Introduction
Lung cancer is one of the most common cancers
globally. An estimated 1.8 million new lung cancer cases occurred
in 2012, accounting for ~13% of all cancer diagnoses. Lung cancer
was the most frequently diagnosed cancer and the leading cause of
cancer death for males and females in that same year (1). In less developed countries in Eastern
Asia, including China, the incidence and mortality of lung cancer
were higher than other cancer types in 2012 (2). The lung cancer burden has significantly
increased in Hebei province, China, which is severely polluted,
from 1973–1975 to 2010–2011, with a rate of increase of 189.15%
(3). Other studies have proposed that
outdoor pollution, which includes increasing concentrations of
SO2 emissions, nitrogen oxide emissions or particulate
matter 2.5, as well as an increase in smoking prevalence, has
contributed to the increasing rate of lung cancer incidence
(4,5).
According to a survey by the European Union, lung cancer had the
highest economic cost (as high as €18.8 billion, or 15% of overall
cancer costs) amongst all cancers in 2009 (6).
Although advances have been made in
multidisciplinary treatments for lung cancer, this cancer continues
to have a poor prognosis due to a general lack of symptoms until it
reaches an advanced stage (7). Due to
discrepancies between results within the same stage, there is an
urgent demand for novel parameters, particularly serum predictive
biomarkers, to combine with low-dose computed tomography, x-ray
examination and other diagnostic tests to complement
tumor-node-metastasis (TNM) staging (8) to accurately predict the prognosis and
provide appropriate preoperative patient counseling (9). White blood cell (WBC) counts and WBC
subtype counts are well established in systemic inflammatory or
infection responses. Wculek and Malanchi hypothesized that an
altered presence of leukocytes within distant tissues of
tumor-bearing hosts may affect specific subsets of disseminating
cancer cells (10). Notably,
leukocytes were demonstrated to accumulate in the lung prior to
infiltration of the tissue by cancer cells (in a pre-metastatic
lung), and their numbers increased during metastatic progression
(in a metastatic lung) (10).
Increasingly, evidence has demonstrated that a systemic
inflammatory response has a prognostic value in various cancers
(11–14). Amongst the convenient and available
markers, a high ratio of neutrophils to lymphocytes (NLR) and
platelets to lymphocytes (PLR), which are calculated from the
complete blood count with differential (15), also have been combined to form
inflammation-based prognostic scores to predict cancer survival
(11). As they are routine laboratory
tests, the NLR and PLR are easy to obtain and may serve an
important function in predicting the survival and monitoring of
cancer progression in patients with lung cancer. Therefore, the
present study aimed to explore the prognostic values of NLR and PLR
in patients with lung cancer.
Materials and methods
Study population
A retrospective analysis was performed on 695
patients diagnosed with lung cancer from high incidence areas in
Hebei Province and treated at the Fourth Hospital of Hebei Medical
University (Shijazhuang, China; also known as the Tumor Hospital of
Hebei Province, a large and comprehensive hospital with the rank of
level three and grade A) from January 1, 2000 to December 31, 2005.
All the patients included were histologically confirmed to have
lung cancer and received a routine laboratory examination at
diagnosis. Patients with pulmonary hypertension and other severe
diseases were excluded.
Clinical data collection
Baseline characteristics were collected, including
age, sex, occupation, family history, tumor data (pathological
type, TNM stage and surgical situation), and laboratory data
(hemoglobin levels, neutrophil count, lymphocyte count, and
platelet count on the first hospitalized day). The NLR was defined
as a simple ratio between the absolute neutrophil count and the
absolute lymphocyte count; similarly, PLR was defined as the ratio
of the absolute platelet count and the absolute lymphocyte
count.
Follow-up studies
The follow-up evaluations were conducted according
to the standard follow-up system of the hospital, i.e., every 6
months after patients were discharged from the hospital. The
deadline for follow-up evaluations was December 31, 2012. The
survival period was measured from the date of admission to the date
of mortality or the date of the follow-up deadline.
Statistical analysis
SPSS 21.0 (IBM Corp., Armonk, NY, USA) and Microsoft
Office Excel 2007 (Microsoft Corporation, Redmond, WA, USA) were
used for statistical analysis. The optimal cutoff values for the
NLR and the PLR were determined from the receiver operating curve
(ROC) analysis. The comparison between high and low NLR and PLR
groups used χ2 tests or the Fisher's exact probability
method to explore the associations between the NLR and PLR and
other clinicopathological variables. The Kaplan-Meier method for
survival analysis was performed. The overall survival (OS) in
different groups was identified using the log-rank test. P<0.05
was considered to indicate a statistically significant difference.
Univariate and multivariate Cox regression models were performed to
analyze the factors that may influence the OS rate.
Results
A total of 695 patients with lung cancer were
included, of which 56 patients were still alive and 639 patients
had succumbed, by the end of the follow-up. The follow-up rate was
98.44%. Overall, 510 (73.38%) cases were male, and 185 (26.62%)
were female with an average age of 61.69±11.07 years (ranging
between 20–89). In total, 232 (33.38%) patients underwent
surgery.
The median absolute neutrophil count was
6.56×106/ml (ranging between 0.01–87.00), the median
absolute platelet count was 276.68×106/ml (ranging
between 6.00–716.00), and the median value of lymphocyte count was
1.89×106/ml (ranging between 0.11–31.60). Then, ROC
curves for OS were used to define the optimal cutoff of NLR and
PLR; the results were 6.0 and 248, respectively. The sensitivity
and specificity were 69.1 and 43.4%, and 55.7 and 49.9%,
respectively.
The associations between the NLR and PLR and the
baseline clinical characteristics of lung cancer patients were
analyzed (Tables I and II). The clinical variables included age,
sex, pathological type, TNM stage, surgery, metastasis, occupation,
and family history. 501 patients, accounting for 72.09% of all
patients, were placed into the low NLR group (NLR<6.0), and 194
patients, accounting for 27.91% of all patients, were placed in the
high NLR group. 549 patients, accounting for 78.99% of all
patients, were placed into the low PLR group (PLR<248.0), and
146 patients, accounting for 21.01% of all patients, were placed
into in the high PLR group. The results revealed that there was no
association between age, sex, pathological type, occupation, or
family history incidence and NLR in different groups, but there was
a statistically significant association between the TNM stage,
surgery, metastasis incidence and NLR (P<0.001). No associations
between age, sex, pathological type, occupation, family history, or
metastasis incidence and PLR was identified, but the association
between PLR and both the TNM stage and surgery incidence was
statistically significant (P<0.001).
 | Table I.Associations between NLR and patient
characteristics. |
Table I.
Associations between NLR and patient
characteristics.
| Factor | Low NLR
(n=501) | High NLR
(n=194) | χ2 | P-value |
|---|
| Age, years | 61.69±11.07 |
|
|
|
| Sex |
|
| 0.744 | 0.388 |
|
Male | 373 | 137 |
|
|
|
Female | 127 | 58 |
|
|
| Age, years |
|
| 3.312 | 0.069 |
|
<40 | 26 |
4 |
|
|
|
≥40 | 475 | 190 |
|
|
| Pathological
type |
|
| 2.290 | 0.130 |
| Small
cell cancer | 46 | 12 |
|
|
|
Non-small cell cancer | 455 | 182 |
|
|
| TNM stage |
|
| 20.041 | 0.000 |
| I | 83 |
9 |
|
|
| II | 158 | 70 |
|
|
|
III | 176 | 68 |
|
|
| IV | 84 | 47 |
|
|
| Surgery |
|
| 41.118 | 0.000 |
|
Yes | 203 | 29 |
|
|
| No | 298 | 165 |
|
|
| Metastasis |
|
| 172.154 | 0.000 |
|
Yes | 220 | 191 |
|
|
| No | 281 |
3 |
|
|
| Occupation |
|
| 0.061 | 0.804 |
|
Labourer | 204 | 77 |
|
|
|
Others | 297 | 117 |
|
|
| Family history |
|
| 0.688 | 0.407 |
|
Yes | 37 | 18 |
|
|
| No | 464 | 176 |
|
|
 | Table II.Associations between PLR and patient
characteristics. |
Table II.
Associations between PLR and patient
characteristics.
| Factor | Low PLR
(n=549) | High PLR
(n=146) | χ2 | P-value |
|---|
| Age, years | 61.69±11.07 |
|
|
|
| Sex |
|
| 2.261 | 0.133 |
|
Male | 410 | 100 |
|
|
|
Female | 139 | 46 |
|
|
| Age, years |
|
| 0.019 | 0.890 |
|
<40 | 24 |
6 |
|
|
|
≥40 | 525 | 140 |
|
|
| Pathological
type |
|
| 1.985 | 0.159 |
| Small
cell cancer | 50 |
8 |
|
|
|
Non-small cell cancer | 499 | 138 |
|
|
| TNM stage |
|
| 51.371 | 0.000 |
| I | 84 |
7 |
|
|
| II | 151 | 77 |
|
|
|
III | 219 | 26 |
|
|
| IV | 95 | 36 |
|
|
| Surgery |
|
| 21.969 | 0.000 |
|
Yes | 207 | 25 |
|
|
| No | 342 | 121 |
|
|
| Metastasis |
|
| 2.106 | 0.147 |
|
Yes | 317 | 94 |
|
|
| No | 232 | 52 |
|
|
| Occupation |
|
| 0.600 | 0.439 |
|
Labourer | 223 | 58 |
|
|
|
Others | 326 | 88 |
|
|
| Family history |
|
| 1.413 | 0.235 |
|
Yes | 40 | 15 |
|
|
| No | 509 | 131 |
|
|
The association between NLR, PLR and the OS rate of
lung cancer patients is presented in Table III. The pathological type, TNM
stage, surgery, metastasis, occupational status and NLR were
demonstrated to affect the OS rate. The 1-, 3-, and 5-year OS rates
of lung cancer patients in the low NLR group were 54.7, 28.9 and
20.9%, respectively, while those in the high NLR group were 43.6,
24.6 and 17.5%, respectively. Notably, patients in the low NLR
group had a significantly longer OS than patients in the high NLR
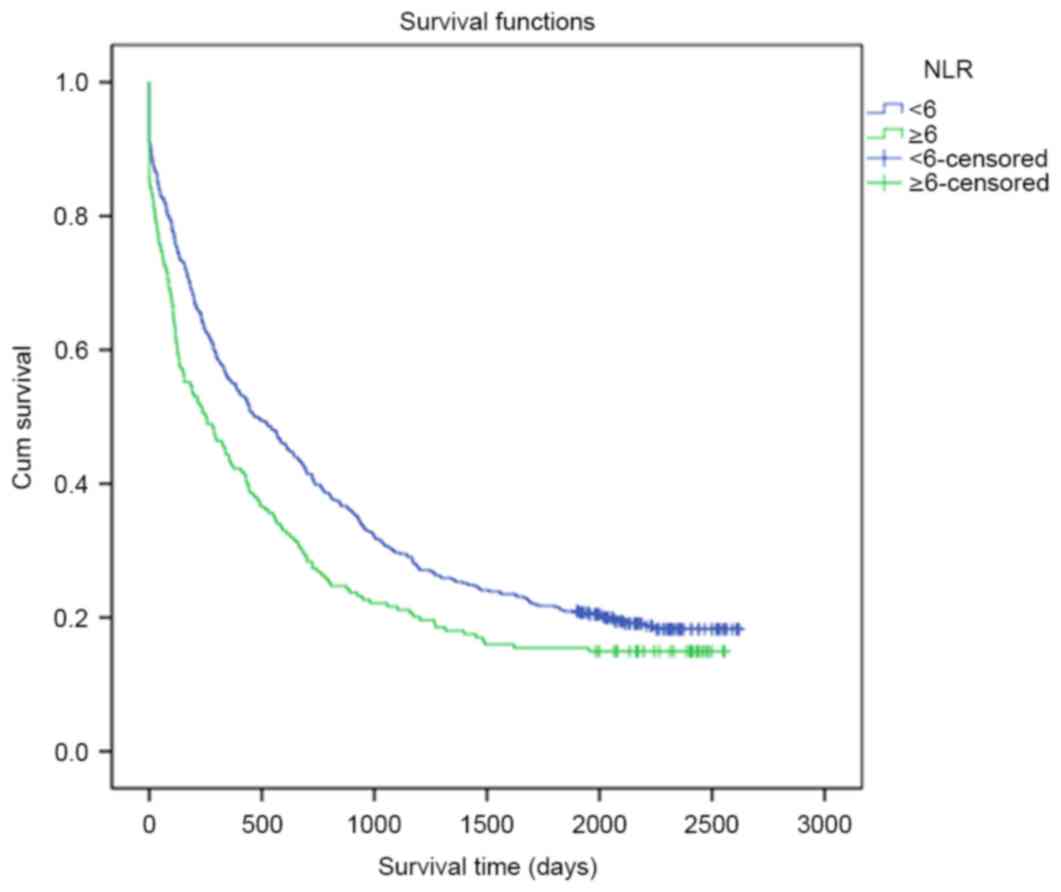
group (819.57 days vs. 629.86 days, P=0.041; Fig. 1). However, there was no significant
difference between patients in the low-PLR or high-PLR groups in
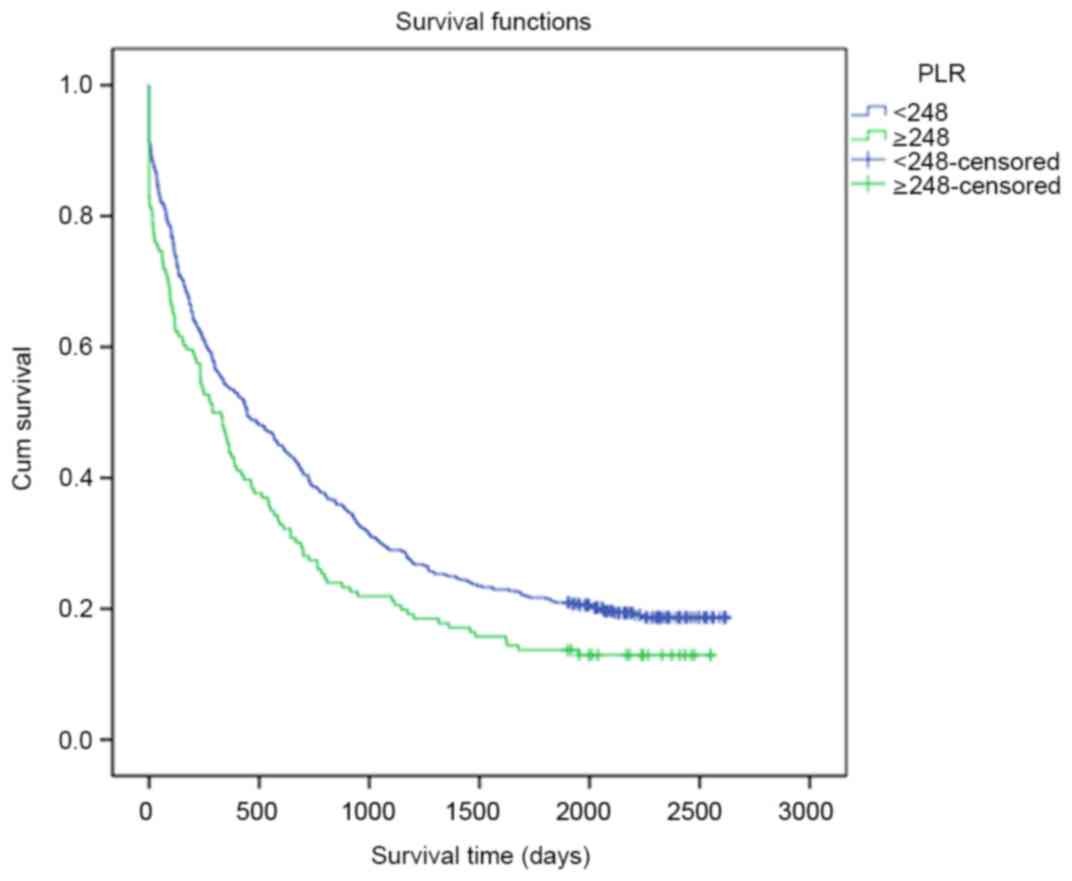
terms of OS rates during the different follow-up periods (P=0.217;
Fig. 2). Furthermore, the potential
prognostic factors for OS rates were explored by conducting
univariate Cox regression analysis (Table IV).
 | Table III.Prognostic factors according to the
univariate analysis. |
Table III.
Prognostic factors according to the
univariate analysis.
|
|
| OS Rate (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Factor | N | 1 year | 3 year | 5 year | χ2 | P-value |
|---|
| Sex |
|
|
|
| 0.234 | 0.628 |
|
Male | 510 | 84.6 | 28.7 | 19.7 |
|
|
|
Female | 185 | 78.9 | 25.4 | 21.1 |
|
|
| Age, years |
|
|
|
| 1.735 | 0.188 |
|
<40 | 30 | 55.7 | 40.0 | 33.3 |
|
|
|
≥40 | 665 | 51.9 | 27.3 | 19.6 |
|
|
| Pathological
type |
|
|
|
| 8.494 | 0.004 |
| Small
cell cancer | 58 | 71.1 | 36.3 | 33.6 |
|
|
|
Non-small cell cancer | 637 | 50.0 | 27.1 | 18.9 |
|
|
| TNM stage |
|
|
|
| 69.095 | 0.000 |
| I | 92 | 83.7 | 66.3 | 52.2 |
|
|
| II | 228 | 50.2 | 24.9 | 19.1 |
|
|
|
III | 245 | 50.8 | 22.2 | 14.0 |
|
|
| IV | 130 | 36.2 | 16.7 | 9.1 |
|
|
| Surgery |
|
|
|
| 65.848 | 0.000 |
|
Yes | 232 | 70.3 | 46.0 | 35.6 |
|
|
| No | 463 | 43.0 | 18.5 | 12.1 |
|
|
| Metastasis |
|
|
|
| 60.599 | 0.000 |
|
Yes | 411 | 43.7 | 17.6 | 10.4 |
|
|
| No | 284 | 63.4 | 42.4 | 33.8 |
|
|
| Occupation |
|
|
|
| 6.099 | 0.014 |
|
Labourer | 281 | 57.7 | 31.3 | 23.5 |
|
|
|
Others | 414 | 48.2 | 25.5 | 17.8 |
|
|
| Family history |
|
|
|
| 0.002 | 0.968 |
|
Yes | 55 | 55.2 | 24.7 | 16.6 |
|
|
| No | 640 | 51.9 | 28.2 | 20.3 |
|
|
| NLR |
|
|
|
| 4.159 | 0.041 |
|
Low | 501 | 54.7 | 28.9 | 20.9 |
|
|
|
High | 194 | 43.6 | 24.6 | 17.5 |
|
|
| PLR |
|
|
|
| 1.523 | 0.217 |
|
Low | 549 | 52.9 | 28.5 | 21.0 |
|
|
|
High | 146 | 47.4 | 25.6 | 16.7 |
|
|
 | Table IV.Prognostic factors according to
multivariate analysis. |
Table IV.
Prognostic factors according to
multivariate analysis.
| Factor | β | SE | Wald | RR (95% CI) | P-value |
|---|
| Pathological
type | 0.476 | 0.166 | 8.241 | 1.069
(1.162–2.227) | 0.004 |
| Surgery | −0.730 | 0.093 | 61.273 | 0.482
(0.401–0.579) | 0.000 |
| Metastasis | 0.673 | 0.089 | 57.536 | 1.960
(1.647–2.332) | 0.000 |
| TNM Stage I |
|
|
| 1.00 |
|
| TNM Stage II | 0.384 | 0.176 | 4.790 | 1.469
(1.041–2.072) | 0.029 |
| TNM Stage III | 0.269 | 0.102 | 6.800 | 1.310
(1.071–1.603) | 0.009 |
| TNM Stage IV | 0.509 | 0.104 | 24.212 | 1.661
(1.355–2.038) | 0.000 |
| NLR | 0.187 | 0.093 | 4.071 | 1.205
(1.005–1.445) | 0.044 |
| PLR | 0.122 | 0.100 | 1.494 | 1.130
(0.929–1.373) | 0.222 |
Discussion
Lung cancer is one of the leading causes of
mortality worldwide in the 21st century (16), with an equally high incidence between
men and women, and between developed countries and less developed
countries (17). The dramatic
increase in the incidence of lung cancer over the last 40 years
(3) established the rationale for
developing biomarkers for the prognosis of lung cancer. This stems
from the fact that, at the population level, the earlier a disease
is predicted, the better the outcome and the lower the health care
cost (18). Multiple studies have
revealed that environmental and occupational causes, including
genetic and sex-specific factors, nutrition, obesity, tobacco
smoking, air pollution and, potentially, human papilloma virus
infection, are involved in increasing the lung cancer burden
(19,20). The International Agency for Research
on Cancer (IARC) has classified air pollution and particulate
matter as carcinogenic to humans (21,22).
Although there have been significant advances in the diagnosis and
comprehensive treatment of lung cancer, the majority of patients
are diagnosed in the late stages of the disease, and the 5-year
survival rate and quality of life of these patients remains low
(23).
Multiple methods to diagnose and predict the
survival of lung cancer have emerged, including pathological
confirmation and tumor biomarkers, which include specific RNA, DNA,
or the mutation/methylation of nucleic acids of cells and tissues
(24–27). Another biomarker, circulating tumor
DNA, offers an alternative, temporal and less-invasive option
(termed a ‘liquid biopsy’ due to the biopsy sample being plasma)
for accurate early detection with high sensitivity for patients
with lung cancer (28). However, the
above approaches also have disadvantages. It is always difficult to
obtain tumor samples; and, in certain tumors, the tissues are only
accessible through fine-needle aspirates, particularly in early
stage cases, and the acquisition of samples from different medical
centers is challenging or time-consuming particularly as they take
a long time to recover. However, NLR and PLR have now been
evaluated for use as prognostic factors, and are simple,
convenient, damage-free and supplementary to the plethora of
biomarkers and other ways with which to predict the survival of
patients with tumors.
Inflammation is a physiological process that is a
crucial function of the innate immune system, as it is a response
to acute or chronic tissue damage, whether resulting from physical
injury, ischemic injury, infection, exposure to toxins, or other
types of trauma. When these situations occur within the body, the
immune system will cause neutrophils, leukocytes, lymphocytes and
other inflammatory cells to be activated and then be attracted to
the site of damage (29). Currently.
there is increasing recognition of the involvement of immune cells
and their supporting pro-inflammatory mediators, typically
associated with the containment of an inflammatory process, in the
pathobiology of multiple types of cancer, including
gastrointestinal, liver and breast cancer (30–32). They
may be involved in tumor suppression by stimulating an antitumor
immune response, but more often, and under certain conditions, this
response appears to stimulate tumor development (29). Bollrath et al (33) discovered that the tumor
microenvironment is comprised not only of tumor cells but also of
stromal cells (including fibroblasts and endothelial cells), cells
from the innate immune system (including macrophages, neutrophils,
mast cells, myeloid-derived suppressor cells, dendritic cells and
natural killer cells) and the adaptive immune cells, T and B
lymphocytes.
Previous studies have begun to unravel the
mechanisms linking the host inflammatory response to tumor growth,
invasion and metastasis in cancers. Based on this relationship
between inflammation and cancer progression, several
inflammation-based scores have been demonstrated to have prognostic
value in numerous types of malignant solid tumor (34), including lung (35) and liver cancer (36). Accumulating evidence has indicated
that long term inflammation-associated factors activate the
occurrence and metastasis of certain types of cancer, as systemic
inflammatory responses and blood NLR, and local inflammatory
responses (including the infiltration of various immune cells and
their subsets in tumors) are associated with the prognosis of
cancer (37). Certain studies have
revealed that the human lung cancer stroma is composed of immune
and inflammatory cells (38,39). These studies demonstrated that, in
human lung cancer sections, there was a significant
elevation/infiltration of total-T lymphocytes (CD3+,
8+, 4+, 20+ etc.) and
tumor-infiltrating lymphocytes compared with in healthy donor
specimens. All these immune cell markers were observed in different
types of lung cancer, and the tumor-infiltrating lymphocytes were
more frequent in poorly differentiated tumors and tumors with
microscopic vascular invasion.
In the present study, it was discovered that the
patients with lung cancer from the low NLR group had a lower OS
rate than patients in the high NLR group, which is in accordance
with results observed in other types of cancers (40,41).
Abbasciano et al (42)
hypothesized that the activation and fibrinolysis of platelets may
trigger colon cancer. The physiological immune response of
circulating leukocytes to various abnormal events is characterized
by an increased neutrophil count and decreased lymphocyte count,
which results in high NLR and PLR levels (43). When the infection or inflammatory
response develops into a chronic disease, the opportunity for
tumorigenesis will increase (44).
Mediators of chronic inflammation inevitably contribute to tumor
initiation, development and progression. As a consequence,
upregulation and overexpression of these molecular mediators often
serve as a prognostic marker for patients with cancer (32). Chronic inflammation predisposes and
potentially initiates cancer in the host by inducing DNA damage,
chromosomal instability, and the production of chemokines,
cytokines and growth factors at the inflammatory sites (45,46). When
a tumor exists in the body, tumor necrosis factors, including
interleukin (IL)-1 and IL-6, will contribute to progression towards
malignancy (47). Meanwhile, systemic
inflammatory responses towards a tumor may also increase metastasis
through the inhibition of apoptosis and augmentation of
angiogenesis (48). Neutrophils
secrete vascular endothelial growth factor in addition to a
pro-angiogenic factor that is involved in tumor development, which
is an absolute requirement in tumor growth and metastatic disease
(49). Therefore, the high level of
NLR may serve a function in predicting the survival of patients
with lung cancer.
As there is a high level of specificity of etiology
and pathogenicity in high risk areas of lung cancer, the
relationship between NLR, PLR and the prognosis of patients with
lung cancer was first proposed relating to the Hebei province
(9), one of the high risk areas in
China, where there was a demonstrable increase in the lung cancer
incidence rate from 1973 to 2011. In 2011, the crude lung cancer
incidence rate in the registry areas was 45.44/100,000, accounting
for 18.44% of all cancers (3).
Comparing the results of the present study with Globocan 2012 data
(50), the lung cancer incidence in
Hebei Province in 2011 (ASRW=39.01/100,000) was 1.08 times that of
China as a whole (36.1/100,000) and 1.69 times that of global rates
(23.1/100,000). The potential association with large-scale samples
was explored in the present study, which was consistent with
results in esophageal cancer (9). The
present study also illustrated that the OS rate of patients with
lung cancer with a high NLR level who live in high-risk areas is
lower than those with a low NLR level. Hence, the NLR may be a
promising approach for predicting the OS rate of lung cancer
patients from a high-risk area.
However, although there is a similar mechanism in
both PLR and NLR, no potential relationship between PLR and the
survival of patients with lung cancer was identified.
Although there is increasing proof that the NLR and
the PLR may be used as prognostic factors in lung cancer, the
mechanisms remain unknown. Further studies are required to confirm
these results and transition these methods from being largely
investigational to potentially being used routinely in lung cancer
prognosis.
Acknowledgements
The present study was supported by grants from the
National Natural Scientific Foundation of China (grant no.
81272682) and the Financial Department of the Hebei Province [grant
no. (2012)2056].
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zuo T, Zeng H, Zhang S
and He J: National cancer incidence and mortality in China, 2012.
Chin J Cancer Res. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Li D, Song G, Li Y, Liang D, Jin J,
Wen D and Shan B: Lung cancer burden has increased during the last
40 years in Hebei Province, China. Thorac Cancer. 7:323–332. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raaschou-Nielsen O, Andersen ZJ, Beelen R,
Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P,
Nieuwenhuijsen MJ, Brunekreef B, et al: Air pollution and lung
cancer incidence in 17 European cohorts: Prospective analyses from
the European Study of Cohorts for Air Pollution Effects (ESCAPE).
Lancet Oncol. 14:813–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamra GB, Guha N, Cohen A, Laden F,
Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P,
Yorifuji T and Loomis D: Outdoor particulate matter exposure and
lung cancer: A systematic review and meta-analysis. Environ Health
Perspect. 122:906–911. 2014.PubMed/NCBI
|
|
6
|
Luengo-Fernandez R, Leal J, Gray A and
Sullivan R: Economic burden of cancer across the European Union: A
population-based cost analysis. Lancet Oncol. 14:1165–1174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Melloni G, Muriana P, Bandiera A, Fontana
R, Maggioni D, Russo V, Doglioni C and Zannini P: Prognostic role
of liver X receptor-alpha in resected stage II and III non-small
cell lung cancer. Clin Respir J Jul. 12:2016.(Epub ahead of
print).
|
|
8
|
Rami-Porta R, Bolejack V, Giroux DJ,
Chansky K, Crowley J, Asamura H and Goldstraw P; International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee, Advisory Board Members and Participating
Institutions, : The IASLC lung cancer staging project: The new
database to inform the eighth edition of the TNM classification of
lung cancer. J Thorac Oncol. 9:1618–1624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yutong H, Xiaoli X, Shumei L, Shan S, Di L
and Baoen S: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with esophageal cancer in a high
incidence area in China. Arch Med Res. 46:557–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wculek SK and Malanchi I: Neutrophils
support lung colonization of metastasis-initiating breast cancer
cells. Nature. 528:413–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Guo J, Feng C, Ke Z, Chen L and
Pan Y: The preoperative platelet-lymphocyte ratio versus
neutrophil-lymphocyte ratio: Which is better as a prognostic factor
in oral squamous cell carcinoma? Ther Adv Med Oncol. 8:160–167.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng Z, Wen H, Bi R, Ju X, Chen X, Yang W
and Wu X: Preoperative neutrophil-to-lymphocyte ratio as a
predictive and prognostic factor for high-grade serous ovarian
cancer. PLoS One. 11:e01561012016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marchioni M, Primiceri G, Ingrosso M,
Filograna R, Castellan P, De Francesco P and Schips L: The clinical
use of the neutrophil to lymphocyte ratio (NLR) in urothelial
cancer: A systematic review. Clin Genitourin Cancer. 14:473–484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda Y, Kawahara T, Koizumi M, Ito H,
Kumano Y, Ohtaka M, Kondo T, Mochizuki T, Hattori Y, Teranishi J,
et al: Lack of an association between neutrophil-to-lymphocyte
ratio and PSA failure of prostate cancer patients who underwent
radical prostatectomy. Biomed Res Int. 2016:61973532016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee H, Um SJ, Kim YS, Kim DK, Jang AS,
Choi HS, Kim YH, Kim TE, Yoo KH and Jung KS: Association of the
neutrophil-to-lymphocyte ratio with lung function and exacerbations
in patients with chronic obstructive pulmonary disease. PLoS One.
11:e01565112016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JL, Lv XD, Ma H, Chen JR and Huang
JA: Detection of cancer embryo antigen and endothelin-1 in exhaled
breath condensate: A novel approach to investigate non-small cell
lung cancer. Mol Clin Oncol. 5:124–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gandara DR, Grimminger P, Mack PC, Lara PN
Jr, Li T, Danenberg PV and Danenberg KD: Association of epidermal
growth factor receptor activating mutations with low ERCC1 gene
expression in non-small cell lung cancer. J Thorac Oncol.
5:1933–1938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atwater T and Massion PP: Biomarkers of
risk to develop lung cancer in the new screening era. Ann Transl
Med. 4:1582016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Field RW and Withers BL: Occupational and
environmental causes of lung cancer. Clin Chest Med. 33:681–703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cruz CS Dela, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Straif K, Cohen A and Samet J: Air
pollution and cancer. International Agency for Research on Cancer;
Lyon, France: pp. 123–148. 2014
|
|
22
|
Oberli LS, Valeri F, Korol D, Rohrmann S
and Dehler S: 31 years of lung cancer in the canton of Zurich,
Switzerland: Incidence trends by sex, histology and laterality.
Swiss Med Wkly. 146:w143272016.PubMed/NCBI
|
|
23
|
Cedrés S, Nuñez I, Longo M, Martinez P,
Checa E, Torrejón D and Felip E: Serum tumor markers CEA,
CYFRA21-1, and CA-125 are associated with worse prognosis in
advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer.
12:172–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Retraction: HMGA2 functions as a competing
endogenous RNA to promote lung cancer progression. Nature.
523:3702015. View Article : Google Scholar
|
|
25
|
Retraction note to: Identification of
featured biomarkers in different types of lung cancer with DNA
microarray. Mol Biol Rep. 42:14812015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agathanggelou A, Honorio S, Macartney DP,
Martinez A, Dallol A, Rader J, Fullwood P, Chauhan A, Walker R,
Shaw JA, et al: Methylation associated inactivation of RASSF1A from
region 3p21.3 in lung, breast and ovarian tumours. Oncogene.
20:1509–1518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdurahman A, Anwar J, Turghun A, Niyaz M,
Zhang L and Awut I: Epidermal growth factor receptor gene mutation
status and its association with clinical characteristics and tumor
markers in non-small-cell lung cancer patients in Northwest China.
Mol Clin Oncol. 3:847–850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherwood JL, Corcoran C, Brown H, Sharpe
AD, Musilova M and Kohlmann A: Optimised pre-analytical methods
improve KRAS mutation detection in circulating tumour DNA (ctDNA)
from patients with non-small cell lung cancer (NSCLC). PLoS One.
11:e01501972016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantovani A and Pierotti MA: Cancer and
inflammation: A complex relationship. Cancer Lett. 267:180–181.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chao C and Hellmich MR: Gastrin,
inflammation, and carcinogenesis. Curr Opin Endocrinol Diabetes
Obes. 17:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adach A, Ellert-Miklaszewska A and
Kaminska B: Molecular characterization of STAT signaling in
inflammation and tumorigenesis. Methods Mol Biol. 512:265–278.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chai EZ, Siveen KS, Shanmugam MK, Arfuso F
and Sethi G: Analysis of the intricate relationship between chronic
inflammation and cancer. Biochem J. 468:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bollrath J and Greten FR: IKK/NF-kappaB
and STAT3 pathways: Central signalling hubs in
inflammation-mediated tumour promotion and metastasis. EMBO Rep.
10:1314–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan QX, Su ZJ, Zhang JH, Wang CR and Ke
SY: A comparison of the prognostic value of preoperative
inflammation-based scores and TNM stage in patients with gastric
cancer. Onco Targets Ther. 8:1375–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiels MS, Pfeiffer RM, Hildesheim A,
Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G,
Caporaso NE, et al: Circulating inflammation markers and
prospective risk for lung cancer. J Natl Cancer Inst.
105:1871–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, risk factors, and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Banat GA, Tretyn A, Pullamsetti SS,
Wilhelm J, Weigert A, Olesch C, Ebel K, Stiewe T, Grimminger F,
Seeger W, et al: Immune and inflammatory cell composition of human
lung cancer stroma. PLoS One. 10:e01390732015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ruffini E, Asioli S, Filosso PL, Lyberis
P, Bruna MC, Macrì L, Daniele L and Oliaro A: Clinical significance
of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac
Surg. 87:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Absenger G, Szkandera J, Pichler M, Stotz
M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H,
Stojakovic T and Gerger A: A derived neutrophil to lymphocyte ratio
predicts clinical outcome in stage II and III colon cancer
patients. Br J Cancer. 109:395–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahn HK, Hwang IC, Lee JS, Sym SJ, Cho EK
and Shin DB: Neutrophil-lymphocyte ratio predicts survival in
terminal cancer patients. J Palliat Med. 19:437–441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abbasciano V, Bianchi MP, Trevisani L,
Sartori S, Gilli G and Zavagli G: Platelet activation and
fibrinolysis in large bowel cancer. Oncology. 52:381–384. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zahorec R: Ratio of neutrophil to
lymphocyte counts-rapid and simple parameter of systemic
inflammation and stress in critically ill. Bratisl Lek Listy.
102:5–14. 2001.(In English, Slovak). PubMed/NCBI
|
|
44
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kundu JK and Surh YJ: Inflammation:
Gearing the journey to cancer. Mutat Res. 659:15–30. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jackson JR, Seed MP, Kircher CH,
Willoughby DA and Winkler JD: The codependence of angiogenesis and
chronic inflammation. FASEB J. 11:457–465. 1997.PubMed/NCBI
|
|
49
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|