonstrates that treatment with 60 µg/ml phloretin for 6,
12, and 24 h induced a time-dependent increase in p53 activity,
with increases of 178.1±17.6, 249.5±18.7 and 261.6±17.6%
respectively in EC-109 cells (*P<0.05, compared with the
standard control group).
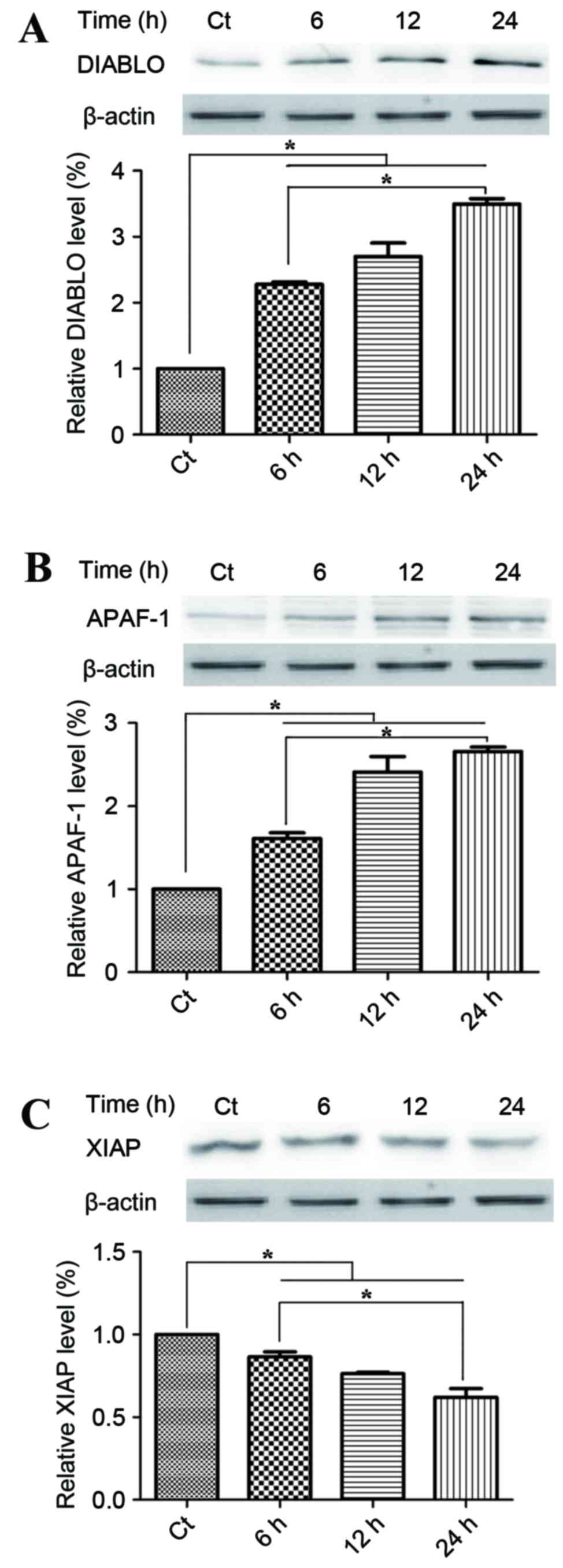
Mitochondrial apoptotic pathways are
involved in the anticancer mechanism of phloretin
To investigate the potential underlying mechanism of
the proapoptotic activities of phloretin on EC-109 cells, the
present study detected changes in the proteins within the
mitochondrial apoptotic pathways. Smac/DIABLO and APAF-1, which
were located the mitochondrial intermembrane space, were
pro-apoptotic with respect to phloretin. Fig. 5A demonstrates that treatment with 60
µg/ml phloretin for 6, 12 and 24 h induced a time-dependent
increase in DIABLO activity, with increases of 225.1±5.6,
280.5±15.7 and 351.6±17.6%, respectively in EC-109 cells,
(P<0.05, compared with the standard control group). Fig. 5B demonstrates that levels of APAF-1
also increased significantly to 160±9.9, 245.5±15.2 and
260.4±10.4%, respectively in the EC-109 cells, subsequent to the
different melatonin treatments (P<0.05, compared with the
standard control group). XIAP is a member of the IAP family of
proteins and inhibits apoptotic cell death. Fig. 5C demonstrates that subsequent to
treatment with 60 µg/ml phloretin for 6, 12 and 24 h, a
time-dependent decrease in the levels of XIAP was observed in the
EC-109 cells, with intracellular levels of XIAP measuring 84.3±4.4,
75±7.0 and 57±6.4%, respectively (P<0.05, compared with the
standard control group).
Discussion
In 1993, Nelson and Falk (13) reported that phloretin restricts tumor
cell growth by inhibiting glucose transmembrane transport.
Furthermore, there is support for the hypothesis that phloretin
serves a pivotal role in numerous anticancer mechanisms. Using an
MTT assay and flow cytometric analysis, the present study
demonstrated that phloretin exhibits antitumor behaviors, including
the inhibition of proliferation and the induction of apoptosis.
Apoptosis, programmed cell death, is required to maintain
homeostasis. Due to decreased rates of apoptosis, tumor cells can
survive indefinitely. Several studies have revealed that anticancer
drugs induce apoptosis, and thus inhibit the proliferation of the
tumor cells (14,15). At present, there are numerous drugs,
including drugs targeting the caspase family of proteins, which can
regulate the apoptotic pathway (16).
The Bcl protein family serves an important role in
apoptosis. This family, which is termed due to their different
functions and structural homology, may be divided into proapoptotic
and antiapoptotic proteins. The antiapoptotic proteins include
Bcl-2, Bcl-extra large (Bcl-xL), Bcl-2-like protein 2 (Bcl-w) and
myeloid leukemia cell differentiation protein (Mcl-1). Equally,
proapoptotic proteins include Bax and Bcl-2 homologous antagonist
killer (Bak), which can provoke mitochondrial damage and promote
cell apoptosis (17). In the present
study, western blot analysis revealed that when phloretin
stimulated the EC-109 cells, the expression of Bax protein
increased, which was accompanied by a decline in the expression of
Bcl-2. Under normal circumstances, levels of the pro-apoptosis
protein Bax and the anti-apoptosis protein Bcl-2 will maintain the
balance of apoptosis. The survival rates of tumor cells depend on
the level of Bcl-2 protein present. The expression of Bcl-2 in
tumor cells is increased in comparison with the expression of Bcl-2
expression within normal cells. Bcl-2 protects cells from
apoptosis, in part due to their ability to bind the Bcl-2 homology
(BH) 3-exposed conformers of Bax and Bak, thereby inhibiting full
activation of the aforementioned proteins. When the level of Bcl-2
decreases and Bcl-2 cannot bind to Bax, cells will trigger
apoptosis. The proapoptotic proteins Bax and Bak serve an important
role in the induction of caspase activation (18). Activated Bax inserts into the
mitochondrial membrane and increases membrane permeability, leading
to an activation of the mitochondrial apoptotic pathway.
p53, as a transcription factor, serves as a tumor
suppresser protein and initiates cell death via the mitochondrial
apoptotic pathway (19). A previous
study revealed that p53 interacts with various members of the Bcl
protein family (20). In the cytosol,
p53 forms an inhibitory complex with Bcl-2 to induce cell death,
whilst the proapoptotic protein Bax may be directly activated to
induce cell death. Activated Bax may form homooligomers, which
participate in forming pores and in the control of the
permeabilization of the outer mitochondrial membrane, leading to
the release of the mitochondrial intermembrane space into the
cytosol, including cytochrome c, APAF-1 and Smac/DIABLO
proteins (21). It has been revealed
that when EC-109 cells are treated with phloretin, levels of
Smac/DIABLO and APAF-1 increase.
APAF-1, which is assembled into a ring-like
platform, is the central component of the apoptosome. In the
presence of dATP/ATP, the cytochrome c released from
mitochondria interacts with the APAF-1 proteins, initiating the
formation of the apoptosome. The apoptosome subsequently begins to
recruit pro-caspase-9, and activates the pro-caspases in the
intrinsic cell-death pathway, in order to initiate apoptosis via
nucleus condensation and/or the degradation of other intracellular
structures (22).
Smac/DIABLO is released from the mitochondria, and
along with cytochrome c, is another important proapoptotic
factor. In contrast, XIAP is a member of the IAP family of
proteins, and inhibits apoptotic cell death. Smac/DIABLO
neutralizes the inhibitory activity of XIAP to promote cytochrome
c-APAF-1-dependent caspase activation (9). From western blot analyses there is a
growing body of evidence indicating that phloretin increases the
levels of the proteins p53, APAF-1 and DIABLO, and reduce the
levels of XIAP protein to induce apoptosis. Previous studies
combined with the results of the present study, have revealed that
phloretin increases the levels of apoptosis of EC-109 cells via the
mitochondria-apoptotic pathway (23–25).
In conclusion, the present study has reported that
phloretin inhibit the proliferation of human esophageal cancer
EC-109 cells, and indicated that phloretin exhibits anticancer
behavior through the activation of the mitochondrial-apoptosis
pathway. In summary, phloretin may be considered to be a promising
chemopreventive agent for the treatment of esophageal cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81200671 and
81172221).
References
|
1
|
Dai T and Shah MA: Chemoradiation in
oesophageal cancer. Best Pract Res Clin Gastroenterol. 29:193–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raymond DP, Seder CW, Wright CD, Magee MJ,
Kosinski AS, Cassivi SD, Grogan EL, Blackmon SH, Allen MS, Park BJ,
et al: Predictors of major morbidity or mortality after resection
for esophageal cancer: A society of thoracic surgeons general
thoracic surgery database risk adjustment model. Ann Thorac Surg.
102:207–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XD, Qin HY, Zhang JE, Zheng MC, Xin MZ,
Liu L, Wu XJ, Jiang CN and Zhang MF: The prevalence and correlates
of symptom distress and quality of life in Chinese oesophageal
cancer patients undergoing chemotherapy after radical
oesophagectomy. Eur J Oncol Nurs. 19:502–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fletcher JI, Meusburger S, Hawkins CJ,
Riglar DT, Lee EF, Fairlie WD, Huang DC and Adams JM: Apoptosis is
triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc
Natl Acad Sci USA. 105:pp. 18081–18087. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
JI, Dumont P, Hafey M, Murphy ME and
George DL: Mitochondrial p53 activates Bak and causes disruption of
a Bak-Mcl1 complex. Nat Cell Biol. 6:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pop C, Timmer J, Sperandio S and Salvesen
GS: The apoptosome activates caspase-9 by dimerization. Mol Cell.
22:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang K and Lin B: Inhibitor of apoptosis
proteins (IAPs) as regulatory factors of hepatic apoptosis. Cell
Signal. 25:1970–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH,
Chen JH, Wu CH and Ho YS: Apple polyphenol phloretin potentiates
the anticancer actions of paclitaxel through induction of apoptosis
in human hep G2 cells. Mol Carcinog. 48:420–431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MS, Kwon JY, Kang NJ, Lee KW and Lee
HJ: Phloretin induces apoptosis in H-Ras MCF10A human breast tumor
cells through the activation of p53 via JNK and p38
mitogen-activated protein kinase signaling. Ann N Y Acad Sci.
1171:479–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Kim EJ, Shin HK, Kwon DY, Kim MS,
Surh YJ and Park JH: Induction of apoptosis in HT-29 colon cancer
cells by phloretin. J Med Food. 10:581–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelson JA and Falk RE: Phloridzin and
phloretin inhibition of 2-deoxy-D-glucose uptake by tumor cells in
vitro and in vivo. Anticancer Res. 13:2293–2299. 1993.PubMed/NCBI
|
|
14
|
Haobo L, Guangfeng Z and Xiao Z: OP0216
Resveratrol ameliorates pulmonary fibrosis and inhibits human lung
fibroblasts activation VIA modulating SIRT1 and GLI1 signaling. Ann
Rheum Dis. 74:152–153. 2015. View Article : Google Scholar
|
|
15
|
Yang YC, Lii CK, Lin AH, Yeh YW, Yao HT,
Li CC, Liu KL and Chen HW: Induction of glutathione synthesis and
heme oxygenase 1 by the flavonoids butein and phloretin is mediated
through the ERK/Nrf2 pathway and protects against oxidative stress.
Free Radic Biol Med. 51:2073–2081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan FK and Lenardo MJ: A crucial role for
p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T
lymphocytes. Eur J Immunol. 30:652–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petros AM, Olejniczak ET and Fesik SW:
Structural biology of the Bcl-2 family of proteins. Biochim Biophys
Acta. 1644:83–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei MC, Lindsten T, Mootha VK, Weiler S,
Gross A, Ashiya M, Thompson CB and Korsmeyer SJ: TBID, a
membrane-targeted death ligand, oligomerizes BAK to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
19
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robbins D, Gu X, Shi R, Liu J, Wang F,
Ponville J, Mccord JM and Zhao Y: The chemopreventive effects of
protandim: Modulation of p53 mitochondrial translocation and
apoptosis during skin carcinogenesis. PLoS One. 5:e119022010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imao T and Nagata S: Apaf-1- and
caspase-8-independent apoptosis. Cell Death Differ. 20:343–352.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao X, Bai N, He K, Ho CT, Yang CS and
Sang S: Apple polyphenols, phloretin and phloridzin: New trapping
agents of reactive dicarbonyl species. Chem Res Toxicol.
21:2042–2050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min J, Huang K, Tang H, Ding X, Qi C, Qin
X and Xu Z: Phloretin induces apoptosis of non-small cell lung
carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol Rep.
34:2871–2879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang WC, Wu SJ, Tu RS, Lai YR and Liou
CJ: Phloretin inhibits interleukin-1β-induced COX-2 and ICAM-1
expression through inhibition of MAPK, Akt and NF-κB signaling in
human lung epithelial cells. Food Funct. 6:1960–1967. 2015.
View Article : Google Scholar : PubMed/NCBI
|