Introduction
Gastric cancer is the third most common type of
cancer and one of the leading causes of cancer-associated mortality
in China (1,2). Patients with gastric cancer often
respond poorly to conventional chemotherapies, and, therefore, more
comprehensive therapy is required (3). In general, gastric cancer remains
difficult to cure, primarily due to gastric cancer cells possessing
high invasion and metastasis capability. Melanoma
differentiation-associated gene-7 (MDA-7), also termed interleukin
(IL)-24, is a member of the IL-10 gene family, and in vitro
and in vivo studies have indicated that MDA-7 overexpression
suppresses tumor growth and causes tumor cell apoptosis in several
types of human cancer, including mesothelioma, osteosarcoma,
melanoma, lung cancer, breast cancer, pancreatic cancer,
glioblastoma and prostate cancer (4–6). It is
known that MDA-7, a cytokine-tumor suppressor gene and the only
tumor suppressor gene in the IL-10 family, not only inhibits tumor
growth but also stimulates the immune system and has an antitumor
effect; these features render the MDA-7 gene a promising option for
the treatment of cancer (7).
Currently, basic studies regarding MDA-7/IL-24 in gastric cancer
have been limited; to the best of our knowledge, whether
MDA-7/IL-24 inhibits gastric cancer cell invasion and metastasis
and the potential underlying mechanisms of action have not been
reported.
The present study evaluated the effect of
MDA-7/IL-24 inhibition on the invasive and metastatic capability of
human gastric cancer AGS cells. Western blotting was used to detect
the expression of epithelial (E)-cadherin, cluster of
differentiation (CD)44, matrix metalloproteinase (MMP)-2 and MMP-9
proteins.
Materials and methods
Cell culture
AGS cells were purchased from the Chinese Academy of
Sciences (Shanghai Institute for Biological Science, Shanghai,
China) and were cultured in Ham's F12 medium (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C and 5% CO2.
Plasmid construction
Total RNA from AGS cells was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RNA
concentration was quantified by assessment of the optical density;
only RNA samples with an A260-A280 ratio between 1.8 and 2.0 were
used to obtain complementary DNA (cDNA). A total of 2 µg of each
RNA was reverse-transcribed into cDNA by using the first-strand
cDNA synthesis kit (Promega Corporation, Madison, WI, USA), and the
complete MDA-7 sequence was amplified from the coding region of the
gene (GenBank accession no. NM_001185156.1). The PCR cycling
conditions were as follows: 94°C for 2 min, 25 cycles of 94°C for
30 sec, 72°C for 30 sec and 56°C for 1 min, followed by a final
extension 72°C for 7 min. The forward and reverse primers used were
5′-ACGCGTCGACGCATGAATTTTCAACAGAGGCTG-3′ (SalI restriction
site underlined) and 5′-CCGCTCGAGGAGCTTGTAGAATTTCTGCATC-3′
(XhoI restriction site underlined), respectively. The
SalI and XhoI sites were cloned into the vector
pShuttle-IRES-hrGFP-1 (Stratagene; Agilent Technologies Inc., USA)
and verified by DNA sequencing. Subsequently, they underwent
homologous recombination with PmeI-linearized plasmid with
the BJ5183 strain backbone carrier pAdEasy-1 (Stratagene, Agilent
Technologies Inc.), and positive recombinants were identified using
the PacI restriction product, termed pAd-MDA-7.
Transfection of MDA-7/IL-24
Following PacI linearization, the recombinant
adenovirus vector pAd-MDA-7 and the empty vector were transfected
with the aid of Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) into a 293A cell line (low passage; Chinese
Academy of Sciences Cell Bank) and were transfected with the
intracellular adenovirus packages Ad-MDA-7 or Ad-Control (empty
negative control). The virus was repeatedly amplified in the 293A
cells, and virus titer was determined by the green fluorescent
protein counting method (8). AGS
cells were subsequently infected with Ad-MDA-7 or Ad-Control using
adenovirus (Stratagene; Agilent Technologies Inc.), and total
cellular protein was collected from the lysed cells after 48 h.
Western blotting was used to detect MDA-7 expression in 30 µg
protein. The primary antibody used was a fusion protein tag
antibody: Anti-FLAG M2 antibody (dilution, 1:500; cat. no. F3165;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and then incubated
with anti-Mouse IgG for 1.5 h at room temperature (dilution,
1:3,000; cat. no. A9044; Sigma-Aldrich; Merck KGaA). The experiment
was performed according to the Press Western breeze kit
(Invitrogen; Thermo Fisher Scientific, Inc.) protocol.
Matrigel Transwell invasion assay
AGS cells were infected with Ad-MDA-7 or Ad-Control.
After 6 h, the cells were harvested by centrifugation at 300 × g at
room temperature for 5 min, and resuspended in serum-free F12
medium, and then transferred to the upper chambers of a
Matrigel-coated Transwell system (25,000 cells/well); the bottom
chambers contained F12 medium with 20% FBS. The cells were
incubated for 24 and 48 h at 37°C, and then invaded to the bottom
surface of the membrane. The membranes were fixed in 4%
paraformaldehyde at room temperature for 30 min, stained with
hematoxylin at room temperature for 1 min and counted using a light
microscope (magnification, ×100); the relative cell number was
calculated. The average number of cells in four random
fields/membrane was used to calculate the relative cell number.
Wound healing assay
AGS cells were infected with Ad-MDA-7 or Ad-Control.
After 24 h, a pipette tip was used to scratch a straight line down
the middle of the cell monolayer. The cells were observed and
images were captured under a microscope at 72 h using light
microscope (magnification, ×100).
Western blot analysis
AGS cells were infected with Ad-MDA-7 or Ad-Control
over 48 h, harvested and lysed by adding ice-cold lysis buffer
containing 1 mM phenylmethylsulphone fluoride to extract the total
proteins. Protein concentrations were determined using the
bicinchoninic acid method. For western blot analysis, 30 µg protein
were separated by 12% SDS-PAGE (110 V, 1.5 h), and the membranes
were blotted by wet transfer (110 V, 1.5 h, 4°C) onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked in 5% non-fat milk solution
in TBS for 1 h at room temperature, and then incubated overnight at
4°C with primary antibodies against CD44 (cat. no. 3578),
epithelial (E-)cadherin (cat. no. 5296), MMP-2 (cat. no. 4022) and
MMP-9 (cat. no. 3852) (dilution for all, 1:1,000; all Cell
Signaling Technology, Inc., Danvers, MA, USA). The membranes were
washed with TBS-Tween (TBST), and then incubated for 1.5 h at room
temperature with a goat anti-Mouse IgG horseradish
peroxidase-conjugated secondary antibody (dilution, 1:1,000; cat.
no. 62-6520; Invitrogen; Thermo Fisher Scientific, Inc.). Following
washing with TBST, the membranes were exposed to X-ray film (1–15
min) to visualize the immunoreactive bands. Densitometric analysis
of specific bands was performed using Image Lab software version
5.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The quantity
of the target protein was calibrated with respect to β-actin, and
the control value and relative intensities were obtained.
Statistical analysis
The results are presented as the mean ± standard
error of the mean. The significance between experimental values was
determined using Student's paired t-tests; one-way analysis
of variance using the statistical software SPSS16.0 (SPSS, Inc.,
Chicago, IL, USA) was used to test differences in repeated measures
across experiments. P<0.05 was considered to indicate a
statistically significant difference. Values were analyzed using
the statistical package SPSS (version 16.0; SPSS, Inc., Chicago,
IL, USA).
Results
Ad-Control or Ad-MDA-7 infection of
AGS cells
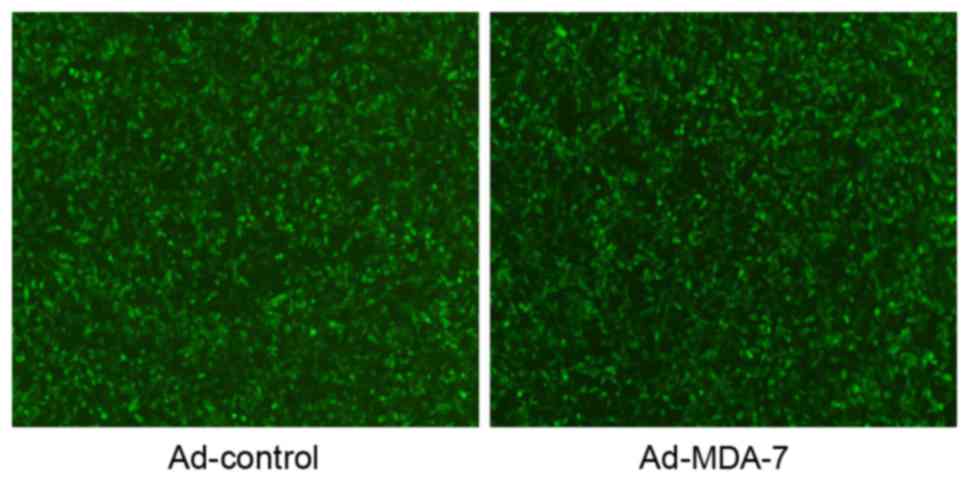
AGS cells infected with Ad-MDA7 or Ad-Control for 48
h were observed under a fluorescence microscope. In total >95%
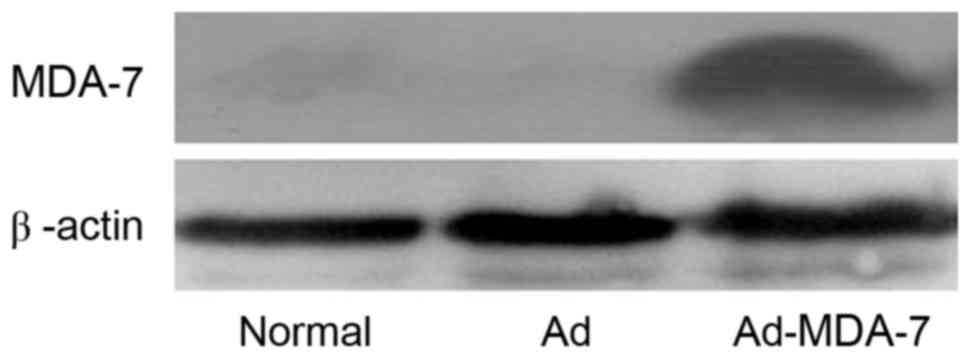
of cells emitted strong green fluorescence (Fig. 1). Western blotting using the anti-FLAG
M2 antibody revealed that cells infected with Ad-MDA-7 expressed
MDA-7 protein, whereas the cells infected with Ad-Control did not
(Fig. 2).
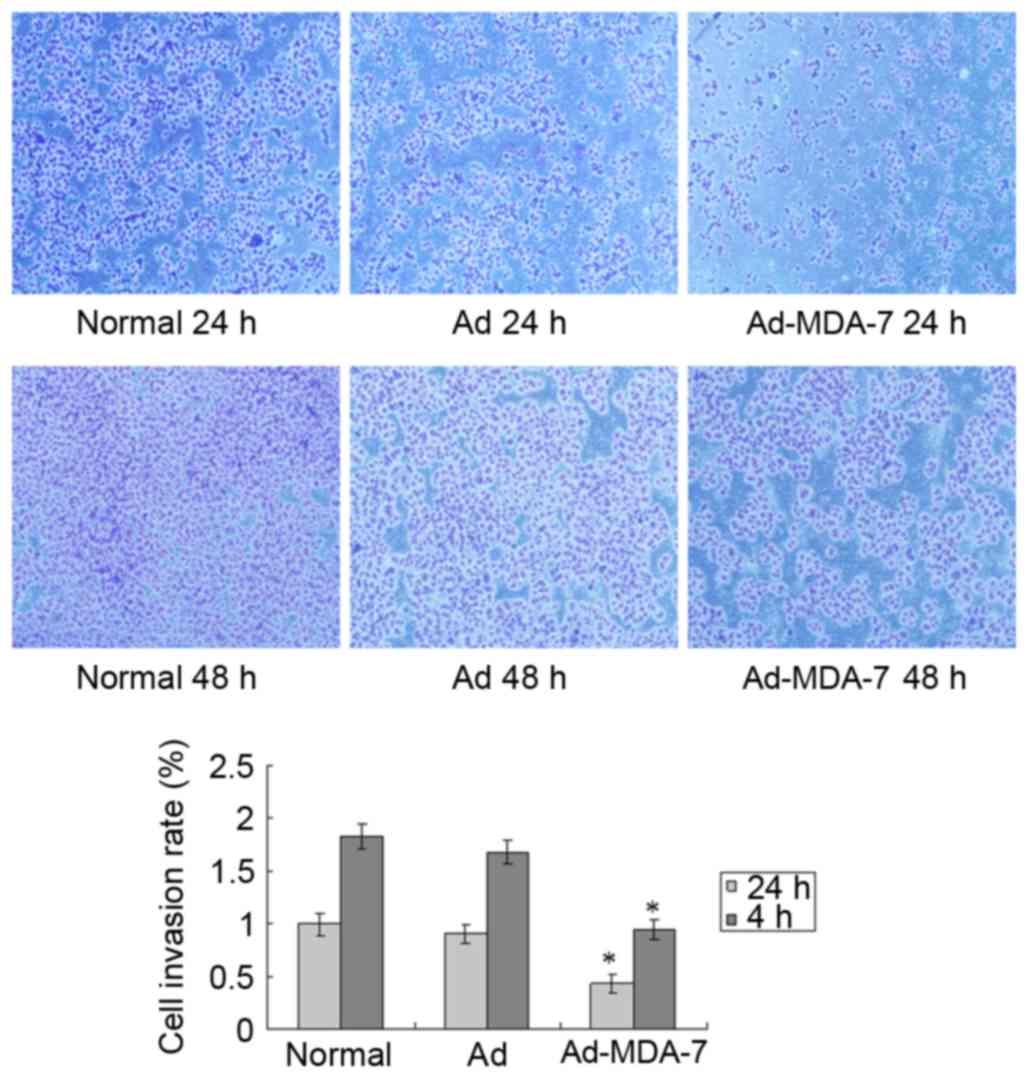
Ad-MDA-7 inhibits AGS cell invasion
and migration
The in vitro Transwell invasion assay
demonstrated that, after 24 and 48 h, there was significantly lower
invasion and metastasis by the AGS cells transfected with the MDA-7
gene in comparison with the normal cells and the Ad-Control group
(P<0.05; Fig. 3).
In the wound healing assay, gap closure after 48 h
by AGS cells transfected with the MDA-7 gene was markedly prolonged
as compared with the normal and Ad-Control groups (Fig. 4).
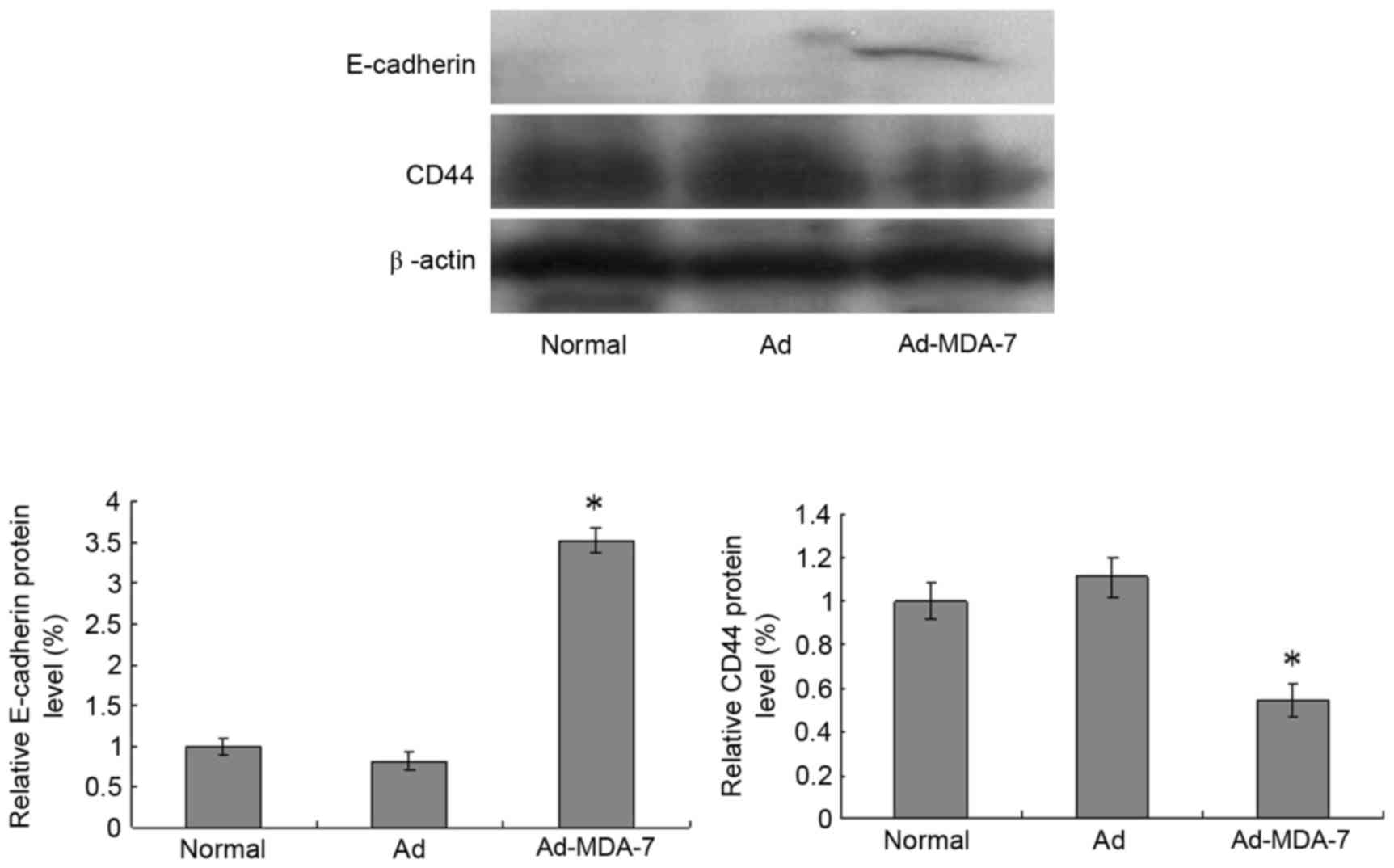
Ad-MDA-7 decreases the expression of
CD44 and E-cadherin proteins
To understand the underlying molecular mechanism of
MDA-7 inhibition on cell invasion and metastasis, CD44 and
E-cadherin expression was examined in the cells (Fig. 5). Compared with the normal and
Ad-Control groups, MDA-7 gene transfection decreased CD44 protein
expression (P<0.05) and increased E-cadherin protein expression
after 48 h (P<0.05).
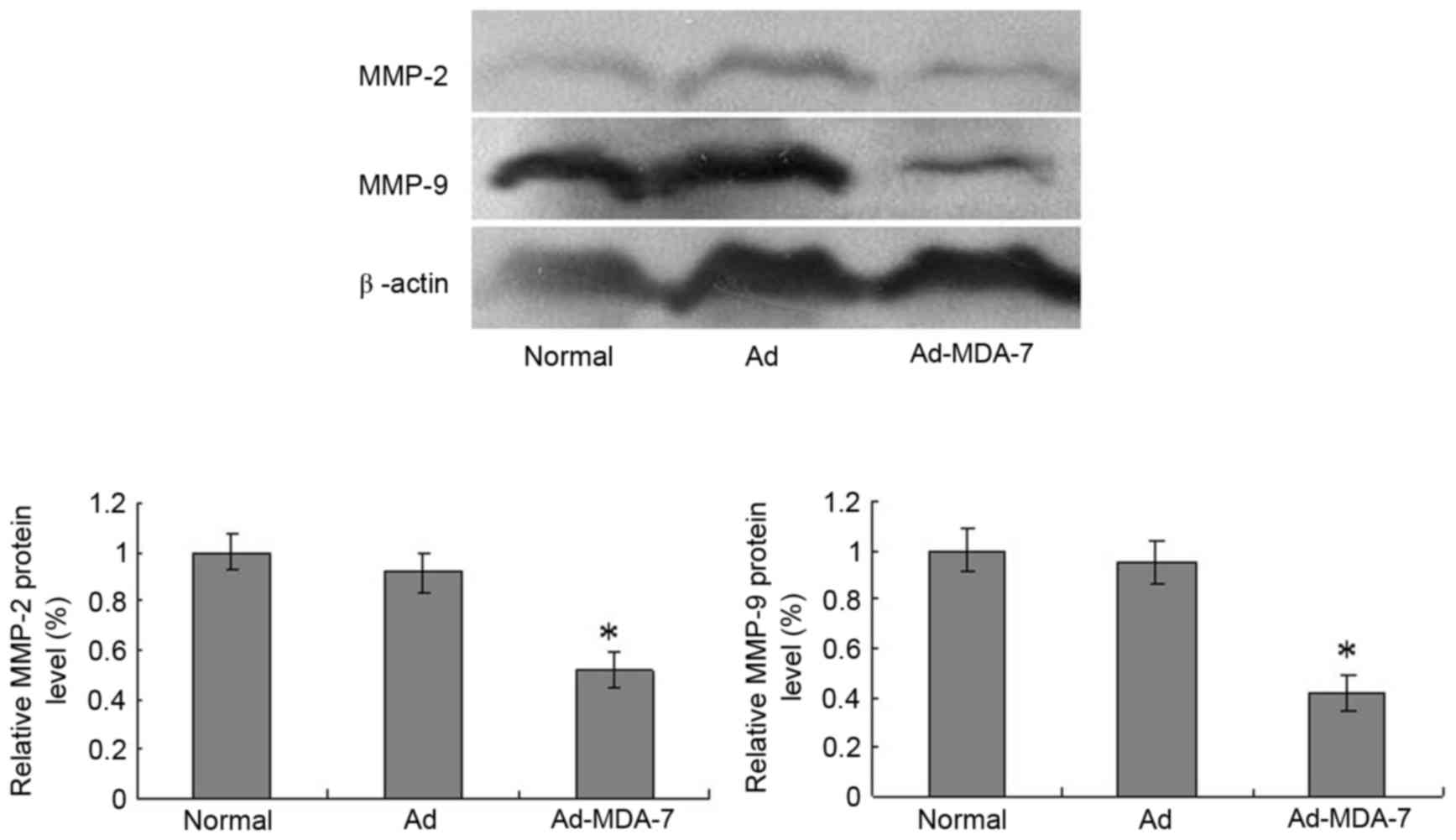
Ad-MDA-7 decreases the expression of
MMP-2 and MMP-9 proteins
Compared with the normal and Ad-Control groups,
MDA-7 gene transfection decreased MMP-2 and MMP-9 expression after
48 h (P<0.05; Fig. 6).
Discussion
Invasion and metastasis are the primary biological
characteristics of malignant tumors and are the primary causes of
surgical, radiotherapy and chemotherapy failure. Invasion and
metastasis are the most important causes of mortality in patients
with cancer (9). The incidence of
gastric cancer is high (10) and the
current means of treating gastric cancer in the clinic is surgery
post-operatively; however, its effect on progressive patients is
not ideal (11). Distal metastasis of
gastric cancer cells is the primary cause of mortality in patients
with gastric cancer; a biological characteristic of gastric cancer,
it is a major obstacle to long-term survival and to improving
prognosis (12).
Tumor metastasis involves multi-gene participation
and is a complex multi-stage evolution of the biological processes.
It was revealed that MDA-7 expression levels were negatively
associated with tumor cell proliferation (13). Jiang et al (14) reported that MDA-7 consists of melanoma
cells and megakaryocytes, and it was demonstrated that the gene
sequence and protein structure of MDA-7 have IL-10 homology domains
belonging to the IL-10 family (15).
MDA-7 inhibits the growth of a variety of tumor cells and induces
features of apoptosis, but does not affect normal cells. The
present study suggests that, as a novel tumor suppressor gene,
MDA-7 is capable of inhibiting tumor cell growth and angiogenesis,
and inducing apoptosis, while stimulating immune cell cytokine
expression (16–18). A number of studies have demonstrated
MDA-7 expression in vitro and in vivo in liver
cancer, lung cancer, pancreatic cancer, breast cancer and
esophageal cancer, where it significantly inhibited tumor growth
and induced apoptosis (19–22).
To study the effect of MDA-7 on invasion and
metastasis in gastric cancer, the MDA-7 gene was inserted into an
adenovirus vector-expressing recombinant adenovirus. The
recombinant adenovirus was used to infect gastric cancer AGS cells
to obtain increased expression of MDA-7. Matrigel, wound healing
and Transwell assays were used to investigate gastric cancer cell
adhesion, migration and invasion ability, respectively. The assays
demonstrated that MDA-7 inhibits gastric cancer cell invasion and
metastasis.
To investigate the underlying molecular mechanism,
the expression of E-cadherin, CD44, MMP-2 and MMP-9 proteins was
examined in gastric cancer cells transfected with MDA-7.
Tumor invasion and metastasis is a complex
multi-step process involving cancer cells shed from the primary
site and the degradation of basement membrane binding. Cancer cells
invade the blood vessels and lymphatic vessels, eventually
colonizing them, and the proliferation forms metastases. Tumor
cells shedding from the primary site, which is associated with
decreased cell adhesion, is the first step in metastasis.
Therefore, mediating cell-cell adhesion of the calcium-dependent
cell adhesion molecule E-cadherin serves an important role in tumor
invasion and metastasis (23). A
study on breast cancer, colorectal cancer, bladder cancer and other
malignant tumors (24) revealed that
E-cadherin is an inhibitory factor in tumor malignant
transformation, invasion and metastasis. E-cadherin downregulation
or loss of function is significantly associated with tumor
differentiation, invasive growth, metastasis and poor
prognosis.
Another important step in tumor invasion and
metastasis is the degradation and destruction of the extracellular
matrix (ECM) and basement membrane structure barrier, which creates
favorable conditions for tumor cell metastasis. MMPs are key
enzymes of ECM catabolism, and MMP-2 and MMP-9 serve an important
role in tumor invasion and metastasis (25). The actions of MMPs are facilitated by
the degradation of ECM and basement membrane type IV collagen
fibers, and they promote tumor angiogenesis and cancer cell
invasion and metastasis (26,27).
Following infiltration from the blood vessels and
lymph vessels by tumor cells and the development of metastases,
cell adhesion is subsequently involved in the process and performs
an important role. CD44 belongs to the adhesion molecule family and
mediates ECM-cell adhesion; its expression is increased in a
variety of tumor cells, promoting tumor cell invasion and
metastasis, and performing an important role in tumor occurrence,
development and metastasis (28,29).
In the present study, CD44, MMP-2 and MMP-9 protein
expression was decreased and E-cadherin protein expression was
increased in gastric cancer cells transfected with the MDA-7 gene.
It was considered that MDA-7 inhibited CD44, MMP-2 and MMP-9
protein expression and promoted E-cadherin protein expression,
consequently inhibiting the invasion and metastasis of gastric
cancer cells. These features render the MDA-7 gene a promising
cancer treatment approach. However, for MDA-7 gene therapy as well
as the means of gene transfer to be effective, in-depth study of
the antitumor capacity, efficiency and persistence of gene
expression is required. The antitumor effect of MDA-7 in Stage I
clinical trials has been demonstrated (30,31); in
the future, the MDA-7 gene may be a novel tool in cancer gene
therapy.
Acknowledgements
The present study was sponsored by the Natural
Science Foundation of Fujian Province (grant nos. 2012J01430,
2016J01403 and 2014J01405), Fujian Provincial Health Systems Young
Backbone Personnel Training Project Funding Plan (grant no.
2013-ZQN-ZD-30), National Health and Family Planning Commission to
Build a Scientific Research Fund - the third round of health
education in Fujian joint research projects (grant no.
WKJ-FJ-37).
References
|
1
|
Hou R, Cao B and Chen Z, Li Y, Ning T, Li
C, Xu C and Chen Z: Association of cytotoxic T
lymphocyte-associated antigen-4 gene haplotype with the
susceptibility to gastric cancer. Mol Biol Rep. 37:515–520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zheng RS, Zhang SW, Zeng HM and
Zou XN: The incidences and mortalities of major cancers in China,
2010. Chin J Cancer. 33:402–405. 2014.PubMed/NCBI
|
|
3
|
Lissoni P, Brivio F, Ardizzoia A, Tancini
G and Barni S: Subcutaneous therapy with low-dose interleukin-2
plus the neurohormone melatonin in metastatic gastric cancer
patients with low performance status. Tumori. 79:401–404.
1993.PubMed/NCBI
|
|
4
|
Bhutia SK, Das SK, Azab B, Menezes ME,
Dent P, Wang XY, Sarkar D and Fisher PB: Targeting breast
cancer-initiating/stem cells with melanoma
differentiation-associated gene-7/interleukin-24. Int J Cancer.
133:2726–2736. 2013.PubMed/NCBI
|
|
5
|
Menezes ME, Shen XN, Das SK, Emdad L, Guo
C, Yuan F, Li YJ, Archer MC, Zacksenhaus E, Windle JJ, et al:
MDA-7/IL-24 functions as a tumor suppressor gene in vivo in
transgenic mouse models of breast cancer. Oncotarget.
6:36928–36942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sauane M, Gupta P, Lebedeva IV, Su ZZ,
Sarkar D, Randolph A, Valerie K, Gopalkrishnan RV and Fisher PB:
N-glycosylation of MDA-7/IL-24 is dispensable for tumor
cell-specific apoptosis and ‘bystander’ antitumor activity. Cancer
Res. 66:11869–11877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong AW, Nemunaitis J, Su D, Zhang Y,
Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, et
al: Intratumoral injection of INGN 241, a nonreplicating
adenovector expressing the melanoma-differentiation associated
gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients.
Mol Ther. 11:160–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Liu D, Wang J, Su Q, Zhou P, Liu
J, Luan M and Xu X: Suppression effect of recombinant adenovirus
vector containing hIL-24 on Hep-2 laryngeal carcinoma cells. Oncol
Lett. 7:771–777. 2014.PubMed/NCBI
|
|
9
|
Hurst DR and Welch DR: Metastasis
suppressor genes at the interface between the environment and tumor
cell growth. Int Rev Cell Mol Biol. 286:107–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao JC, Mansfield PF, Pisters PW, Feig BW,
Janjan NA, Crane C and Ajani JA: Combined modality the therapy for
gastric cancer. Semin Surg Oncol. 21:223–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu Y, Zhou C, Zhang J, Cai Q, Li J, Du T,
Zhu Z, Cui X and Liu B: The metastasis suppressor SOX11 is an
independent prognostic factor for improved survival in gastric
cancer. Int J Oncol. 44:1512–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panneerselvam J, Munshi A and Ramesh R:
Molecular targets and signaling pathways regulated by interleukin
(IL)-24 in mediating its antitumor activities. J Mol Signal.
8:152013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
15
|
Chada S, Sutton RB, Ekmekcioglu S,
Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK,
Fuson KL, et al: Mda-7/IL-24 is a unique cytokine-tumor suppressor
in the IL-10 family. Int Immunopharmacol. 4:649–667. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lebedeva IV, Emdad L, Su ZZ, Gupta P,
Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, et al:
mda-7/IL-24, novel anticancer cytokine: Focus on bystander
antitumor, radiosensitization and antiangiogenic properties and
overview of the phase I clinical experience (Review). Int J Oncol.
31:985–1007. 2007.PubMed/NCBI
|
|
17
|
Menezes ME, Bhatia S, Bhoopathi P, Das SK,
Emdad L, Dasgupta S, Dent P, Wang XY, Sarkar D and Fisher PB:
MDA-7/IL-24: Multifunctional cancer killing cytokine. Adv Exp Med
Biol. 818:127–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie Y, Sheng W, Xiang J, Ye Z, Zhu Y, Chen
X and Yang J: Recombinant human IL-24 suppresses lung carcinoma
cell growth via induction of cell apoptosis and inhibition of tumor
angiogenesis. Cancer Biother Radiopharm. 23:310–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma G, Kawamura K, Shan Y, Okamoto S, Li Q,
Namba M, Shingyoji M, Tada Y, Tatsumi K, Hiroshima K, et al:
Combination of adenoviruses expressing melanoma
differentiation-associated gene-7 and chemotherapeutic agents
produces enhanced cytotoxicity on esophageal carcinoma. Cancer Gene
Ther. 21:31–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan X, Sheng W, Zhu Q, Xie Y, Ye Z, Xiang
J, Li D and Yang J: Inhibition of pancreatic carcinoma growth by
adenovirus-mediated human interleukin-24 expression in animal
model. Cancer Biother Radiopharm. 23:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Liu D, Wang J, Su Q, Zhou P, Liu
J, Luan M and Xu X: Suppression effect of recombinant adenovirus
vector containing hIL-24 on Hep-2 laryngeal carcinoma cells. Oncol
Lett. 7:771–777. 2014.PubMed/NCBI
|
|
22
|
Patani N, Douglas-Jones A, Mansel R, Jiang
W and Mokbel K: Tumour suppressor function of MDA-7/IL-24 in human
breast cancer. Cancer Cell Int. 10:292010.PubMed/NCBI
|
|
23
|
Shamir ER and Ewald AJ: Adhesion in
mammary development: Novel roles for E-cadherin in individual and
collective cell migration. Curr Top Dev Biol. 112:353–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sarrió D, Palacios J, Hergueta-Redondo M,
Gómez-López G, Cano A and Moreno-Bueno G: Functional
characterization of E-and P-cadherin in invasive breast cancer
cells. BMC Cancer. 9:742009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sounni NE and Noel A: Membrane type-matrix
metalloproteinases and tumor progression. Biochimie. 87:329–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao L, Li Z, Lin L, Lei Y, Hongyuan Y,
Hongwei J, Yang L and Chuize K: MMP1, 2, 3, 7, and 9 gene
polymorphisms and urinary cancer risk: A meta-analysis. Genet Test
Mol Biomarkers. 19:548–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belotti D, Paganoni P, Manenti L, Garofalo
A, Marchini S, Taraboletti G and Giavazzi R: Matrix
metalloproteinases (MMP9 and MMP2) induce the release of vascular
endothelial growth factor (VEGF) by ovarian carcinoma cells:
Implications for ascites formation. Cancer Res. 63:5224–5229.
2003.PubMed/NCBI
|
|
28
|
Bánky B, Rásó-Barnett L, Barbai T, Tímár
J, Becságh P and Rásó E: Characteristics of CD44 alternative splice
pattern in the course of human colorectal adenocarcinoma
progression. Mol Cancer. 11:832012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paulis YW, Huijbers EJ, van der Schaft DW,
Soetekouw PM, Pauwels P, Tjan-Heijnen VC and Griffioen AW: CD44
enhances tumor aggressiveness by promoting tumor cell plasticity.
Oncotarget. 6:19634–19646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dent P, Yacoub A, Grant S, Curiel DT and
Fisher PB: MDA-7/IL-24 regulates proliferation, invasion and tumor
cell radiosensitivity: A new cancer therapy? J Cell Biochem.
95:712–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cunningham CC, Chada S, Merritt JA, Tong
A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards
D, et al: Clinical and local biological effects of an intratumoral
injection of mda-7 (IL24; INGN 241) in patients with advanced
carcinoma: A phase I study. Mol Ther. 11:149–159. 2005. View Article : Google Scholar : PubMed/NCBI
|