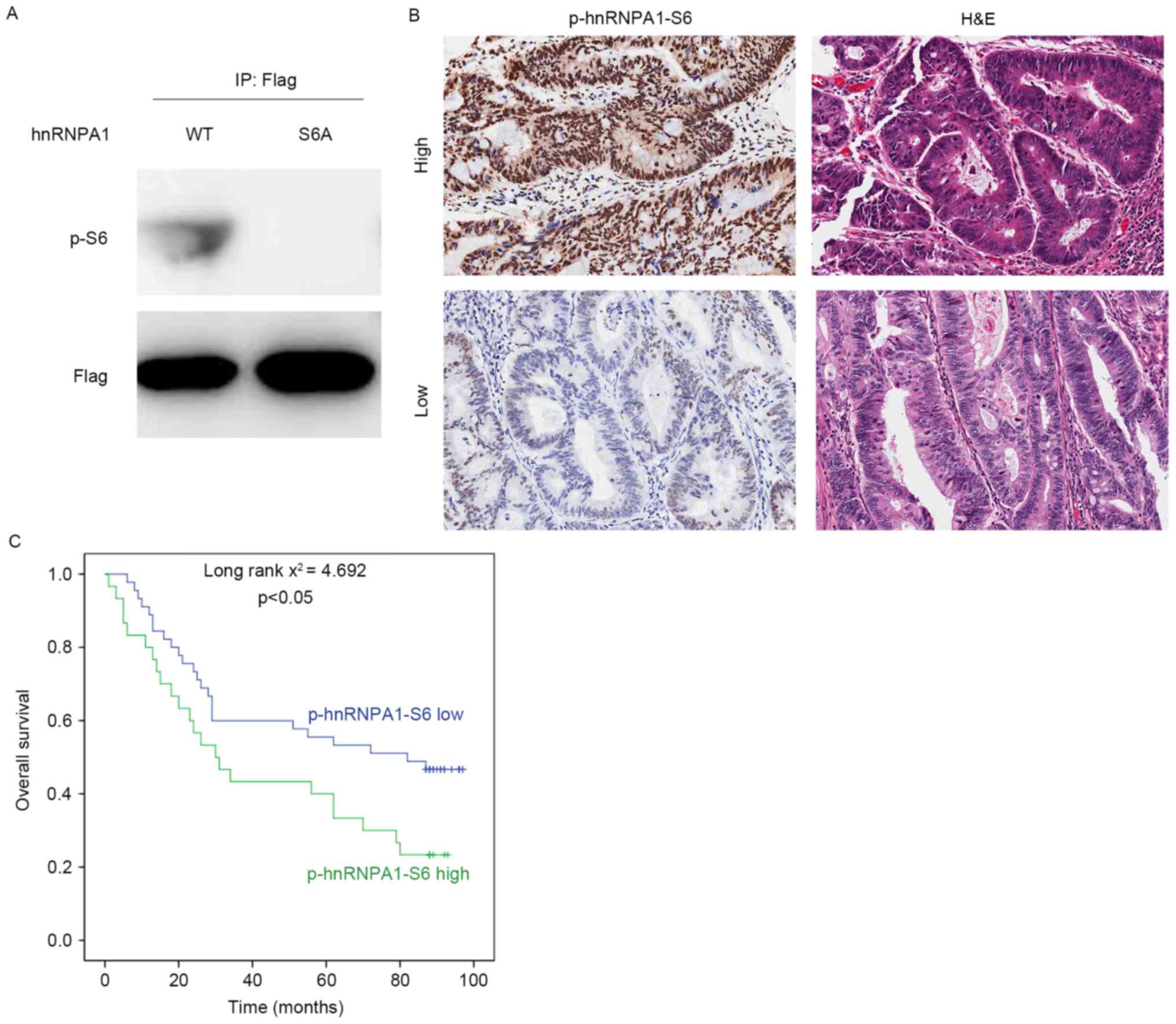
|
1
|
Tange TO, Damgaard CK, Guth S, Valcárcel J
and Kjems J: The hnRNP A1 protein regulates HIV-1 tat splicing via
a novel intron silencer element. EMBO J. 20:5748–5758. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang W, Xia Y, Hawke D, Li X, Liang J,
Xing D, Aldape K, Hunter T, Yung Alfred WK and Lu Z: PKM2
phosphorylates histone h3 and promotes gene transcription and
tumorigenesis. Cell. 150:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calabretta S, Bielli P, Passacantilli I,
Pilozzi E, Fendrich V, Capurso G, Fave GD and Sette C: Modulation
of PKM alternative splicing by PTBP1 promotes gemcitabine
resistance in pancreatic cancer cells. Oncogene. 35:2031–2039.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Zhao X, Zhou Y and Hu Y: miR-124,
miR-137 and miR-340 regulate colorectal cancer growth via
inhibition of the Warburg effect. Oncol Rep. 28:1346–1352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pearce LR, Komander D and Alessi DR: The
nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol.
11:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magnuson B, Ekim B and Fingar DC:
Regulation and function of ribosomal protein S6 kinase (S6K) within
mTOR signalling networks. Biochem J. 441:1–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardo OE and Seckl MJ: S6K2: The neglected
s6 kinase family member. Front Oncol. 3:1912013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Savinska LO, Lyzogubov VV, Usenko VS,
Ovcharenko GV, Gorbenko ON, Rodnin MV, Vudmaska MI, Pogribniy PV,
Kyyamova RG, Panasyuk GG, et al: Immunohistochemical analysis of
S6K1 and S6K2 expression in human breast tumors. Eksp Onkol.
26:24–30. 2004.PubMed/NCBI
|
|
17
|
Lyzogubov VV, Lytvyn DI, Dudchenko TM,
Lubchenko NV, Pogrybniy PV, Nespryadko SV, Vinnitska AB, Usenko VS,
Gout IT and Filonenko VV: Immunohistochemical analysis of S6K1 and
S6K2 expression in endometrial adenocarcinomas. Exp Oncol.
26:287–293. 2004.PubMed/NCBI
|
|
18
|
Pardo OE, Wellbrock C, Khanzada UK, Aubert
M, Arozarena I, Davidson S, Bowen F, Parker PJ, Filonenko VV, Gout
IT, et al: FGF-2 protects small cell lung cancer cells from
apoptosis through a complex involving PKCepsilon, B-Raf and S6K2.
EMBO J. 25:3078–3088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roy R, Durie D, Li H, Liu BQ, Skehel JM,
Mauri F, Cuorvo LV, Barbareschi M, Guo L, Holcik M, et al: hnRNPA1
couples nuclear export and translation of specific mRNAs downstream
of FGF-2/S6K2 signalling. Nucleic Acids Res. 42:12483–12497. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, He W, Luo M, Zhou Y, Chang G, Ren
W, Wu K, Li X, Shen J, Zhao X and Hu Y: SREBP1 regulates
tumorigenesis and prognosis of pancreatic cancer through targeting
lipid metabolism. Tumour Biol. 36:4133–4141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Zhao X, Yao Y, Qi X, Yuan Y and Hu
Y: Connexin 43 interacts with Bax to regulate apoptosis of
pancreatic cancer through a gap junction-independent pathway. Int J
Oncol. 41:941–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Zhao X, Luo M, Zhou Y, Ren W, Wu K,
Li X, Shen J and Hu Y: The pro-apoptotic role of the regulatory
feedback loop between miR-124 and PKM1/HNF4alpha in colorectal
cancer cells. Int J Mol Sci. 15:4318–4332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Jeon HY, Rigo F, Bennett CF and
Krainer AR: Manipulation of PK-M mutually exclusive alternative
splicing by antisense oligonucleotides. Open Biol. 2:1201332012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anastasiou D, Yu Y, Israelsen WJ, Jiang
JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, et al:
Pyruvate kinase M2 activators promote tetramer formation and
suppress tumorigenesis. Nat Chem Biol. 8:839–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang S and Fang X: Advances in glucose
metabolism research in colorectal cancer. Biomed Rep. 5:289–295.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vellinga TT, Borovski T, de Boer VC,
Fatrai S, van Schelven S, Trumpi K, Verheem A, Snoeren N, Emmink
BL, Koster J, et al: SIRT1/PGC1α-dependent increase in oxidative
phosphorylation supports chemotherapy resistance of colon cancer.
Clin Cancer Res. 21:2870–2879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even Warburg did not anticipate.
Cancer cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirschey MD, DeBerardinis RJ, Diehl AME,
Drew JE, Frezza C, Green MF, Jones LW, Ko YH, Le A, Lea MA, et al:
Dysregulated metabolism contributes to oncogenesis. Semin Cancer
Biol. 35 Suppl:S129–S150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou CF, Li XB, Sun H, Zhang B, Han YS,
Jiang Y, Zhuang QL, Fang J and Wu GH: Pyruvate kinase type M2 is
upregulated in colorectal cancer and promotes proliferation and
migration of colon cancer cells. IUBMB Life. 64:775–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong N, De Melo J and Tang D: PKM2, a
central point of regulation in cancer metabolism. Int J Cell Biol.
2013:2425132013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jean-Philippe J, Paz S and Caputi M: hnRNP
A1: The Swiss army knife of gene expression. Int J Mol Sci.
14:18999–19024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goina E, Skoko N and Pagani F: Binding of
DAZAP1 and hnRNPA1/A2 to an exonic splicing silencer in a natural
BRCA1 exon 18 mutant. Mol Cell Biol. 28:3850–3860. 2008. View Article : Google Scholar : PubMed/NCBI
|