Introduction
Nodular disease of the thyroid gland is a relatively
common malignancy worldwide and is present in 4–7% of
North-American adults (1,2). Clinical studies suggest that the
diagnosis of thyroid nodules may increase from 20–70% in the
general population, based on the increased use of ultrasound
techniques, and their presence may reach up to 50% in patients
undergoing autopsy (3). Although the
majority of nodules are benign and asymptomatic, there is an ~10%
risk of the presence of underlying malignant disease, which
requires patients to undergo additional procedures, including
surgical intervention (4). The
majority of malignant thyroid neoplasms have a good prognosis;
however, several studies have identified factors that significantly
affect the patient survival rate and have long-term implications
(5–7).
Therefore, it is crucial that a distinction between benign and
malignant lesions is reliably made pre-surgically using techniques
including fine needle biopsy and/or post-surgically (8,9).
Consequently, novel techniques that unambiguously aid
distinguishing between benign and malignant disease are
required.
The thyroid has been the focus of
immunohistochemical studies comprising large numbers of antigens
and antibodies in order to characterize benign and malignant
lesions (10). While certain
antibodies have demonstrated notable potential, particularly when
used together to increase their impact, a marker with high
sensitivity and specificity remains to be identified.
The search for tumor-associated antigens capable of
eliciting an immune response and that may be used in the
development of cancer vaccines has been the primary effort in the
field of tumor immunology over the last 2 decades (11). Several tumor antigens have been
identified as having the ability to elicit cellular and/or humoral
immune responses in the autologous host (12). One such group of tumor-associated
antigens is referred to as cancer/testis (CT) antigens. They are
expressed in a number of types of cancer; however, in normal adult
tissue, CT antigens are solely present in testicular germ cells and
occasionally in the placenta (13).
There have been >100 CT antigens and CT antigen-families
identified to date and melanoma associated antigen (MAGE) A1
remains the prototype. Classical CT antigens that map to chromosome
X and with largely unknown functions may be distinguished from
non-classical CT antigens that have known functions and map to
autosomes (14,15). CT antigens are considered valuable
target antigens for vaccine-based immunotherapeutic approaches due
to their cancer-associated expression pattern and their lack of
expression in almost all normal tissues except germ cells (6,16). Their
exclusive presence in malignant tumors has been confirmed in
numerous studies and in various tumor types (17); however, little is known about the
presence of CT antigens in thyroid neoplasms.
Among CT antigens, particular antigens have been
studied more extensively. MAGE-A antigens are the most highly
expressed in tumors, including head and neck cancer (18–23). In
recent years, members of the MAGE-A family, particularly MAGE-A3,
have been studied as target antigens in vaccine clinical trials for
numerous types of cancer (24) and
current data suggest that MAGE-C1 may serve an important role in
tumorigenesis (22,25). In myeloma for example, the expression
of MAGE-C1 is correlated with disease progression and resistance to
apoptosis and its expression was reported to be a strong prediction
marker for lymph node metastases in melanoma (26,27). New
York esophageal squamous cell carcinoma 1 (NY-ESO-1) is not highly
expressed compared with other CT antigens; however, it is a
cytoplasmic highly immunogenic molecule present in numerous
malignant cells and has been the subject of translational research
in patients with melanoma (28–31).
Additionally, it has been demonstrated that the G antigen (GAGE)
family is associated with specific clinical characteristics in
certain types of cancer, including poor prognosis and increasing
cellular resistance to apoptosis (29,32).
Consequently, in the present study the in
situ protein expression of the CT antigens MAGE-A, MAGE-C1/CT7,
GAGE and CTAG1B were measured in benign and malignant lesions of
the thyroid gland and the potential associations with
clinicopathological and prognostic variables was analyzed.
Materials and methods
Patient group
In the present study, data from patients who
underwent total thyroidectomy at the Departments of Head and Neck
Surgery and Otorhinolaryngology of A.C. Camargo Cancer Center, São
Paulo as well as the Medical Center of the University of São Paulo
at Ribeirao Preto between January 1962 and December 2011 were
analyzed. Inclusion criteria were: Availability for pathological
specimens and complete clinical data, patient age and gender,
nodule size, status of potential vascular and capsular invasions,
extraglandular extension, presence of ganglionic metastasis and
distant metastasis. A total of 117 patients were enrolled in the
study based on the inclusion criteria; 86 patients were from the
Ribeirao Preto Medical School Hospital and 31 patients from the AC
Camargo Hospital. The 117 cases consisted of the following lesions:
22 colloid goiters; 9 follicular adenomas; 9 follicular carcinomas;
28 papillary carcinomas; 28 medullary carcinomas; 8 poorly
differentiated carcinomas; and 13 anaplastic carcinomas. In
addition, thyroid tissue from 8 necropsy cases without any thyroid
disease was analyzed. All patients provided written informed
consent and the study has been approved by the Ethical Committee of
the Faculty of Medicine of Ribeirão Preto, University of São Paulo
and A.C. Camargo Cancer Center (protocols no. 13.141/2009 and
1.645/12).
Histological preparation and
immunohistochemical staining
Surgical specimens were fixed in 10% buffered
formalin for a maximum of 48 h at room temperature. Paraffin blocks
with representative areas of tumor, in 4-µm sections, were selected
for immunohistochemical analysis following confirmation of the
presence of tumor on a hematoxylin and eosin stained section.
Readings were performed by 2 independent observers, surgical
pathologists with experience in the area who were unaware of the
identity of the cases, prior to inclusion in the study without, and
using tissue microarray technology.
For the detection of CT antigens, the following
antibodies were employed. CTAG1B was detected by monoclonal
antibody (mAb) E978 and mAb CT7-33 was used for MAGE-C1/CT7; the
two mAbs had been previously generated by our group (28,33). GAGE
was detected with a commercial reagent clone #26 (BD Transduction
Laboratories; BD Biosciences, Franklin Lakes, NJ, USA). To analyze
MAGE-A antigens, a cocktail consisting of mAb MA454 for MAGE-A1,
mAb 57B for MAGE-A4 and mAb 6C1 for MAGE-A1, -A3/6, -A4, -A10 and
A12 was used to detect a broad spectrum of MAGE-A antigens
(18–20,34). All
slides were subjected to heat-induced antigen retrieval prior to
application of the primary antibodies. The antibodies,
concentrations and conditions are listed in Table I. All primary antibodies, with the
exception of mAb E978, were detected with a biotinylated
horse-anti-mouse-secondary antibody (dilution, 1:200; Vector Labs,
Inc., Burlingame, CA, USA) followed by an avidin-biotin-complex
tertiary (dilution, 1:70; ABC-Elite, Vector Laboratories, Inc.).
mAb E978 was detected with the PowerVision kit (Leica Microsystems,
Inc., Buffalo Grove, IL, USA). Diaminobenzidine (DAB) served as a
chromogen (Biogenex, Fremont, CA, USA) and hematoxylin (Gill II)
was used for counterstaining. Immunostaining was assessed
semi-quantitatively and graded based on the estimated amount of
immunopositive tumor cells as follows: Negative, no staining; focal
(f), <5%; 1+, 5–25%; 2+, >25–50%; 3+, >50-75%; 4+,
>75%.
 | Table I.Primary antibodies. |
Table I.
Primary antibodies.
| Monoclonal
antibody | Antigen | Dilution | Buffer |
|---|
| MA454a,c | MAGE-A1 | 1:200 | EDTA, 1 mM, pH
8.0 |
| 57Bb,c | MAGE-A4 | 1:4,000 | EDTA, 1 mM, pH
8.0 |
| 6C1a,c | MAGE-A 1, −2, −3,
−4, −6, −10 and −12 | 1:20 | Citrate, 10 mM, pH
6.0 |
| CT7-33a | CT7 (MAGE-C1) | 1:32,000 | Citrate, 10 mM, pH
6.0 |
| #26d | GAGE | 1:80,000 | EDTA, 1 mM, pH
8.0 |
| E978a | CTAG1B | 1:3,200 | High pH retrieval
solution |
Statistical analysis
Cases were divided into four groups: i) Benign
diseases (colloid goiters and follicular adenomas); ii) follicular
and papillary carcinomas; and iii) medullary carcinomas; and iv)
poorly differentiated carcinomas.
Statistical analysis evaluated the significance of
CT antigen expression and clinicopathological variables associated
with the patient (gender and age) and tumor (histological type,
size, tumor stage, positive lymph node, metastasis, stage grouping,
angiolymphatic invasion and extra-thyroidal extension). Variables
were grouped as follows: i) Age: Patients were divided into two
groups, patients <45 years old and patients ≥45 years; ii) tumor
classification: Tumors were analyzed in two separate groups, T1/T2
vs. T3/T4 tumors and T4 tumors, which were defined as poorly
differentiated and anaplastic carcinomas; and iii) staging: Tumors
were analyzed in two separate groups, stage I/II patients vs. stage
III/IV patients (poorly differentiated and anaplastic carcinomas
were all considered clinical stage IV). Following pathological
analysis, the cases were divided into the 4 aforementioned groups.
On the basis of the contingency table of the observed frequencies,
the expected frequencies were calculated. The χ2 was
used in the present study, involving the sum of all the results
that are obtained by dividing the square result of the difference
between the observed and expected frequencies by the expected
frequency. The obtained value of the χ2 test is compared
with the border value for the determined number of the degree of
freedom and from the χ2 table, the probability of the
zero hypotheses is read. The significance of the correlation of
gene expression with histopathologic and clinical characteristics
was analyzed by the Fisher exact test (P<0.05 was considered to
indicate a statistically significant difference).
Results
Clinical and immunostaining
variables
There were 31/117 patients with benign diseases
consisting of 22 goiters and 9 follicular adenomas, of which the
vast majority (30/31; 96.7%) were women. The average age in this
group was 51.1 years, with a standard deviation (SD) of 19.46 years
and median age of 55 years. Clinical evaluation demonstrated that
the average nodule diameter was 3.0±2.15 cm (0.6–10 cm).
Benign samples, immunostaining
variable
None of the 31 cases with benign lesions exhibited
any expression of the CT antigens tested. The eight healthy thyroid
tissue samples were negative for all CT antigens tested.
Papillary and follicular carcinomas,
clinical and immunostaining variables
The clinical data as well as the immunohistochemical
staining for patients with papillary and follicular carcinoma are
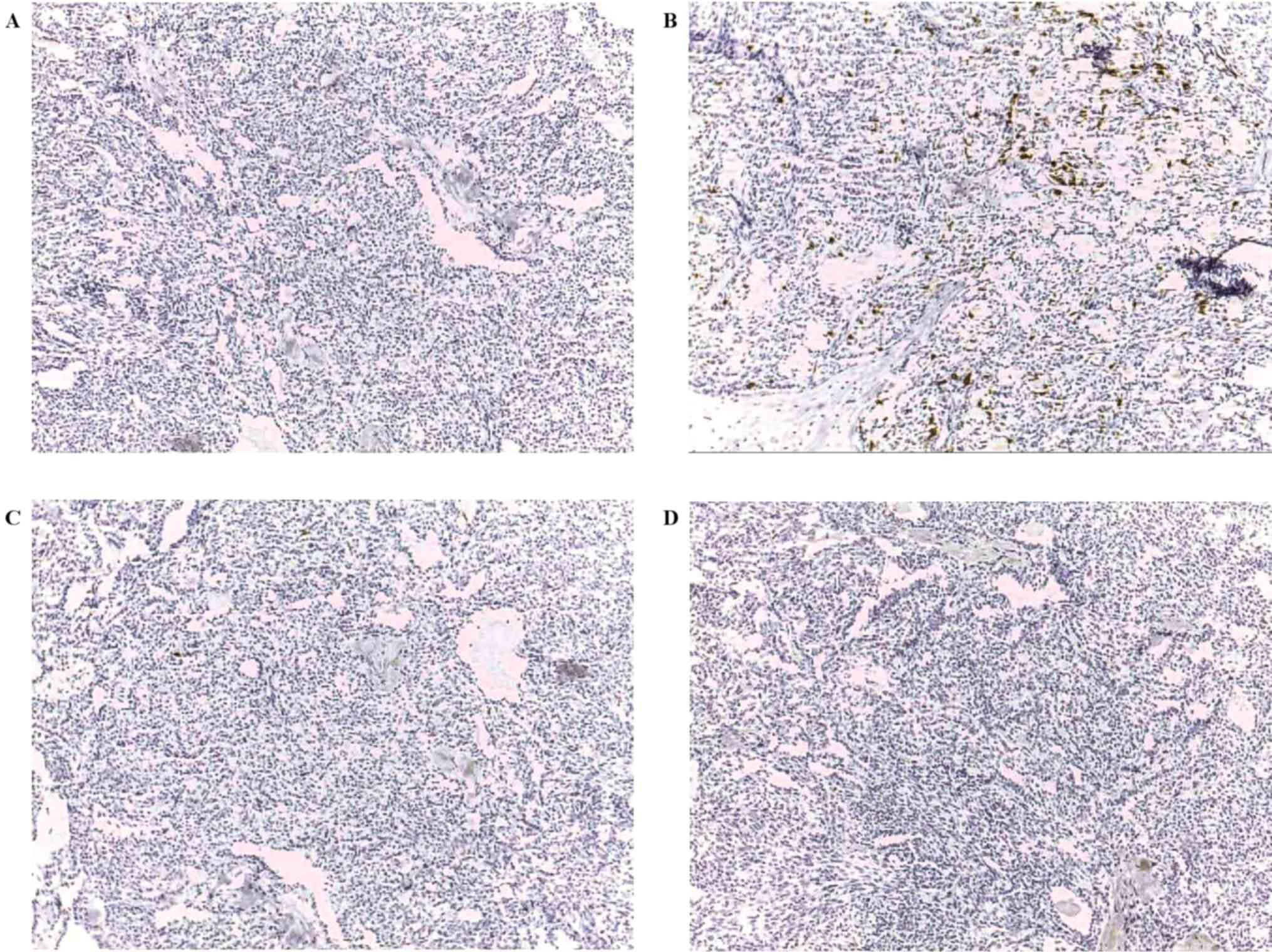
presented in Table II and Fig. 1. There were 37 patients, of which 30
(81.5%) were women, with a ratio of women to men of 8.1:1.9. The
average age of patients with this disease was 47.13 years, with an
SD of 14.75 years and a median age of 46 years. Clinically, the
average diameter of the nodules was 2.8±1.36 cm (0.5–5 cm). There
were 9 follicular and 28 papillary carcinomas. In the group with
follicular carcinoma, there was no predominance in tumor location
between the right and left side (2:1). Among the 28 cases of
papillary carcinomas, tumor location was in the left lobe in 10 and
in the right lobe in 18 cases respectively. GAGE and MAGE-A were
most commonly expressed in 4/37 (10.8%) and 3/37 (8.1%) cases,
respectively. In 6/37 samples (16.2%), ≥1 CT antigen was present.
One case of papillary carcinoma was positive for three CT antigens
(MAGE-A, GAGE and MAGE-C1/CT7) and another papillary carcinoma was
positive for two CT antigens (MAGE-A and CTAG1B). In papillary and
follicular carcinomas, there was no association between the
expression of any CT antigens and the variables analyzed.
 | Table II.CT antigen expression in patients
with papillary and follicular carcinoma. |
Table II.
CT antigen expression in patients
with papillary and follicular carcinoma.
| P | Gender | Age, years | Disease | Size, cm | Tumor stage | Positive lymph
node | Metastasis | Stage grouping | Angiolymphatic
invasion | Extra-thyroidal
extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|
| 1 | F | 23 | Papillary | 3.0 | T2N1aM0 | Yes | Yes | III | No | No | – | – | – | – |
| 2 | F | 54 | Papillary | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 3 | F | 34 | Papillary | 1.9 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 4 | F | 45 | Papillary | 3.2 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 5 | F | 47 | Papillary | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 6 | M | 76 | Papillary | 3.5 | T2N1aM0 | Yes | Yes | III | Yes | Yes | – | – | – | – |
| 7 | F | 49 | Papillary | 2.7 | T2N1bM0 | Yes | Yes | IV | Yes | No | – | – | – | – |
| 8 | F | 13 | Papillary | 3.5 | T2N0M0 | No | No | I | No | No | – | – | f | – |
| 9 | F | 33 | Papillary | 1.5 | T1N0M0 | No | No | I | Yes | No | 1+ | f | f | – |
| 10 | F | 43 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 11 | M | 45 | Papillary | 1.0 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 12 | F | 30 | Papillary | 1.4 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 13 | M | 37 | Papillary | 0.5 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 14 | F | 68 | Papillary | 4.5 | T3N0M0 | No | No | III | No | No | – | – | f | – |
| 15 | M | 39 | Papillary | 1.7 | T1N0M0 | No | No | I | Yes | No | – | – | – | – |
| 16 | M | 73 | Papillary | 7.0 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 17 | M | 47 | Papillary | 0.6 | T1N1aM0 | Yes | Yes | III | No | No | – | – | – | – |
| 18 | F | 51 | Papillary | 2.2 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 19 | F | 48 | Papillary | 1.1 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 20 | F | 71 | Papillary | 4.0 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 21 | M | 62 | Papillary | 5.0 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 22 | F | 28 | Papillary | 1.6 | T1N0M0 | No | No | I | Yes | No | – | – | – | – |
| 23 | F | 37 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 24 | F | 37 | Papillary | 2.3 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 25 | F | 66 | Papillary | 2.6 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 26 | F | 47 | Papillary | 1.7 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 27 | F | 46 | Papillary | 1.8 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 28 | F | 41 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 29 | F | 63 | Follicular | 4.0 | T2N0M0 | No | No | II | No | No | 1+ | – | – | 4+ |
| 30 | F | 61 | Follicular | 3.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 31 | F | 70 | Follicular | 1.4 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 32 | F | 44 | Follicular | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 33 | F | 46 | Follicular | 4.5 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 34 | F | 43 | Follicular | 2.9 | T2N0M0 | No | No | II | No | No | 4+ | – | – | – |
| 35 | F | 33 | Follicular | 3.1 | T2N0M0 | No | No | I | Yes | Yes | – | – | – | – |
| 36 | F | 30 | Follicular | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 37 | F | 50 | Follicular | 4.5 | T3N0M0 | No | No | III | No | No | – | – | – | – |
Medullary carcinoma, clinical and
immunostaining variables
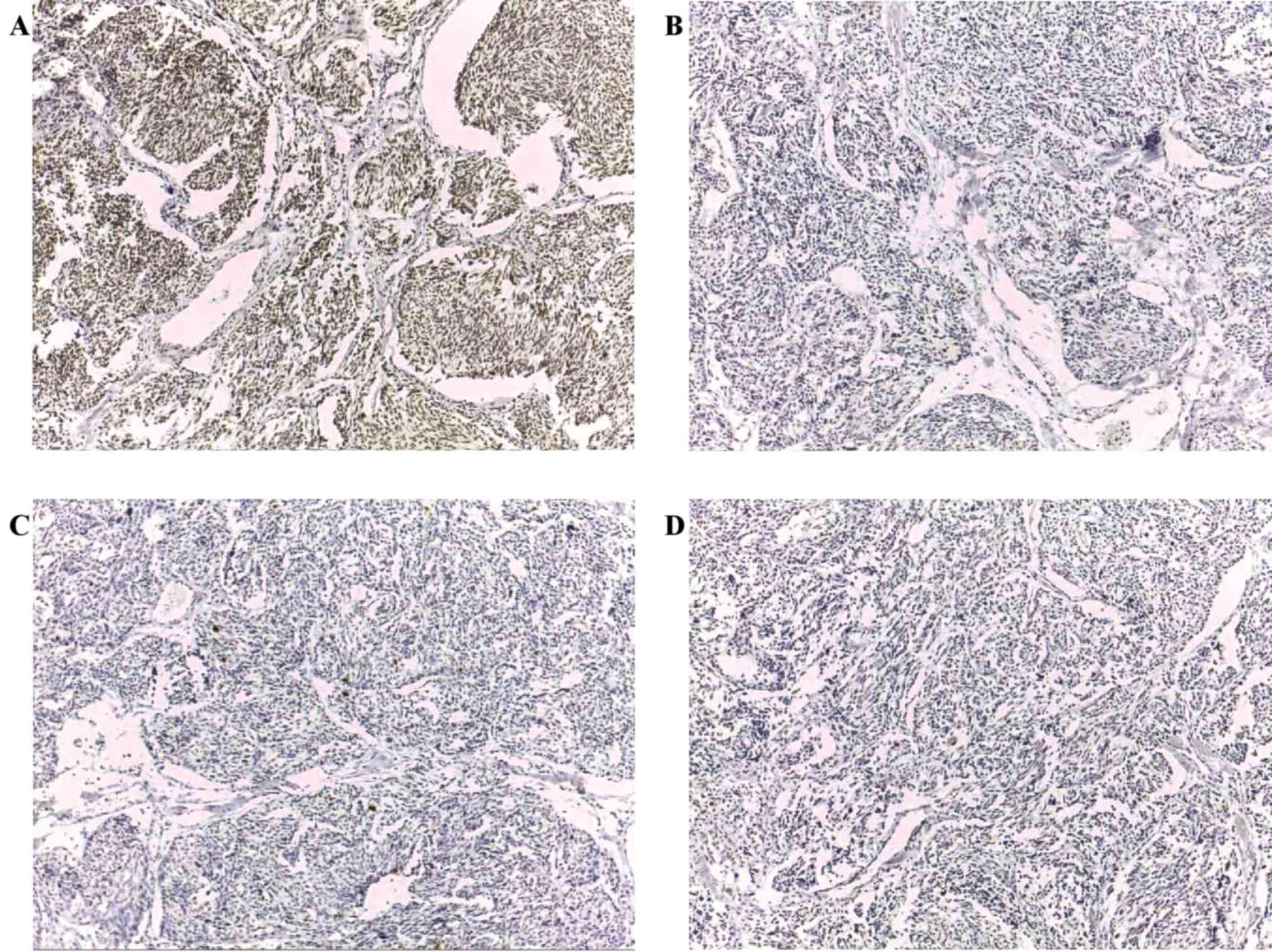
Table III summarizes
the clinical and CT antigen expression data for the 28 patients
with medullary thyroid carcinoma. Distribution by gender indicates
that 67.9% of patients were women, with a ratio of women to men of
6.8:3.2. The average patient age was 47.5 years, with an SD of
15.68 years and a median of 51 years and the average nodule
diameter was 1.9±1.02 cm (0.5–4.2 cm). While the expression of CT
antigens in papillary and follicular carcinoma was low, a
completely different pattern was present in medullary carcinoma.
GAGE was the most prevalent antigen and present in 26/28 (92.9%)
cases. MAGE-A and MAGE-C1/CT7 were both expressed in ~50% of cases
[MAGE-A, 12/28 (42.9%); MAGE-C1/CT7, 13/28 (46.4%)]. CTAG1B was
poorly expressed and was detected in only 2/28 (7.1%) cases. Only 2
cases were completely negative. One (3.6%) case, tested positive
for all four tested CT antigens and 9 cases (32.1%) expressed three
CT antigens (Fig. 2 and Table IV). Among cases of medullary
carcinoma, the variables patient gender as well as patient clinical
stage exhibited a statistically significant association with the
expression of MAGE-C1/CT7 (P=0.029 and 0.031, respectively). GAGE
expression was observed in almost all cases, but there was no
statistically significant association with any of the variables
investigated. MAGE-A and MAGE-C1/CT7 were widely expressed, but
without statistical significance.
 | Table III.CT antigen expression in patients
with medullary carcinoma. |
Table III.
CT antigen expression in patients
with medullary carcinoma.
| P | Gender | Age, years | Size, cm | Tumor stage | Positive lymph
node | Metastasis | Stage grouping | Angiolymphatic
invasion | Extra-thyroidal
extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|
| 1 | F | 40 | 3.0 | T1N0M0 | No | No | I | No | No | 1+ | 1+ | f | – |
| 2 | F | 57 | 2.4 | T1N1bM0 | No | No | II | No | No | – | – | f | – |
| 3 | F | 38 | 2.7 | T1N1aM0 | No | No | II | No | No | f | f | f | – |
| 4 | F | 67 | 3.4 | T3N0M0 | Yes | Yes | III | No | No | 1+ | f | f | – |
| 5 | F | 67 | 2.5 | T1N0M0 | No | No | II | No | No | – | f | f | – |
| 6 | F | 40 | 3.0 | T2N1bM1 | No | No | I | Yes | Yes | f | – | f | – |
| 7 | M | 56 | 1.5 | T1N0M0 | No | No | I | No | No | f | f | f | – |
| 8 | M | 54 | 0.5 | T2N1bM0 | Yes | No | IV | No | No | f | f | f | – |
| 9 | M | 53 | 1.2 | T2N0M0 | Yes | No | III | Yes | No | f | f | f | f |
| 10 | F | 48 | 4.2 | T1N0M0 | No | No | III | No | No | f | f | f | – |
| 11 | F | 42 | 1.0 | T1N0M0 | No | No | I | No | No | 1+ | – | f | – |
| 12 | M | 65 | 3.0 | T4bN0M1 | Yes | Yes | IV | Yes | No | – | f | f | – |
| 13 | F | 17 | 0.5 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 14 | F | 50 | 2.0 | T1N1bM0 | Yes | No | IV | Yes | No | – | – | f | – |
| 15 | F | 33 | 2.3 | T1N0M0 | No | No | II | No | No | – | – | – | – |
| 16 | F | 60 | 1.7 | T1N0M0 | No | No | I | No | Yes | – | – | f | – |
| 17 | F | 63 | 1.3 | T2N1aM0 | No | No | I | No | No | – | – | f | – |
| 18 | M | 29 | 1.0 | T2N1bM0 | No | Yes | IV | Yes | Yes | 1+ | f | f | – |
| 19 | F | 77 | 0.6 | T2N1aM0 | No | No | I | No | No | – | – | f | – |
| 20 | M | 14 | 1.2 | T2N0M0 | Yes | No | IV | Yes | Yes | f | f | f | – |
| 21 | M | 53 | 0.5 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 22 | M | 35 | 0.6 | T2N0M0 | No | No | I | No | No | – | – | f | – |
| 23 | F | 31 | 2.5 | T1N0M0 | Yes | No | III | Yes | No | f | – | f | – |
| 24 | F | 25 | 2.5 | T1N1bM0 | Yes | No | IV | Yes | No | – | f | f | – |
| 25 | F | 49 | 3.2 | T1N1aM0 | Yes | No | III | Yes | Yes | – | – | f | – |
| 26 | F | 60 | 2.8 | T3N0M0 | No | No | II | No | No | – | f | f | – |
| 27 | M | 55 | 1.4 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 28 | F | 54 | 2.0 | T2N1bM1 | No | No | II | No | No | – | – | f | – |
 | Table IV.Summary of CT antigen expression
patients with medullary carcinoma. |
Table IV.
Summary of CT antigen expression
patients with medullary carcinoma.
| Antigen | Expression, % |
|---|
| Multi MAGE-A | 42.9 |
| CT7 (MAGE-C1) | 46.4 |
| GAGE | 92.9 |
| CTAG1B |
3.6 |
Poorly differentiated carcinomas,
clinical and immunostaining variables
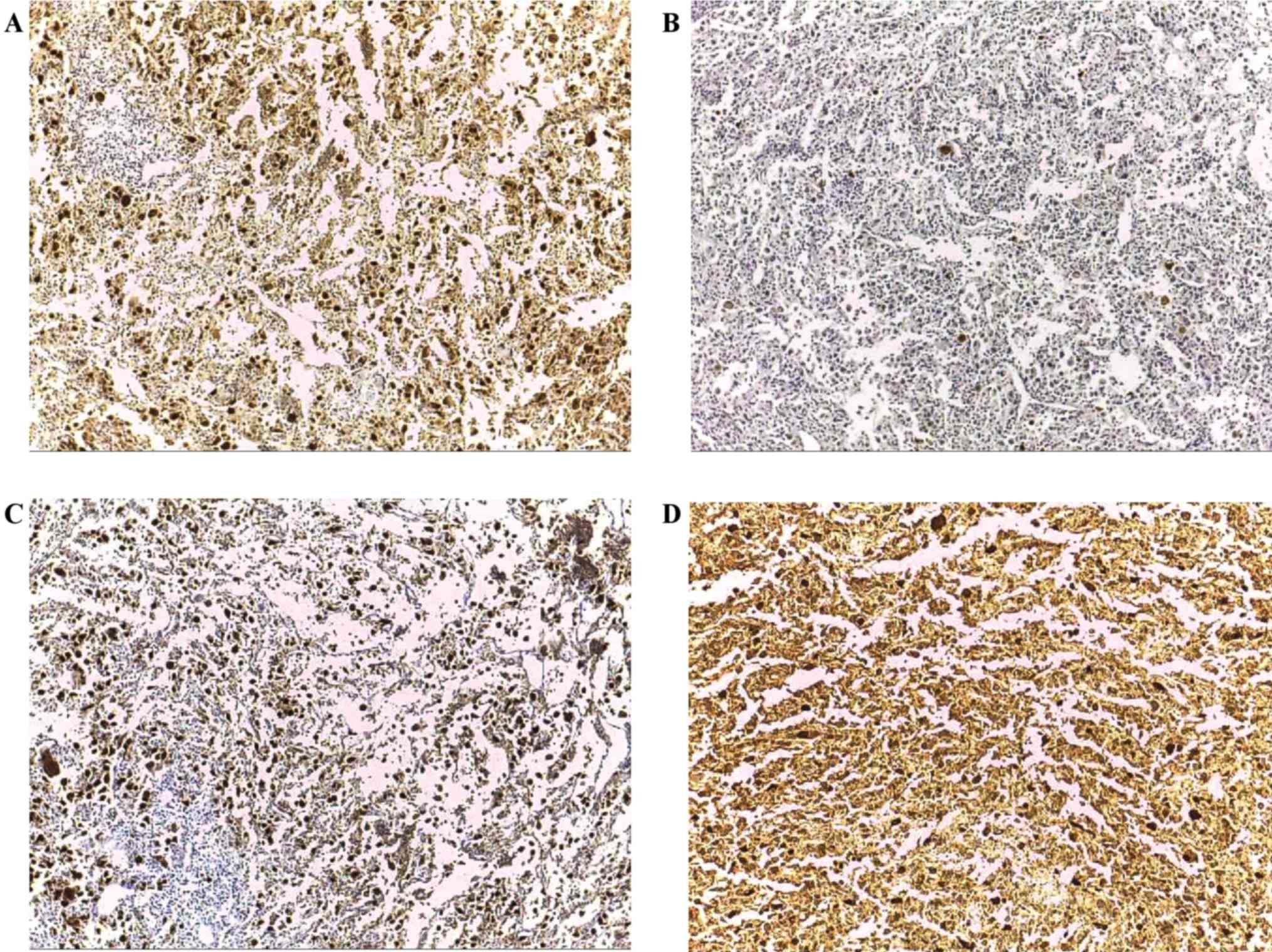
Clinical and protein expression data for the 21
cases of poorly differentiated and anaplastic carcinoma are
summarized in Table V. There were 10
women and 11 men (1.0:1.1). The mean age of patients with this
disease was 65.3 years, with a SD of 11.4 years and a median of 65
years. The mean tumor size was 2.7±1.49 cm (0.8–7.0 cm). Among the
tested CT antigens, GAGE demonstrated the highest incidence (14/21;
66.7%), which was similar to the incidence of MAGE-A (13/21; 61.9%)
and MAGE-C1/CT7 (12/21; 57.1%); 19/21 (90.5%) tumors expressed ≥1
CT antigen (Fig. 3 and Table VI). Notably, there were 3 cases that
were positive for all four tested CT antigens and 5 cases expressed
three CT antigens. Among the cases of poorly differentiated and
anaplastic carcinomas, there was an association between GAGE
expression and gender (P=0.043). An increased expression of MAGE-A
and MAGE-C1/CT7 in all variables was observed, but the difference
was not significant.
 | Table V.CT antigen expression in patients
with poorly differentiated and anaplastic carcinomas. |
Table V.
CT antigen expression in patients
with poorly differentiated and anaplastic carcinomas.
| P | Gender | Age | Disease | Size (cm) | Tumor stage | Positive lymph
node | Metastasis | Angiolymphatic
invasion | Extra-thyroidal
extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|
| 1 | M | 61 | anaplastic | 2.0 | T4aN0M0 | No | No | No | No | f | f | f | – |
| 2 | M | 73 | anaplastic | 1.5 | T4aN0M0 | No | No | No | No | f | f | – | – |
| 3 | F | 82 | anaplastic | 0.8 | T4bN0M0 | No | No | Yes | Yes | 4+ | f | f | – |
| 4 | F | 83 | anaplastic | 4.7 | T4bN0M0 | No | No | Yes | Yes | – | – | – | – |
| 5 | M | 53 | anaplastic | 1.2 | T4bN0M0 | No | No | No | Yes | 1+ | f | – | – |
| 6 | F | 70 | anaplastic | 2.5 | T4aN1bM0 | Yes | No | Yes | Yes | f | 1+ | 2+ | – |
| 7 | F | 61 | anaplastic | 1.5 | T4bN0M0 | No | Yes | Yes | Yes | 4+ | f | f | f |
| 8 | F | 59 | anaplastic | 2.5 | T4aN0M0 | No | No | No | No | – | – | f | – |
| 9 | M | 59 | anaplastic | 4.0 | T4aN0M0 | No | Yes | No | No | – | – | f | – |
| 10 | M | 57 | anaplastic | 2.2 | T4aN1bM0 | Yes | No | Yes | No | – | f | f | – |
| 11 | M | 70 | anaplastic | 3.5 | T4aN0M0 | No | No | No | No | 4+ | – | – | – |
| 12 | M | 79 | anaplastic | 4.0 | T4aN0M0 | No | No | No | No | – | – | f | – |
| 13 | F | 78 | anaplastic | 7.0 | T4bN1bM1 | Yes | Yes | Yes | Yes | – | – | 3+ | – |
| 14 | F | 74 | p.
differentiated | 3.2 | T4aN0M0 | No | No | No | No | f | f | f | – |
| 15 | M | 44 | p.
differentiated | 1.8 | T4bN0M0 | No | No | No | Yes | 1+ | f | f | – |
| 16 | F | 57 | p.
differentiated | 2.4 | T4bN1bM0 | Yes | No | Yes | Yes | 4+ | f | f | 4+ |
| 17 | M | 80 | p.
differentiated | 1.2 | T4aN0M1 | No | Yes | No | No | – | – | – | – |
| 18 | M | 52 | p.
differentiated | 1.8 | T4aN0M0 | No | No | Yes | No | 4+ | – | – | – |
| 19 | M | 65 | p.
differentiated | 1.6 | T4aN0M0 | No | No | No | No | 4+ | – | – | – |
| 20 | F | 66 | p.
differentiated | 4.3 | T4aN0M0 | No | No | No | No | – | f | f | – |
| 21 | F | 50 | p.
differentiated | 3.0 | T4aN1bM0 | Yes | No | Yes | Yes | 4+ | f | f | 3+ |
 | Table VI.Summary of CT antigen expression in
patients with poorly differentiated and anaplastic carcinomas. |
Table VI.
Summary of CT antigen expression in
patients with poorly differentiated and anaplastic carcinomas.
| Antigen | Expression (%) |
|---|
| Multi MAGE-A | 61.9 |
| MAGE-C1 | 57.1 |
| GAGE | 66.7 |
| CTAG1B | 14.3 |
Patients were followed from the day of surgery
(stipulated as the start of follow-up) until June 2012; the average
follow-up period was 73.8 months (range, 1–168 months). The
analysis of evolution and survival was assessed in four steps: i)
patients who did not express any CT antigens vs. those who
expressed 1 CT antigen; ii) patients who did not express any CT
antigens vs. those who expressed 2 CT antigens; iii) patients who
did not express any CT antigens vs. those who expressed 3 CT
antigens; and iv) patients who did not express any CT antigen vs.
those who expressed 4 CT antigens. Furthermore, the association
between the extent of immunopositive areas based on the
immunohistochemical grading for each CT antigen and clinical data
was assessed. However, there was no association between the extent
of protein expression for any of the tested CT antigens and
clinical variables.
During the follow-up period, regional recurrence
occurred in 3 cases of papillary carcinoma, 1 case of follicular
carcinoma and 2 cases of medullary carcinoma. Distant metastasis
was identified in 4 cases of papillary carcinoma, 3 cases of
medullary carcinoma, 1 case of poorly differentiated carcinoma and
3 cases of anaplastic carcinoma. Regarding patient mortality, 2
patients with papillary carcinoma, 3 patients with medullary
carcinoma and all 21 patients with poorly differentiated and
anaplastic carcinoma succumbed during the follow-up period.
A statistically significant association between
clinical variables including recurrence, metastases or survival and
the presence of any CT antigen, including co-expression was not
identified. This lack of association was observed for all samples
studied.
Discussion
The present study aimed to characterize the
expression of various CT antigens in thyroid neoplasms. Though
numerous studies have been performed to investigate the presence of
CT antigens in a wide variety of malignant tumors, little is known
about the expression of these antigens in thyroid tumors,
particularly about any associations with clinical parameters. In
the present study, the antibodies selected were previously
generated by our group or by collaborators, the majority of which
are now available commercially and have been used in a wide variety
of studies (18,19,25,28,35).
Only the antibody to GAGE antigens was a commercial product, which
has been employed in several previous studies (29,36,37). To
detect MAGE-A, a cocktail of several antibodies was used, thus
covering a wide spectrum of MAGE-A antigens. As in previous
studies, the lack of specificity and the ability to identify single
MAGE-A antigens was intended to ensure the detection of any
low-level MAGE-A expression in the present tumor series (34,38).
The current study confirms the cancer-restricted
expression of classical CT antigens in the thyroid. No expression
of any of the CT antigens was detected in normal thyroid tissue or
benign lesions. As with tumors in other organs, this has important
implications since the expression of any CT antigens in thyroid
tissue would indicate malignancy. Though malignancy-associated
expression has been demonstrated in a number of neoplasms, the
diagnostic potential of CT antigens as immunohistochemical markers
of malignancy has yet to be exploited by pathologists (21,39,40).
The most striking finding of the current study is
the apparent dichotomy of high and low CT antigen-expressing
thyroid cancer. Extremely low expression of all tested antigens in
papillary and follicular carcinoma was observed. In this group,
GAGE and MAGE-A were the most prevalent and present in ~11 and 8%
of cases respectively. Expression of MAGE-C1/CT7 and CTAG1B was
even lower. This level of expression is similar to other tumors
that express low levels of CT antigens, such as colon carcinoma,
renal cell carcinoma and lymphoma (28,25,41). The
results of the current study are supported by Melo et al
(42), who identified a lack of
expression of MAGE-A and MAGE-C1/CT7 in a series of papillary and
follicular thyroid carcinomas. Since an association of CT antigens
with the biology of tumor stem cells was being assessed and due to
the scarcity of potential stem cells within tumor tissue, a
threshold level was not set and any number of immunostained tumor
cells was regarded as positive in previous studies and the present
study (43,44). The majority of positive cases
demonstrated focal expression (expression in <5% of tumor cells)
only, which may explain the slightly higher number of positive
cases in the current study. The current study and the previous
study by Melo et al (42)
identified low CT antigen levels in papillary and follicular
carcinoma, which contrasts with results from two previous studies
detecting a much higher level of MAGE-A expression of up to 80% in
the two tumor types (21,39), despite employing the same antibodies
used in the current study. There is no clear explanation for these
major discrepancies, except perhaps geographical differences in the
patient population. However, it is unlikely that ethnic differences
are the reason for such large differences in CT antigen
expression.
Cheng et al (39) demonstrated CT antigen expression in
healthy tissue, a feature not consistent with the present study and
numerous previous analyses of the expression of classical CT
antigens, including in the thyroid (15). Milkovic et al reported
extremely high MAGE-A expression in thyroid tumors exceeding
measurements of MAGE-A expression in any other study of epithelial
cancer to date (21). However, each
study employed high antibody concentrations, and figures provided
in the studies suggest unspecific immunoreactivity.
The low expression of CT antigens in papillary and
follicular neoplasms contrasts with the high expression detected in
medullary and anaplastic/poorly differentiated thyroid carcinomas.
The highest prevalence was observed for GAGE, which was present in
~90% of all medullary carcinomas analyzed. To the best of our
knowledge, no previous analyses of GAGE antigens have demonstrated
a similar high expression on a protein level (22,29,45). The
present study used a commercial reagent used in several previous
studies and was generated to a consensus region of the GAGE-family.
Consequently, it cannot be determined if a particular GAGE antigen
was the most prevalent. Notably, all GAGE-positive medullary
carcinomas exhibited exclusively focal immunopositivity,
occasionally comprising only a single positive tumor cell. The same
predominant focal expression pattern was present for the other
antigens in the majority of the tested medullary tumors. GAGE was
again the most prevalent antigen in anaplastic/poorly different
carcinomas and its expression pattern was mostly focal.
Immunohistochemistry has demonstrated that the majority of CTs are
focally expression, meaning that tumor cells are heterogeneous
(18). There are a number of studies
demonstrating that immune targets may include surface or
cytoplasmic antigens, which are different in tumor cells and normal
cells (46). Previous studies have
demonstrated that the same CT antigen may be expressed in different
subcellular compartments, nuclear and/or cytoplasmic, of tumor
cells and this pattern of expression has been observed regarding
MAGE, CTAG1B and MAGE-C1/CT7 (24,29,31).
Furthermore, patients with plasma cell myeloma and only cytoplasmic
MAGE-C1/CT7 expression had a better prognosis than patients with
nuclear or combined nuclear and cytoplasmic MAGE-C1/CT7 expression
(47). The high expression of
MAGE-C1/CT7 and MAGE-A in medullary and anaplastic/poorly
differentiated carcinomas was in the range of what has been
reported in other malignant tumors (23,25,48).
Notably, CTAG1B exhibited the lowest expression of all tested CT
antigens in medullary as well as anaplastic/poorly differentiated
tumors. This matches the previous expression pattern in epithelial
tumors, where CTAG1B is among the lowest expressed CT antigens
(34,36,38). Its
low incidence of expression in numerous tumors is associated with
high immunogenicity, as CTAG1B is the most immunogenic CT antigen
in various types of cancer (30,40). The
reverse pattern is observed in CT antigens, including MAGE-A
antigens that exhibit high expression but low immunogenicity in
several tumor types (49,50). Unfortunately, there was no serum
available to test for immunity in the present tumor series.
However, a protocol has been initiated that will allow for the
collection of tissue specimens as well as peripheral blood in
patients with thyroid tumors.
Notably, no association between CT antigen
expression and the major clinical parameters was observed. The
current study did identify two associations: One between the
cytoplasmic expression of MAGE-A and the number of lymph node
metastasis, and one between gender and the presence of MAGE-C1/CT7
or GAGE. However in the current study there was no association
between CT antigen immunostaining and recurrence, metastasis or
mortality. One possible reason could be the good overall prognosis
of papillary/follicular carcinomas and the extremely low expression
of CT antigens in these types of tumors. By contrast, there was
high expression of particular CT antigens in medullary and
anaplastic/poorly differentiated carcinomas and GAGE was present in
almost all tumors. However, the survival time of patients with
medullary and anaplastic/poorly differentiated tumors is extremely
short and the sample size of the current study may have been too
small to identify any associations between clinicopathological
parameters and the presence of CT antigens.
In conclusion, the present study identifies a
distinct expression pattern of CT antigens in malignant thyroid
tumors. The expression of CT antigens is low in papillary and
follicular carcinoma, whereas in medullary and anaplastic/poorly
differentiated carcinomas the expression of particular CT antigens
is extremely high, with GAGE being the most prevalent. A GAGE
commercial reagent clone is commercially available, which means
that GAGE proteins could be used to predict cancer prognosis; high
GAGE expression is correlated with poor prognosis in stomach
cancer, esophageal carcinoma and neuroblastoma (32). However, this correlation between GAGE
expression and clinical characteristics is controversial, since it
has not been identified in a previous study (29). Thus, the reliability of commercial
GAGE monoclonal antibody as a prognostic marker is unclear and
additional studies are required. Though the current study did not
identify an association with clinical parameters in the individual
patient, the prevalence of CT antigens in high-grade carcinomas
suggests a biological role within the more malignant tumor
entities.
Acknowledgment
This study was supported by São Paulo Research
Foundation (FAPESP) (grant nos. 2012/00588-5 and 13/08135-2).
Glossary
Abbreviations
Abbreviations:
|
CT antigens
|
cancer/testis antigens
|
|
MAGE-A
|
melanoma associated-antigen A
|
|
MAGE-C1/CT7
|
melanoma-associated antigen C1
|
|
CTAG1B
|
cancer/testis antigen 1B
|
|
GAGE
|
G antigen
|
|
mAb
|
monoclonal antibody
|
References
|
1
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI and
Tuttle RM: American Thyroid Association Guidelines Taskforce:
Management guidelines for patients with thyroid nodules and
differentiated thyroid cancer. Thyroid. 16:109–142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, ; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American thyroid association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan GH and Gharib H: Thyroid
incidentalomas: Management approaches to nonpalpable nodules
discovered incidentally on thyroid imaging. Ann Intern Med.
126:226–231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singer PA, Cooper DS, Daniels GH, Ladenson
PW, Greenspan FS, Levy EG, Braverman LE, Clark OH, McDougall IR,
Ain KV and Dorfman SG: Treatment guidelines for patients with
thyroid nodules and well-differentiated thyroid cancer. American
thyroid association. Arch Intern Med. 156:2165–2172. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito Y, Kudo T, Kobayashi K, Miya A,
Ichihara K and Miyauchi A: Prognostic factors for recurrence of
papillary thyroid carcinoma in the lymph nodes, lung and bone:
Analysis of 5,768 patients with average 10-year follow-up. World J
Surg. 36:1274–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang IC, Chou FF, Liu RT, Tung SC, Chen
JF, Kuo MC, Hsieh CJ and Wang PW: Long-term outcomes of distant
metastasis from differentiated thyroid carcinoma. Clin Endocrinol
(Oxf). 76:439–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konturek A, Barczyński M, Nowak W and
Richter P: Prognostic factors in differentiated thyroid cancer-a
20-year surgical outcome study. Langenbecks Arch Surg. 397:809–815.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung CC, Carydis B, Ezzat S, Bedard YC
and Asa SL: Analysis of ret/PTC gene rearrangements refines the
fine needle aspiration diagnosis of thyroid cancer. J Clin
Endocrinol Metab. 86:2187–2190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pizzolanti G, Russo L, Richiusa P, Bronte
V, Nuara RB, Rodolico V, Amato MC, Smeraldi L, Sisto PS, Nucera M,
et al: Fine-needle aspiration molecular analysis for the diagnosis
of papillary thyroid carcinoma through BRAF V600E mutation and
RET/PTC rearrangement. Thyroid. 17:1109–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faggiano A, Caillou B, Lacroix L, Talbot
M, Filetti S, Bidart JM and Schlumberger M: Functional
characterization of human thyroid tissue with immunohistochemistry.
Thyroid. 17:203–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beatty PL, Cascio S and Lutz E: Tumor
immunology: Basic and clinical advances. Cancer Res. 71:4338–4343.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boon T and Old LJ: Cancer tumor antigens.
Curr Opin Immunol. 9:681–683. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
15
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodey B: Cancer-testis antigens: Promising
targets for antigen directed antineoplastic immunotherapy. Expert
Opin Biol Ther. 2:577–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fratta E, Coral S, Covre A, Parisi G,
Colizzi F, Danielli R, Nicolay HJ, Sigalotti L and Maio M: The
biology of cancer testis antigens: Putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jungbluth AA, Stockert E, Chen YT, Kolb D,
Iversen K, Coplan K, Williamson B, Altorki N, Busam KJ and Old LJ:
Monoclonal antibody MA454 reveals a heterogeneous expression
pattern of MAGE-1 antigen in formalin-fixed paraffin embedded lung
tumours. Br J Cancer. 83:493–497. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landry C, Brasseur F, Spagnoli GC, Marbaix
E, Boon T, Coulie P and Godelaine D: Monoclonal antibody 57B stains
tumor tissues that express gene MAGE-A4. Int J Cancer. 86:835–841.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rimoldi D, Salvi S, Schultz-Thater E,
Spagnoli GC and Cerottini JC: Anti-MAGE-3 antibody 57B and
anti-MAGE-1 antibody 6C1 can be used to study different proteins of
the MAGE-A family. Int J Cancer. 86:749–751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Milkovic M, Sarcevic B and Glavan E:
Expression of MAGE tumor-associated antigen in thyroid carcinomas.
Endocr Pathol. 17:45–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inaoka RJ, Jungbluth AA, Baiocchi OC,
Assis MC, Hanson NC, Frosina D, Tassello J, Bortoluzzo AB, Alves AC
and Colleoni GW: An overview of cancer/testis antigens expression
in classical Hodgkin's lymphoma (cHL) identifies MAGE-A family and
MAGE-C1 as the most frequently expressed antigens in a set of
Brazilian cHL patients. BMC Cancer. 11:4162011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jungbluth AA, Busam KJ, Kolb D, Iversen K,
Coplan K, Chen YT, Spagnoli GC and Old LJ: Expression of
MAGE-antigens in normal tissues and cancer. Int J Cancer.
85:460–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krishnadas DK, Shusterman S, Bai F, Diller
L, Sullivan JE, Cheerva AC, George RE and Lucas KG: A phase I trial
combining decitabine/dendritic cell vaccine targeting MAGE-A1,
MAGE-A3 and NY-ESO-1 for children with relapsed or
therapy-refractory neuroblastoma and sarcoma. Cancer Immunol
Immunother. 64:1251–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jungbluth AA, Chen YT, Busam KJ, Coplan K,
Kolb D, Iversen K, Williamson B, Van Landeghem FK, Stockert E and
Old LJ: CT7 (MAGE-C1) antigen expression in normal and neoplastic
tissues. Int J Cancer. 99:839–845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curioni-Fontecedro A, Knights AJ, Tinguely
M, Nuber N, Schneider C, Thomson CW, von Boehmer L, Bossart W,
Pahlich S, Gehring H, et al: MAGE-C1/CT7 is the dominant
cancer-testis antigen targeted by humoral immune responses in
patients with multiple myeloma. Leukemia. 22:1646–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Curioni-Fontecedro A, Nuber N,
Mihic-Probst D, Seifert B, Soldini D, Dummer R, Knuth A, van den
Broek M and Moch H: Expression of MAGE-C1/CT7 and MAGE-C2/CT10
predicts lymph node metastasis in melanoma patients. PLoS One.
6:e214182011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jungbluth AA, Chen YT, Stockert E, Busam
KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N and Old
LJ: Immunohistochemical analysis of NY-ESO-1 antigen expression in
normal and malignant human tissues. Int J Cancer. 92:856–860. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gjerstorff MF, Pøhl M, Olsen KE and Ditzel
HJ: Analysis of GAGE, NY-ESO-1 and SP17 cancer/testis antigen
expression in early stage non-small cell lung carcinoma. BMC
Cancer. 13:4662013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ademuyiwa FO, Bshara W, Attwood K,
Morrison C, Edge SB, Karpf AR, James SA, Ambrosone CB, O'Connor TL,
Levine EG, et al: NY-ESO-1 cancer testis antigen demonstrates high
immunogenicity in triple negative breast cancer. PLoS One.
7:e387832012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cilensek ZM, Yehiely F, Kular RK and Deiss
LP: A member of the GAGE family of tumor antigens is an
anti-apoptotic gene that confers resistance to Fas/CD95/APO-1,
interferon-gamma, taxol and gamma-irradiation. Cancer Biol Ther.
1:380–387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gnjatic S, Nishikawa H, Jungbluth AA, Güre
AO, Ritter G, Jäger E, Knuth A, Chen YT and Old LJ: NY-ESO-1:
Review of an immunogenic tumor antigen. Adv Cancer Res. 95:1–30.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Curigliano G, Viale G, Ghioni M, Jungbluth
AA, Bagnardi V, Spagnoli GC, Neville AM, Nolè F, Rotmensz N and
Goldhirsch A: Cancer-testis antigen expression in triple-negative
breast cancer. Ann Oncol. 22:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang R, Zhu Y, Fang L, Liu XS, Tian Y,
Chen LH, Ouyang WM, Xu XG, Jian JL, Güre AO, et al: Generation of
monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2.
Cancer Immun. 6:72006.PubMed/NCBI
|
|
36
|
Sharma P, Shen Y, Wen S, Bajorin DF,
Reuter VE, Old LJ and Jungbluth AA: Cancer-testis antigens:
Expression and correlation with survival in human urothelial
carcinoma. Clin Cancer Res. 12:5442–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jungbluth AA, Silva WA Jr, Iversen K,
Frosina D, Zaidi B, Coplan K, Eastlake-Wade SK, Castelli SB,
Spagnoli GC, Old LJ and Vogel M: Expression of cancer-testis (CT)
antigens in placenta. Cancer Immun. 7:152007.PubMed/NCBI
|
|
38
|
Grigoriadis A, Caballero OL, Hoek KS, da
Silva L, Chen YT, Shin SJ, Jungbluth AA, Miller LD, Clouston D,
Cebon J, et al: CT-X antigen expression in human breast cancer.
Proc Natl Acad Sci USA. 106:pp. 13493–13498. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng S, Liu W, Mercado M, Ezzat S and Asa
SL: Expression of the melanoma-associated antigen is associated
with progression of human thyroid cancer. Endocr Relat Cancer.
16:455–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noguchi T, Kato T, Wang L, Maeda Y, Ikeda
H, Sato E, Knuth A, Gnjatic S, Ritter G, Sakaguchi S, et al:
Intracellular tumor-associated antigens represent effective targets
for passive immunotherapy. Cancer Res. 72:1672–1682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Inaoka RJ, Jungbluth AA, Gnjatic S, Ritter
E, Hanson NC, Frosina D, Tassello J, Etto LY, Bortoluzzo AB, Alves
AC and Colleoni GW: Cancer/testis antigens expression and
autologous serological response in a set of Brazilian non-Hodgkin's
lymphoma patients. Cancer Immunol Immunother. 61:2207–2214. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Melo DH, Mamede RC, Neder L, Saggioro FP,
Figueiredo DL, da Silva WA Jr, Jungbluth AA and Zago MA: Expression
of MAGE-A4 and MAGE-C1 tumor-associated antigen in benign and
malignant thyroid diseases. Head Neck. 33:1426–1432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sigalotti L, Covre A, Nicolay HJ, Coral S
and Maio M: Cancer testis antigens and melanoma stem cells: New
promises for therapeutic intervention. Cancer Immunol Immunother.
59:487–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saldanha-Araujo F, Haddad R, Zanette DL,
De Araujo AG, Orellana MD, Covas DT, Zago MA and Panepucci RA:
Cancer/Testis antigen expression on mesenchymal stem cells isolated
from different tissues. Anticancer Res. 30:5023–5017.
2010.PubMed/NCBI
|
|
45
|
Chen YT, Ross DS, Chiu R, Zhou XK, Chen
YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O and Neville AM:
Multiple cancer/testis antigens are preferentially expressed in
hormone-receptor negative and high-grade breast cancers. PLoS One.
6:e178762011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gunda V, Frederick DT, Bernasconi MJ,
Wargo JA and Parangi S: A potential role for immunotherapy in
thyroid cancer by enhancing NY-ESO-1 cancer antigen expression.
Thyroid. 24:1241–1250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tinguely M, Jenni B, Knights A, Lopes B,
Korol D, Rousson V, Fontecedro A Curioni, Cogliatti SB, Bittermann
AG, Schmid U, et al: MAGE-C1/CT-7 expression in plasma cell
myeloma: Sub-cellular localization impacts on clinical outcome.
Cancer Sci. 99:720–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Barrow C, Browning J, MacGregor D, Davis
ID, Sturrock S, Jungbluth AA and Cebon J: Tumor antigen expression
in melanoma varies according to antigen and stage. Clin Cancer Res.
12:764–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Groeper C, Gambazzi F, Zajac P, Bubendorf
L, Adamina M, Rosenthal R, Zerkowski HR, Heberer M and Spagnoli GC:
Cancer/testis antigen expression and specific cytotoxic T
lymphocyte responses in non small cell lung cancer. Int J Cancer.
120:337–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Atanackovic D, Arfsten J, Cao Y, Gnjatic
S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C,
Dierlamm J, et al: Cancer-testis antigens are commonly expressed in
multiple myeloma and induce systemic immunity following allogeneic
stem cell transplantation. Blood. 109:1103–1112. 2007. View Article : Google Scholar : PubMed/NCBI
|