Introduction
Advanced-stage pancreatic cancer is the most lethal
human malignancy, with an overall 5-year survival rate of <5%
(1). This poor outcome is largely due
to the late diagnosis, and as conventional therapeutics, including
surgical resection, chemotherapy and radiotherapy, have limited
efficacy (2). Although several
strategies are widely used to treat advanced pancreatic cancer,
such as using gemcitabine alone or in combination with other drugs,
the emergence of drug resistance is becoming a problem for treating
advanced pancreatic cancer (3,4).
Cellular tumor antigen p53 (p53) is an important
transcription factor that actives or represses the expression of
numerous genes, including those involved in cell cycle and cell
survival (5,6). Previous studies have shown that p53
causes a significant antitumor effect inducing cell cycle arrest,
senescence and apoptosis in response to stress stimulus such as
oncogene activation and DNA damage (7–11). A
recent study reported that >75% of patients with advanced
pancreatic cancer possessed a tumor protein p53 (TP53) gene
alteration (12). Although there have
been a number of widely reported adverse events, gene therapy is
becoming a new paradigm and an important part of combined therapy
regimens for tumors, and has shown enormous therapeutic potential
(13–15). TP53 is by far the most commonly
transferred tumor suppressor gene in cancer trials (16), but clinical trials based on wild-type
TP53 in patients with pancreatic cancer are lacking.
The present study demonstrated that administration
of recombinant human adenovirus p53 agent (rAd-p53) could not only
reduce the size of the tumor, but also relieve the symptoms induced
by pancreatic cancer.
Patients and methods
Patients and ethics statement
The present study was approved by the Ethics
Committee of Taizhou Municipal Central Hospital (Taizhou, China)
and conducted according to the Ethical Guidelines for Human
Genome/Gene Research enacted by the Helsinki Declaration and the
Chinese Government. Written informed consent was obtained from all
patients. All 23 cases of patients who did not receive surgical
resection were collected between January 2010 and January 2012 at
the Department of Hepatobiliary Surgery of Taizhou Central Hospital
(Taizhou, China). None of the patients received local or systemic
treatment prior to rAd-p53 treatment.
Treatment
First, the patient was laid flat in the digital
subtraction angiography room for a celiac arteriography via the
right femoral artery using the Seldinger method to identify the
trunk of the celiac artery. The arteriography was superselected to
the gastroduodenal artery in 18 cases, and to the superior
pancreaticoduodenal artery in the remaining 5 cases. A total of 4
ml rAd-p53 (2×1012 virus particles;
Gendicine®; Benda Pharmaceutical, Inc., Wuhan, China) in
6 ml normal saline was pushed (intravenous bolus) into each patient
per cycle. After sealing the indwelling catheter with heparin, the
patient was sent back to the ward. rAd-p53 was administered at a
3-day interval for a total of 4 cycles, so that a total dose of
8×1012 virus particles were administered to each
patient. Next, the perfusion catheter was removed and the incision
was pressure-dressed locally.
Response evaluation
Temperature was checked three times daily, blood and
urine routine three times per week, liver and kidney function was
assessed three times per week, and pancreatic computed tomography
(CT) was performed at 2, 8 and 16 weeks post-rAd-p53
administration.
Criteria for therapeutic effect
evaluation
Therapeutic effect was graded as follows: i)
Complete response (CR), disappearance of all visible lesions for at
least 4 weeks; ii) partial response (PR), a >50% decrease in the
product of the maximum diameter and the maximum vertical diameter
of the lesion for at least 4 weeks; iii) minimal response (MR), a
>25% and <50% decrease in the product of the two diameters
without occurrence of new lesions; iv) stable disease (SD), a
<25% decrease or a <25% increase in the product of the two
diameters without occurrence of new lesions; and v) progressive
disease (PD), a >25% increase in the product of the two
diameters or occurrence of new lesions. The overall response rate
was calculated as the CR plus the PR, without including MR and SD
(17).
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Values are
expressed as the mean ± standard deviation. One-way analysis of
variance was used to compare the differences between the groups
using Dunnett's post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
The present study included 23 patients diagnosed
with pancreatic cancer by biopsy, including 17 males and 6 females,
with a mean age of 56.8 years (Table
I). The time of disease onset was 6–18 months before diagnosis.
All patients reported varying degrees of abdominal pain and
radiating pain to the back on admission. Of the 23 patients, 19
patients presented with jaundice, 17 patients looked extremely
lean, and 5 cases were complicated with lung metastasis and 3 with
bone metastasis. Of the 23 pancreatic cancer cases, 15 cases were
located in the head of the pancreas arising from the uncinate
process, and the remaining 8 cases were located in the junction of
the head and body.
 | Table I.Clinical features, treatment doses and
outcomes of the 23 pancreatic cancer patients. |
Table I.
Clinical features, treatment doses and
outcomes of the 23 pancreatic cancer patients.
| Case no. | Sex | Age | Location | Size, cm | Jaundice | Pain | Lean | LM | BM | rAdp53 (vp) | Shrinkage, % |
|---|
| 1 | F | 68 | Head | 4.6×6.3 | Yes | Yes | No | Yes | No |
8.0×1012 | 26 |
| 2 | F | 79 | Body tail | 7.0×4.3 | No | No | Yes | No | Yes |
8.0×1012 | 40 |
| 3 | M | 73 | Head | 4.1×3.6 | No | Yes | Yes | No | No |
8.0×1012 | 32 |
| 4 | M | 50 | Head | 4.0×2.7 | Yes | Yes | No | No | No |
8.0×1012 | 60 |
| 5 | F | 62 | Head | 9.0×6.0 | No | Yes | No | Yes | Yes |
8.0×1012 | 43 |
| 6 | M | 45 | Head | 4.3×5.0 | No | No | Yes | No | No |
8.0×1012 | 50 |
| 7 | M | 53 | Body tail | 13.7×9.7 | No | Yes | No | Yes | No |
8.0×1012 | 58 |
| 8 | M | 67 | Head | 3.4×5.5 | No | Yes | Yes | No | No |
8.0×1012 | 0 |
| 9 | F | 82 | Head | 4.7×3.4 | Yes | Yes | No | No | No |
8.0×1012 | 55 |
| 10 | M | 47 | Head | 5.4×4.2 | Yes | Yes | No | No | No |
8.0×1012 | 25 |
| 11 | M | 63 | Head | 6.0×5.3 | Yes | Yes | Yes | No | No |
8.0×1012 | 50 |
| 12 | F | 57 | Head | 4.7×6.3 | Yes | Yes | Yes | Yes | No |
8.0×1012 | 32 |
| 13 | M | 48 | Head | 5.6×7.3 | Yes | Yes | Yes | No | Yes |
8.0×1012 | 0 |
| 14 | M | 73 | Head | 4.9×6.5 | Yes | No | Yes | No | No |
8.0×1012 | 40 |
| 15 | M | 81 | Body | 4.6×5.6 | No | Yes | Yes | No | No |
8.0×1012 | 50 |
| 16 | F | 75 | Head | 4.5×6.3 | Yes | Yes | Yes | No | No |
8.0×1012 | 32 |
| 17 | M | 57 | Body | 4.6×5.8 | No | Yes | Yes | No | Yes |
8.0×1012 | 50 |
| 18 | M | 74 | Body tail | 5.2×6.7 | No | Yes | Yes | Yes | No |
8.0×1012 | 40 |
| 19 | M | 68 | Head | 4.5×5.2 | Yes | Yes | Yes | No | No |
8.0×1012 | 50 |
| 20 | M | 71 | Head | 5.6×6.8 | Yes | Yes | No | No | Yes |
8.0×1012 | 40 |
| 21 | M | 86 | Body tail | 9.5×8.3 | No | Yes | Yes | No | No |
6.0×1012 | 50 |
| 22 | M | 56 | Body | 5.6×4.2 | No | No | No | No | No |
8.0×1012 | 10 |
| 23 | M | 61 | Head | 3.8×6.0 | No | Yes | No | No | No |
8.0×1012 | 25 |
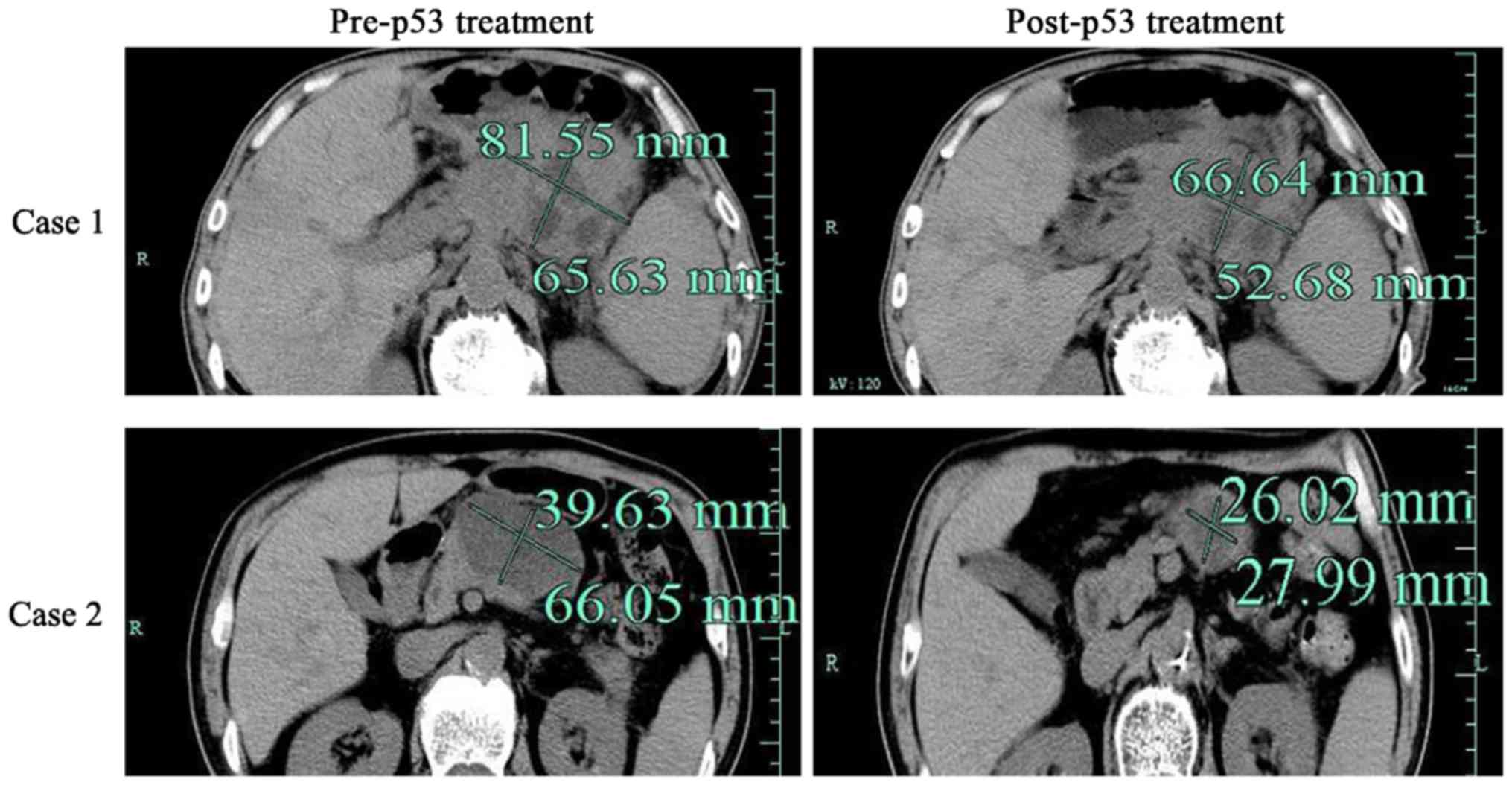
Clinical outcome
Among the 23 pancreatic cancer patients (Table I), 17 patients experienced marked
tumor shrinkage (>20%), 14 patients reported relief from the
abdominal and back pain (Fig. 1 and
Table II). The patient's general
condition, including appetite, was improved in 13 patients, weight
gain was reported in 9 patients, jaundice was lessened in 13
patients and ascites subsided in 10 patients (Table III). Clinical characteristics,
including hematological profiles, and liver and kidney function,
were not significantly different. The clinical outcome was poor in
2 patients, in whom a CT re-checkup showed no marked change in the
tumor volume. These 2 patients succumbed within 6 months.
 | Table II.Therapeutic outcome evaluation. |
Table II.
Therapeutic outcome evaluation.
| Outcome
evaluation | CR (%) | PR (%) | MR (%) | SD (%) | PD (%) |
|---|
| At the end of
treatment | 0 (0.0) | 9 (39.1) | 13 (56.5) | 1 (4.3) | 0 (0.0) |
| CT
confirmation | 0 (0.0) | 9 (39.1) | 12 (52.2) | 2 (8.7) | 0 (0.0) |
 | Table III.Changes in hematological profiles and
liver/kidney function (mean ± standard deviation). |
Table III.
Changes in hematological profiles and
liver/kidney function (mean ± standard deviation).
| Time | WBC,
×109/l | RBC,
×1012/l | HB, g/l | Pt,
×1012/l | SGPT, µ/l | Plasma protein,
g/l | Albumin, g/l | BUN, mol/l | Bilirubin level,
µmol/l |
|---|
| Prior to
treatment | 6.12±2.6 | 445±44 | 129±19 | 256±130 | 24±17 | 71.6±73 | 41.7±4.1 | 5.3±1.0 | 65.7±4.1 |
| After 1st
injection | 8.16±4.6 | 435±60 | 128±18 | 256±93 | 19±6 | 67.6±6.9 | 38±4.9 | 5.9±1.0 | 56.3±4.1 |
| After 4th
injection | 7.26±4.0 | 425±84 | 124±26 | 308±14 | 21±12 | 69.8±8.6 | 39.4±4.9 | 4.8±1.5 |
38.4±4.1a |
Discussion
Patients with pancreatic cancer, particularly
mid-late stage pancreatic head cancer, often lose the opportunity
for surgical intervention, while the therapeutic efficacy of
chemotherapy and radiotherapy is usually unsatisfactory (1,18). The
prognosis of these patients is extremely poor due to advanced TNM
stage and is often complicated with systemic jaundice, ascites, and
intolerable abdominal and back pain (19).
The key to TP53 gene therapy lies in obtaining
exogenous wild-type TP53, introducing it into tumor cells, and
inducing its expression in the cells safely and effectively,
usually using a virus, adenovirus or liposome as the vector
(4). Current studies have
demonstrated that introduction of wild-type TP53 into tumor cells
can induce cell cycle arrest, promote apoptosis and inhibit tumor
angiogenesis, so as to cause tumor cells of various origins to
undergo apoptosis, eventually resulting in tumor disappearance
(20–22).
Numerous studies have indicated that the expression
of TP53 is altered and frequently mutated in pancreatic cancer,
which may be associated with the more malignant biological behavior
of the cancer (23–26). TP53 gene therapy, particularly
adenovirus-mediated rAd-P53 gene therapy, has been used for the
clinical treatment of head and neck squamous cell carcinoma, with
good clinical outcomes, determined by increased survival times
(27,28). The arterial infusion approach can
ensure that large amounts of the high-concentration drug can access
to tumor cells, with only small amounts of the drug entering the
circulation, thus maximizing the therapeutic efficacy and reducing
the systemic adverse effects (29).
Further laboratory tests showed that there were no significant side
effects following adenovirus-mediated rAd-p53 gene therapy by the
arterial infusion approach in terms of hematological profiles, and
liver and kidney function. In the present study, the clinical
symptoms were relieved in 21 of the 23 patients who received
trans-arterial p53 perfusion therapy, suggesting that this method
can be used clinically on a larger scale, although the number of
cases in the study was relatively small and further clinical trials
are required.
References
|
1
|
Nelson NJ: Pancreatic cancer research
matures. J Natl Cancer Inst. 99:1432–1434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Vervenne WL, Bennouna J,
Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang
A, Cosaert J and Moore MJ: Phase III trial of bevacizumab in
combination with gemcitabine and erlotinib in patients with
metastatic pancreatic cancer. J Clin Oncol. 27:2231–2237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirao A, Kong YY, Matsuoka S, Wakeham A,
Ruland J, Yoshida H, Liu D, Elledge SJ and Mak TW: DNA
damage-induced activation of p53 by the checkpoint kinase Chk2.
Science. 287:1824–1827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaguchi K, Herrera JE, Saito S, Miki T,
Bustin M, Vassilev A, Anderson CW and Appella E: DNA damage
activates p53 through a phosphorylation-acetylation cascade. Genes
Dev. 12:2831–2841. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kastan MB, Canman CE and Leonard CJ: P53,
cell cycle control and apoptosis: Implications for cancer. Cancer
Metastasis Rev. 14:3–15. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghaneh P, Greenhalf W, Humphreys M, Wilson
D, Zumstein L, Lemoine NR and Neoptolemos JP: Adenovirus-mediated
transfer of p53 and p16(INK4a) results in pancreatic cancer
regression in vitro and in vivo. Gene Ther. 8:199–208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, Zhu Y, Xu C, Xu H, Zhou X, Yang J,
Xie Y and Tao M: Adenovirus-mediated p53 and ING4 gene co-transfer
elicits synergistic antitumor effects through enhancement of p53
acetylation in breast cancer. Oncol Rep. 35:243–252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenfeldt MT, O'Prey J, Morton JP, Nixon
C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, et al:
p53 status determines the role of autophagy in pancreatic tumour
development. Nature. 504:296–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weill D, Mack M, Roth J, Swisher S,
Proksch S, Merritt J and Nemunaitis J: Adenoviral-mediated p53 gene
transfer to non-small cell lung cancer through endobronchial
injection. Chest. 118:966–970. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012 - an
update. J Gene Med. 15:65–77. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merritt JA, Roth JA and Logothetis CJ:
Clinical evaluation of adenoviral-mediated p53 gene transfer:
Review of INGN 201 studies. Semin Oncol. 28 5 Suppl 16:S105–S114.
2001. View Article : Google Scholar
|
|
16
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu TJ, Zhang WW, Taylor DL, Roth JA,
Goepfert H and Clayman GL: Growth suppression of human head and
neck cancer cells by the introduction of a wild-type p53 gene via a
recombinant adenovirus. Cancer Res. 54:3662–3667. 1994.PubMed/NCBI
|
|
21
|
Shimada H, Shimizu T, Ochiai T, Liu TL,
Sashiyama H, Nakamura A, Matsubara H, Gunji Y, Kobayashi S, Tagawa
M, et al: Preclinical study of adenoviral p53 gene therapy for
esophageal cancer. Surg Today. 31:597–604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang FF, Bruner JM, Fuller GN, Aldape K,
Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR,
et al: Phase I trial of adenovirus-mediated p53 gene therapy for
recurrent glioma: Biological and clinical results. J Clin Oncol.
21:2508–2518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ternovoi VV, Curiel DT, Smith BF and
Siegal GP: Adenovirus-mediated p53 tumor suppressor gene therapy of
osteosarcoma. Lab Invest. 86:748–766. 2006.PubMed/NCBI
|
|
24
|
Scarpa A, Capelli P, Mukai K, Zamboni G,
Oda T, Iacono C and Hirohashi S: Pancreatic adenocarcinomas
frequently show p53 gene mutations. Am J Pathol. 142:1534–1543.
1993.PubMed/NCBI
|
|
25
|
Tomaszewska R, Karcz D and Stachura J: An
immunohistochemical study of the expression of bcl-2 and p53
oncoproteins in pancreatic intraepithelial neoplasia and pancreatic
cancer. Int J Pancreatol. 26:163–171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weissmueller S, Manchado E, Saborowski M,
JP IV Morris, Wagenblast E, Davis CA, Moon SH, Pfister NT,
Tschaharganeh DF, Kitzing T, et al: Mutant p53 drives pancreatic
cancer metastasis through cell-autonomous PDGF receptor β
signaling. Cell. 157:382–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang SW, Xiao SW, Liu CQ, Sun Y, Su X, Li
DM, Xu G, Cai Y, Zhu GY, Xu B and Lü YY: Treatment of head and neck
squamous cell carcinoma by recombinant adenovirus-p53 combined with
radiotherapy: A phase II clinical trial of 42 cases. Zhonghua Yi
Xue Za Zhi. 83:2023–2083. 2003.(In Chinese). PubMed/NCBI
|
|
28
|
Nemunaitis J, Swisher SG, Timmons T,
Connors D, Mack M, Doerksen L, Weill D, Wait J, Lawrence DD, Kemp
BL, et al: Adenovirus-mediated p53 gene transfer in sequence with
cisplatin to tumors of patients with non-small-cell lung cancer. J
Clin Oncol. 18:609–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Zhou J, Weng D, Zhang C, Li L, Wang
B, Song Y, He Q, Lin D, Chen D, et al: Adjuvant adenovirus-mediated
delivery of herpes simplex virus thymidine kinase administration
improves outcome of liver transplantation in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 13:5847–5854. 2007.
View Article : Google Scholar : PubMed/NCBI
|