Introduction
Gastric cancer is one of the most common types of
malignant tumors, with the highest incidence and mortality rates
among all malignant tumors globally, and accounting for ~160,000
mortalities annually in China (1).
The proportion of postoperative survival >5 years is usually low
(2). In addition, ~84% of patients
with gastric cancer will exhibit advanced disease with a median
survival time of 3–4 months if they are not treated with
chemotherapy (1).
Several previous reports have indicated that the
pathogenesis of gastric cancer involves complex molecular
mechanisms, and multiple genetic and epigenetic alterations to
oncogenes and tumor suppressor genes (3,4). Signaling
pathway alterations are currently considered to be important in the
development of gastric cancer (5).
The aberrant activation of signaling systems may cause cancer
(6). Among the signaling pathways
associated with cell apoptosis, the phosphatidylinositol
3-kinase-(PI3K)-RAC-α serine/threonine kinase (Akt) signaling
pathway is currently considered to be important in cell survival,
which is closely associated with the occurrence and development of
multiple tumors (7,8). Additionally, it serves an important role
in promoting the growth, malignant proliferation and metastasis of
tumor cells, inhibiting apoptosis, accelerating angiogenesis, and
inducing resistance to chemotherapy and radiotherapy (9). As an essential effector in the PI3K/Akt
signaling pathway, activated Akt exerts a range of biological
effects by facilitating the phosphorylation of downstream
substrates, including glycogen synthase kinase-3β (10). It has been demonstrated that the
enhanced activity of Akt kinase is present in gastric cancer, and
that Akt overexpression is associated with the poor prognoses and
high recurrence rates of patients with gastric cancer (11). Therefore, inhibiting the activated
PI3K/Akt signaling pathway may effectively inhibit the growth of
gastric cancer cells.
LY294002 is the specific inhibitor of PI3K. A
previous study demonstrated that LY294002 administered in
combination with certain anti-tumor drugs may enhance curative
effects and reduce drug resistance (12). CecropinXJ was isolated from the larvae
of Bombyx mori, which possesses a 37-amino acid cationic
antimicrobial peptide with specific amphipathic α-helices (13). CecropinXJ elicits a broad spectrum of
effects against bacteria and fungi (14,15). Our
previous studies indicated that cecropinXJ exhibits anti-tumor and
anti-proliferation activities, and an apoptosis-promoting effect on
human gastric cancer AGS (16), and
BGC823 cells (17). In addition,
apoptosis is mediated by the mitochondrial apoptotic pathway.
However, the anti-tumor effect of the CecropinXJ antibacterial
peptide involves multiple mechanisms. Therefore, the present study
primarily aimed to identify the effect of cecropinXJ alone or in
combination with LY294002 on the PI3K/Akt signaling pathway in
BGC823 cells, and to demonstrate whether they synergistically
facilitate the inhibition and apoptosis-promoting effect on cell
proliferation, thus providing an experimental basis for a novel
treatment of gastric cancer.
Materials and methods
Chemicals and reagents
LY294002, MTT, Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were purchased from Gibco;
Thermo Fisher Scientific, Ins. (Waltham, MA, USA). All other
chemicals used were of analytical grade, available locally.
Preparation of antimicrobial peptide
cecropinXJ
CecropinXJ of B. mori was prepared through
the Saccharomyces cerevisiae eukaryotic expression system and
purified to homogeneity by a nickel-chelating Sepharose column as
described previously (14). The
concentration of purified recombinant cecropinXJ protein was
detected using the Bradford protein assay method. Prior to use, the
peptide was dissolved in DMEM at a concentration of 1 mg/ml and
sterilized by filtration through a 0.22 µm filter.
Cell Culture
The human gastric cancer cell line BGC823 was
provided by Professor Youyong Lv (Beijing Cancer Hospital, Beijing,
China). The BGC823 cells were cultured in DMEM medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C under a humidified atmosphere in a 5% CO2
incubator. Cells in mid-logarithmic growth were used for the
following experiments.
Cell viability assay
The viability of cells treated with cecropinXJ and
LY294002 were measured using an MTT assay. The experiments divided
into four groups: Group I, control group (only medium); Group II,
cecropinXJ alone treatment (20, 50 or 100 µg/ml); Group III,
LY294002 alone treatment (25 µmol/l); Group IV, cecropinXJ in
combination with LY294002 treatment. During the logarithmic growth
phase, cells were collected and seeded in 96-well plates at a
density of 5×104 cells/well, and cultured. Following 12
h of incubation, the cells were treated with medium only,
cecropinXJ (20, 50 and 100 µg/ml), LY294002 (25 µmol/l), or
cecropinXJ (20, 50 and 100 µg/ml) and LY294002 (25 µmol/l) for 12,
24 and 48 h. Subsequent to treatment, 20 µl MTT solution (5 mg/ml)
was added to each well and the cells were then incubated at 37°C
for 4 h. The culture medium was then replaced with 100 µl dimethyl
sulfoxide. The absorbance of the solution at 490 nm was measured
with a microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA). The cell inhibitory rate (%) was calculated as follows: (A490
control-A490 sample)/(A490 control-A490 blank) ×100%.
Annexin V/PI staining assay for
apoptosis
The cells of the four groups were treated for 24 h.
Subsequently, cells were collected following digestion with 0.25%
trypsin (HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
washed with cold PBS (pH 7.4; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) twice and suspended in 400 µl
binding buffer from the Annexin V-FITC/PI apoptosis assay kit
(BestBio Biotechnologies, Shanghai, China). Cell suspensions were
stained with 5 µl Annexin V-FITC and 10 µl PI and incubated at 4°C
for 30 min in the dark according to the manufacturer's protocol
(BestBio Biotechnologies). Cells were analyzed using a FACScan flow
cytometer with CellQuest software version 3.0 (BD Biosciences,
Franklin Lakes, NJ, USA).
Protein extraction and western blot
analysis
Proteins from the cells were extracted using
CytoBuster™ protein extraction reagent (Merck Millipore, Darmstadt,
Germany) with a cocktail of proteinase inhibitors (Roche Applied
Science, Rotkreuz, Switzerland) and a cocktail of phosphatase
inhibitors (Roche Applied Science) according to the manufacturer's
protocol. The concentration of protein was determined by BCA assay.
For western blot analysis, the protein lysates (40 µg/lane) were
separated by 12% SDS-PAGE and transferred to nitrocellulose
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked with 5% skimmed milk at room temperature for
1 h, incubated with specific primary antibodies against Akt (cat.
no., 4691), p-Akt (cat. nos., 4046, 13038), Bad (cat. no., 9239),
p-Bad (cat. no., 5284), Bak (cat. no., 12105), Bid (cat. no.,
8762), Bik (cat. no., 4592), Puma (cat. no., 12450), Bim (cat. no.,
2933), Bax (cat. no., 14796), Bcl-2 (cat. no., 15071), caspase-3
(cat. no., 9664), cytochrome c (cat. no., 11940) and GAPDH (cat.
no., 2118; all dilutions, 1:1,000; all from Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight and followed
by incubation with horseradish peroxidase-conjugated
goat-anti-rabbit (cat. no., 7074) or goat-anti-mouse (cat. no.,
7076) antibodies (dilutions, 1:2,000; Cell Signaling Technology,
Inc.) for 1 h at room temperature. Subsequent to washing three
times with TBST buffer, the protein signals were visualized with an
enhanced chemiluminescence immunoblotting detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). Equal loading of
samples was confirmed using probes for GAPDH.
Statistical analyses
At least three replicates were performed for each
experiment. All data were presented as mean ± SD deviation. The
differences between experimental groups were compared using one-way
analysis of variance followed by Student-Newman-Keuls. P<0.05
were considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS version 16.0 (SPSS
Inc., Chicago, IL, USA).
Results
Inhibiting effect of cecropinXJ on Akt
phosphorylation
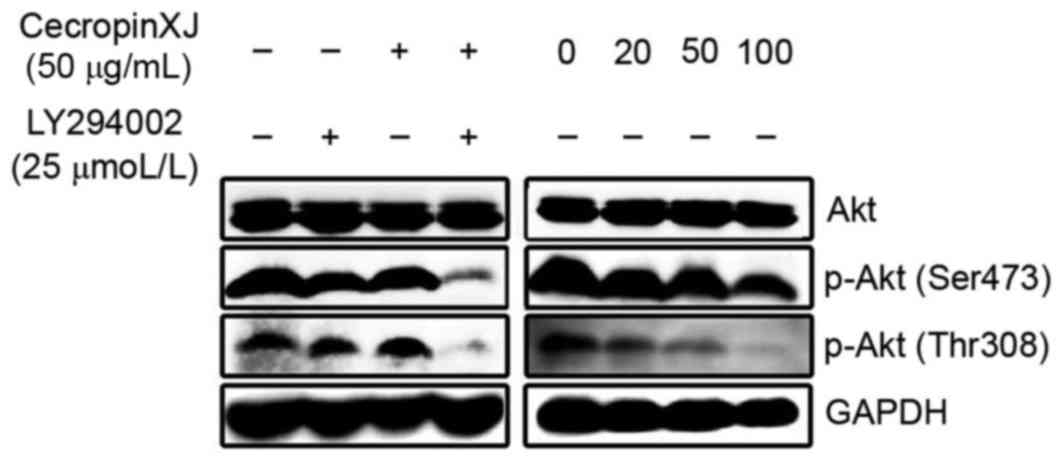
To determine whether cecropinXJ reduced Akt
activation in BGC823 cells, the expression level of total Akt
protein and p-Akt in BGC823 cells 24 h after intervention with
different drugs was detected by western blot analysis (Fig. 1). The results demonstrated that the
p-Akt protein band was marked in untreated BGC823 cells, while the
expression of the p-Akt protein bands in the cecropinXJ treatment
and LY294002 treatment groups were downregulated by different
concentrations. In addition, the concentration of p-Akt protein in
the cecropinXJ treatment group was significantly downregulated in a
concentration-dependent manner. Only a faint protein band was
observed in the cecropinXJ and LY294002 combined treatment group.
The phosphorylation of Akt protein at the Ser473 and Thr308 sites
was inhibited, while total Akt protein in each group did not
demonstrated any marked changes, indicating that cecropinXJ
inhibited Akt activation and consequently the PI3K/Akt signaling
pathway. In combination with LY294002, the results suggest that the
inhibitory effect of cecropinXJ on the PI3K/Akt signaling pathway
was enhanced (Fig. 1).
Inhibiting effect of alone and
combined cecropinXJ at different concentrations and LY294002 on
cell viability
As summarized in Table
I, 12, 24 and 48 h after the treatment of BGC823 cells with
cecropinXJ at different concentrations, the viability of cells were
significantly inhibited. LY294002 alone exhibited a weak inhibitory
effect on cell viability. However, cecropinXJ in combination with
LY294002 exhibited a significantly higher inhibitory effect on cell
viability compared with cecropinXJ alone. Additionally, the cell
survival rate was decreased with the increase of cecropinXJ
concentration in a dose- and time-dependent manner. It indicated
that the toxic effect of cecropinXJ in combination with LY294002
was greater compared with cecropinXJ alone, which may inhibit the
in vitro growth of gastric cancer BGC823 cells more
effectively.
 | Table I.Inhibitory effects of different
concentrations of cecropinXJ in combination with LY294002 on the
viability of BGC823 cells. |
Table I.
Inhibitory effects of different
concentrations of cecropinXJ in combination with LY294002 on the
viability of BGC823 cells.
|
|
| Cell viability,
% |
|---|
|
|
|
|
|---|
| Group | Dose | 12 h | 24 h | 48 h |
|---|
| Control | Dulbecco's
modified | 100 | 100 | 100 |
|
| Eagle's medium |
|
|
|
| CecropinXJ | 20 µg/ml | 88.96±9.67 |
75.20±3.27a |
52.58±6.04a |
|
| 50 µg/ml |
78.80±7.01a |
68.02±6.39a |
44.81±2.84a |
|
| 100 µg/ml |
40.46±6.95a |
35.98±7.38a |
16.30±1.42a |
| LY294002 | 25 µmol/l | 91.01±11.18 |
83.51±9.74a |
57.90±14.33a |
| CecropinXJ and
LY294002 | 20 µg/ml
cecropinXJ |
70.56±7.10a–c |
59.36±8.48a–c |
32.14±10.64a–c |
|
| +25 µmol/l |
|
|
|
|
| LY294002 |
|
|
|
|
| 50 µg/ml
cecropinXJ |
62.23±12.26a–c |
49.34±9.86a–c |
19.31±9.30a–c |
|
| +25 µmol/l |
|
|
|
|
| LY294002 |
|
|
|
|
| 100 µg/ml
cecropinXJ |
23.90±11.71a–c |
16.23±6.95a–c |
2.64±1.88a–c |
|
| +25 µmol/l |
|
|
|
|
| LY294002 |
|
|
|
Enhancement of LY294002 on
cecropinXJ-mediated apoptosis
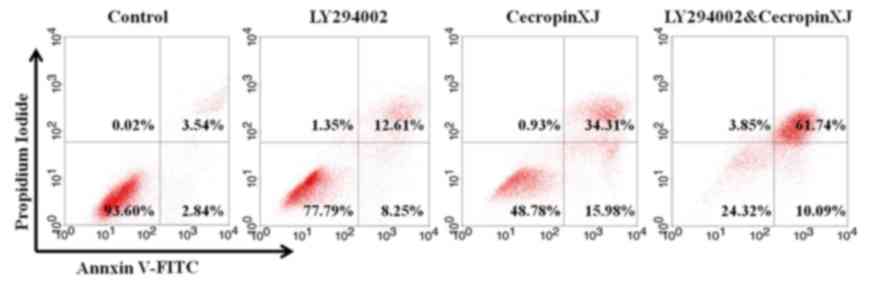
The results of the flow cytometry analysis
demonstrated that the apoptosis rate of BGC823 cells in each
treatment group was significantly increased in comparison with the
control group (Fig. 2; Table II): The early apoptosis and total
apoptosis rates of the combined treatment group were significantly
higher compared with the two single-treatment groups (Table II), indicating that LY294002 in
combination with cecropinXJ exhibited a synergistic
apoptosis-promoting effect.
 | Table II.Apoptotic effects of cecropinXJ alone
and in combination with LY294002 in BGC823 cells. |
Table II.
Apoptotic effects of cecropinXJ alone
and in combination with LY294002 in BGC823 cells.
|
| Apoptosis rate,
% |
|---|
|
|
|
|---|
| Group | Early | Late | Total |
|---|
| Control | 3.03±0.78 | 5.32±1.82 | 8.35±2.30 |
| LY294002 | 11.11±2.96 | 12.14±0.81 |
23.25±2.42a |
| CecropinXJ | 18.03±3.42 | 32.51±2.55 |
50.54±5.97a |
| CecropinXJ and
LY294002 | 9.44±0.69 | 60.91±1.46 |
70.34±2.15a,b |
Inhibiting effect of cecropinXJ on Bad
phosphorylation
The results demonstrated that p-Akt may
phosphorylate Bad. Bad phosphorylation leads to the decomposition
of protein complex consisting of anti-apoptotic proteins in the Bad
and Bcl-2 protein families, and promotes cell survival (18). The expression level of p-Bad and Bad
in the BGC823 cells that were treated with different interventions
was detected by western blot analysis (Fig. 3). The results indicated that the p-Bad
protein band in the single-compound treatment groups of cecropinXJ
and LY294002 was downregulated by varying degrees, and Bad protein
expression was markedly upregulated. In the combined treatment
group of cecropinXJ and LY294002, no p-Bad protein band was
observed and Bad protein expression was slightly upregulated in
comparison with the two single-treatment groups. These results
indicate that cecropinXJ inhibited the growth of BGC823 cells,
mediated by the PI3K/Akt signaling pathway by downregulating Bad
phosphorylation.
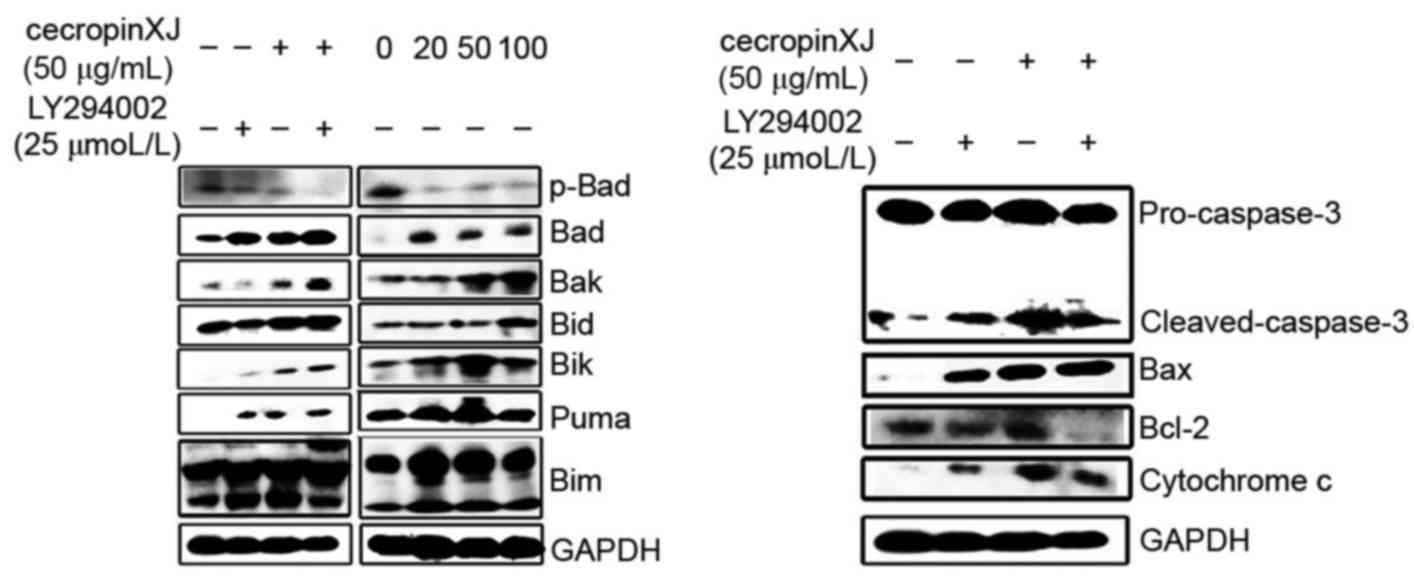 | Figure 3.Western blot analyses of BAD, p-BAD,
Bak, Bid, Bik, Puma, Bim, caspase-3, Bax, Bcl-2 and cytochrome c
expressions following treatment with cecropinXJ alone and in
combination with LY294002 in BGC823 cells. Representative data from
several independent experiments are presented. Bcl-2, B-cell
lymphoma 2; Bad, Bcl-2-associated death promotor; p,
phosphorylated; Bak, Bcl-2 homologous antagonist killer, Bid, BH3
interacting-domain death agonist; Bik, Bcl-2 interacting killer;
Puma, p53 upregulated modulator of apoptosis; Bim, Bcl-2-like
protein 11; Bax, Bcl-2-like protein 4. |
Effect of cecropinXJ on expression of
Bcl-2 family, caspase-3 and cytochrome C
The expression of pro-apoptotic and anti-apoptotic
proteins in BGC823 cells was detected by western blot analysis 24 h
following different treatments (Fig.
3). The results indicated that the expression levels of
pro-apoptotic proteins were upregulated following single treatments
with cecropinXJ and LY294002, and that the upregulated expression
of pro-apoptotic protein expression levels in the cecropinXJ
treatment group was concentration-dependent. In the combined
treatment group of cecropinXJ and LY294002, the expression of
pro-apoptotic proteins was markedly upregulated in comparison with
the two single-treatment groups. Concurrently, cecropinXJ induced
apoptosis by activating the mitochondrial pathway, while LY294002,
as the inhibitor of PI3K, synergistically increased the proportion
of cecropinXJ-induced Bax/Bcl-2, but did not synergistically
promote the shearing of caspase-3 and the release of cytochrome
C.
Discussion
The PI3K/Akt signaling pathway serves an important
in regulating cell proliferation, growth and apoptosis. Due to its
important role in cancer, there is great interest in the
development of inhibitors able to target on the signaling pathways
in preclinical trials. Sukawa et al (19) detected the expression of p-Akt in the
tissue specimens of 231 patients with gastric cancer, and
identified that 53% gastric cancer tissue specimens exhibit p-Akt
expression and that patients with p-Akt expression demonstrate poor
prognoses. An additional previous study suggested that the
expression of p-Akt in 45 gastric cancer tissue specimens was up to
82.2%, and associated with tumor growth and metastasis (20).
Phosphorylated Akt exhibits a range of biological
effects, including preventing apoptosis and promoting cell survival
by phosphorylating substrates containing Ser/Thr residues. At
present, several studies have indicated that chemotherapy drugs,
including 5-fluorouracil, adriamycin and cis-platinum may increase
p-Akt expression levels, and induce gastric cancer cells to
generate chemotherapy resistance (21,22).
Therefore, a decrease in p-Akt expression may effectively inhibit
tumor cell proliferation, promote tumor cell apoptosis and reduce
levels of chemoresistance. The present study identified that the
phosphorylation level of Akt in BGC823 cells was high. Individual
treatment with cecropinXJ or the inhibitor LY294002 inhibited the
expression of p-Akt in BGC823 cells to varying degrees, and the
expression levels exhibited a dose-dependent decrease while the
expression of total Akt protein was not affected. The combined
treatment significantly decreased the expression of p-Akt. It was
suggested that protein kinases are the target of antibacterial
peptides, and certain antibacterial peptides, including defensins,
such as human neutrophil peptide (HNP)-1, HNP-2 and HNP-3, are
potential inhibitors of protein kinase C (23). Antibacterial protein PR-39 is an
antibacterial peptide rich in proline and arginine. It participates
in different cell activities, including cell adhesion and migration
by combining with adaptor protein p130Cas. In addition, it may also
inhibit the activity of PI3K by combining with a subunit of PI3K
(24). As Akt is the downstream
target of PI3K, the inhibiting effect of cecropinXJ on Akt
phosphorylation demonstrates that cecropinXJ may also inhibit the
activity of PI3K.
Previous studies have indicated that antibacterial
peptides may significantly inhibit gastric cancer development and
promote apoptosis (25,26). The mechanism of antibacterial peptides
inducing cell apoptosis is complex. Certain studies have suggested
that the antibacterial peptides may lyse tumor cells to directly
kill cells (27) or induce tumor cell
apoptosis through Fas death receptor (28) and the mitochondrial pathway (29). In addition, antibacterial peptides may
inhibit the proliferation and growth of tumor cells by regulating
multiple signaling pathways, including the PI3K/Akt signaling
(30,31), mitogen-activated protein kinase
signaling (32) and endoplasmic
reticulum stress-mediated apoptotic pathways (33). PI3K is a proto-oncogene and LY294002
is its specific inhibitor. In vitro and in vivo
experiments have indicated that LY294002 may inhibit PI3K from
phosphorylating Akt in order to inhibit the downstream pathways and
increase apoptosis rate (34). In
combination with routine chemotherapy and radiotherapy, LY294002
exhibits a synergistic effect on chemosensitization, reduces
cytotoxicity, and effectively inhibits tumor cell growth (35,36).
Additional studies have revealed that single anti-tumor drugs
exhibit lower efficiencies in inhibiting tumor growth and inducing
apoptosis compared with multi-targeted inhibition (37,38). In
previous years, the combination of inhibitors targeting signaling
pathways and anti-tumor drugs has suggested a novel method of tumor
treatment, and has gained attention. The present study indicated
that cecropinXJ exhibited a significant inhibitory effect on the
viability of BGC823 cells, while the inhibitory effect of
PI3K-specific inhibitor LY294002 is less significant compared with
cecropinXJ. In comparison with cecropinXJ alone, the combination of
cecropinXJ and LY294002 demonstrated a significant increased
inhibitory effect on cell viability, which was concentration and
time-dependent. It was also observed that at the same dose of
cecropinXJ, the rate of apoptosis of BGC823 cells was significantly
increased following treatment with LY94002, suggesting that
LY294002 may promote the inhibitory effect and apoptosis-inducing
effect of cecropinXJ on BGC823 cells. This indicates that LY294002
may enhance the sensitivity of BGC823 cells to cecropinXJ and
increase cecropinXJ-induced apoptosis of BGC823 cells.
Apoptosis involves the activation and regulation of
the expression of a series of genes, including the regulation of
the Bcl-2 protein and caspase protein families. Over-activated Akt
achieves its anti-apoptotic effects by phosphorylating various
substrates such as Bad (39) and
caspase-9 (40). Previous studies
have revealed that cecropinXJ may inhibit Bad phosphorylation
(16,17). p-Bad may combine with Bcl-2 or Bcl-XL
on the mitochondrial membrane to prevent the release of cytochrome
c from the mitochondria and the activation of caspase-9 (41). A previous study indicated that p-Akt
downregulates the pro-apoptotic proteins in the Bcl-2 family,
including Bax and Bak, and upregulates the anti-apoptotic proteins
in Bcl-2 family, including Bcl-2 and Bcl-XL (42). The inhibition of Akt phosphorylation
may significantly downregulate the expression of Bcl-2 and Bcl-XL
(43). The results of the present
study demonstrated that in BGC823 cells treated with cecropinXJ in
combination with LY294002, the pro-apoptotic proteins in the Bcl-2
family, including Bax, were markedly upregulated and the
anti-apoptotic proteins, including Bcl-2, were downregulated. This
resulted in an increase in the Bax/Bcl-2 ratio, an increase in
mitochondrial membrane permeability, promotion of cytochrome c
release and activation of caspase-3 (44). The inhibitory effect on Akt activation
of PI3K-specific inhibitors may additionally increase
cecropinXJ-induced Bax/Bcl-2 ratio.
In conclusion, the present study provided a novel
therapeutic regimen for the use of the cecropinXJ in combination
with LY294002 for the treatment of gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31500752), the
Doctoral Start-up Fund of Xinjiang University (grant no. BS150241)
and the High-Tech Research and Development Program of Xinjiang
(grant no. 201110101).
References
|
1
|
Chen XZ, Jiang K, Hu JK, Zhang B, Gou HF,
Yang K, Chen ZX and Chen JP: Cost-effectiveness analysis of
chemotherapy for advanced gastric cancer in China. World J
Gastroenterol. 14:2715–2722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartmann W, Küchler J, Koch A, Friedrichs
N, Waha A, Endl E, Czerwitzki J, Metzger D, Steiner S, Wurst P, et
al: Activation of phosphatidylinositol-3′-kinase/AKT signaling is
essential in hepatoblastoma survival. Clin Cancer Res.
15:4538–4545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C, Li S and Wang MW:
Evodiamine-induced human melanoma A375-S2 cell death was mediated
by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and
augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro.
24:898–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Downward J: PI3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LQ, Wong KY, Rosèn A and Chim CS:
Epigenetic silencing of tumor suppressor miR-3151 contributes to
Chinese chronic lymphocytic leukemia by constitutive activation of
MADD/ERK and PIK3R2/AKT signaling pathways. Oncotarget.
6:44422–44436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong S, Kim S, Kim HY, Kang M, Jang HH and
Lee WS: Targeting the PI3K signaling pathway in KRAS mutant colon
cancer. Cancer Med. 5:248–255. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ang KL, Shi DL, Keong WW and Epstein RJ:
Upregulated Akt signaling adjacent to gastric cancers: Implications
for screening and chemoprevention. Cancer Lett. 225:53–59. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang Z, Kong D, Li R, Sarkar SH and
Sarkar FH: Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network
by isoflavone in prostate cancer cells. J Biol Chem.
283:27707–27716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Han L, Shi Z, Zhang K, Liu Y,
Zheng Y, Jiang T, Pu P, Jiang C and Kang C: LY294002 enhances
cytotoxicity of temzolomide in glioma by down-regulation of the
PI3K/Akt pathway. Mol Med Report. 5:575–579. 2012.
|
|
12
|
Liang J, Ge F, Guo C, Luo G, Wang X, Han
G, Zhang D, Wang J, Li K, Pan Y, et al: Inhibition PI3K/Akt
partially leads to the inhibition of PrP(C)-induced drug resistance
in gastric cancer cells. FEBS J. 276:685–694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Zhang F, Cai L, Zhao G and Wang B:
Studies on the properties of cecropin-XJ expressed in yeast from
Xinjiang silkworm. Wei Sheng Wu Xue Bao. 43:635–641. 2003.(In
Chinese). PubMed/NCBI
|
|
14
|
Xia L, Zhang F, Liu Z, Ma J and Yang J:
Expression and characterization of cecropinXJ, a bioactive
antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in
Escherichia coli. Exp Ther Med. 5:1745–1751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia L, Liu Z, Ma J, Sun S, Yang J and
Zhang F: Expression, purification and characterization of cecropin
antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae.
Protein Expr Purif. 90:47–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Xia L and Zhang F: Inhibition of
CecropinXJ on proliferation of human gastric cancer AGS cells. Chin
J Cell Bio. 36:1355–1361. 2014.
|
|
17
|
Wu YL, Xia LJ, Li JY and Zhang FC:
CecropinXJ inhibited cell proliferation of human gastric cancer
BGC823 cells and induces cell death in vitro and in vivo. Int J
Oncol. 46:2181–2193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
She QB, Solit DB, Ye Q, O'Reilly KE, Lobo
J and Rosen N: The BAD protein integrates survival signaling by
EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor
cells. Cancer Cell. 8:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sukawa Y, Yamamoto H, Nosho K, Kunimoto H,
Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M, et
al: Alterations in the human epidermal growth factor receptor
2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer.
World J Gastroenterol. 18:6577–6586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye B, Jiang LL, Xu HT, Zhou DW and Li ZS:
Expression of PI3K/AKT pathway in gastric cancer and its blockade
suppresses tumor growth and metastasis. Int J Immunopathol
Pharmacol. 25:627–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oki E, Baba H, Tokunaga E, Nakamura T,
Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y and
Maehara Y: Akt phosphorylation associates with LOH of PTEN and
leads to chemoresistance for gastric cancer. Int J Cancer.
117:376–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li
JH, Xu XM, Liu S, Chen J, Liu F, et al: Phosphoinositide
3-kinase/Akt pathway plays an important role in chemoresistance of
gastric cancer cells against etoposide and doxorubicin induced cell
death. Int J Cancer. 122:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Charp PA, Rice WG, Raynor RL, Reimund E,
Kinkade JM Jr, Ganz T, Selsted ME, Lehrer RI and Kuo JF: Inhibition
of protein kinase C by defensins, antibiotic peptides from human
neutrophils. Biochem Pharmacol. 37:951–956. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Fujimoto Y, Suzuki M, Suzuki Y,
Ohtake T, Saito H and Kohgo Y: PI3-kinase p85alpha is a target
molecule of proline-rich antimicrobial peptide to suppress
proliferation of ras-transformed cells. Jpn J Cancer Res.
92:959–967. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H and Cui H: Study on
antiproliferative effects of antibiotic peptide from skin of
Chinese forest frog on gastric cancer cell lines. Chin J Public
Health. 23:913–914. 2007.
|
|
26
|
Pan WR, Chen PW, Chen YL, Hsu HC, Lin CC
and Chen WJ: Bovine lactoferricin B induces apoptosis of human
gastric cancer cell line AGS by inhibition of autophagy at a late
stage. J Dairy Sci. 96:7511–7520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Zhou Y, Li S, Li H, Tian L, Wang H
and Shang D: Anticancer mechanisms of temporin-1CEa, an amphipathic
α-helical antimicrobial peptide, in Bcap-37 human breast cancer
cells. Life Sci. 92:1004–1014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin X, Mei H, Li X, Ma Y, Zeng AH, Wang Y,
Lu X, Chu F, Wu Q and Zhu J: Apoptosis-inducing activity of the
antimicrobial peptide cecropin of Musca domestica in human
hepatocellular carcinoma cell line BEL-7402 and the possible
mechanism. Acta Biochim Biophys Sin (Shanghai). 42:259–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Risso A, Braidot E, Sordan MC, Vianello A,
Macrì F, Skerlavaj B, Zanetti M, Gennaro R and Bernardi P: BMAP-28,
an antibiotic peptide of innate immunity, induces cell death
through opening of the mitochondrial permeability transition pore.
Mol Cell Biol. 22:1926–1935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu XX, Jiang HR, Li HB, Zhang TN, Zhou Q
and Liu N: Apoptosis of stomach cancer cell SGC-7901 and regulation
of Akt signaling way induced by bovine lactoferrin. J Dairy Sci.
93:2344–2350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang
JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, et al: Melittin
suppresses EGF-induced cell motility and invasion by inhibiting
PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem
Toxicol. 68:218–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huh JE, Kang JW, Nam D, Baek YH, Choi DY,
Park DS and Lee JD: Melittin suppresses VEGF-A-induced tumor growth
by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway.
J Nat Prod. 75:1922–1929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan Q, Hu Y, Pang H, Sun J, Wang Z and Li
J: Melittin protein inhibits the proliferation of MG63 cells by
activating inositol-requiring protein-1α and X-box binding protein
1-mediated apoptosis. Mol Med Rep. 9:1365–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xing C, Zhu B, Liu H, Yao H and Zhang L:
Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates
autophagy and induces apoptosis through p53 pathway in gastric
cancer cell line SGC7901. Acta Biochim Biophys Sin (Shanghai).
40:194–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwase M, Yoshiba S, Uchid M, Takaoka S,
Kurihara Y, Ito D, Hatori M and Shintani S: Enhanced susceptibility
to apoptosis of oral squamous cell carcinoma cells subjected to
combined treatment with anticancer drugs and phosphatidylinositol
3-kinase inhibitors. Int J Oncol. 31:1141–1147. 2007.PubMed/NCBI
|
|
36
|
Li C, Liu VW, Chan DW, Yao KM and Ngan HY:
LY294002 and metformin cooperatively enhance the inhibition of
growth and the induction of apoptosis of ovarian cancer cells. Int
J Gynecol Cancer. 22:15–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Małecki JM, Bentke A, Ostrowska B and
Laidler P: Cytochalasin D, LY294002 and olomoucine synergize in
promoting death of melanoma cells through activation of caspase-3
and apoptosis. Melanoma Res. 20:52–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kennedy SG, Kandel ES, Cross TK and Hay N:
Akt/protein kinase B inhibits cell death by preventing the release
of cytochrome c from mitochondria. Mol Cell Biol. 19:5800–5810.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals tothe cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chong ZZ and Maiese K: Targeting WNT,
protein kinase B, and mitochondrial membrane integrity to foster
cellular survival in the nervous system. Histol Histopathol.
19:495–504. 2004.PubMed/NCBI
|
|
42
|
Han Z, Hong L, Han Y, Wu K, Han S, Shen H,
Li C, Yao L, Qiao T and Fan D: Phospho Akt mediates multidrug
resistance of gastric cancer cells through regulation of P-gp,
Bcl-2 and Bax. J Exp Clin Cancer Res. 26:261–268. 2007.PubMed/NCBI
|
|
43
|
Lee WS, Yi SM, Yun JW, Jung JH, Kim DH,
Kim HJ, Chang SH, Kim G, Ryu CH, Shin SC, et al: Polyphenols
isolated from Allium cepa L. Induces apoptosis by induction of p53
and suppression of Bcl-2 through inhibiting PI3K/Akt signaling
pathway in AGS human cancer cells. J Cancer Prev. 19:14–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du L, Mei HF, Yin X and Xing YQ: Delayed
growth of glioma by a polysaccharide from Aster tataricus involve
upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and
downregulation of the Akt. Tumour Biol. 35:1819–1825. 2014.
View Article : Google Scholar : PubMed/NCBI
|