Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth
most common cancer type and the third leading cause of
cancer-associated mortality worldwide (1,2). The
majority of patients with HCC are diagnosed at an advanced disease
stage and are not amenable to potentially curative therapies,
including surgery, transplantation, and radiofrequency ablation
(3). Several therapies, including
transarterial chemoembolization and radiation therapy are currently
available for patients with locally advanced HCC (4). With recent advances in radiotherapy
technology, a substantial dose of radiation can be precisely
delivered to tumors, thereby reducing adverse effects on the
surrounding normal tissues. It has been reported that radiotherapy
yields remarkable local tumor control and has a potential
beneficial survival impact in well-selected patients with HCC
(5). However, the emergence of
radioresistant tumor cells commonly leads to therapeutic failure.
Therefore, understanding the mechanism underlying the resistance of
HCC cells to radiation is of importance in improving the efficacy
of radiotherapy.
Mucin 1 (MUC1) is a heterodimeric epithelial cell
glycoprotein that is aberrantly overexpressed in a range of human
cancer types (6). Mucin 1 protein has
been implicated in various aspects of tumor development, including
angiogenesis (7), proliferation
(8), migration (9), survival (10) and metastasis (11). Accumulating evidence indicates a
causal association between MUC1 expression and chemoresistance of
tumor cells (12,13). For instance, Nath et al
(12) reported that MUC1 contributes
to drug resistance in pancreatic cancer cells through upregulation
of multidrug resistance genes, including ATP binding cassette
subfamily C member (ABCC)1, ABCC3, ABCC5 and ABCB1. Overexpression
of the oncogenic MUC1-C subunit has been identified to confer
tamoxifen resistance in MCF-7 breast cancer cells (13). However, few studies have addressed the
role of MUC1 in the development of radioresistance of tumor
cells.
MUC1 serves as an oncogene in HCC and has been
demonstrated to facilitate HCC cell migration and invasion
(9). In the present study, the
possible implication of MUC1 in the radioresistance of HCC cells
was evaluated. The janus kinase/signal transducer and activator of
transcription (JAK/STAT) signaling pathway serves an essential role
in the growth and survival of HCC (14). Inhibition of STAT3 signaling has been
reported to enhance radiation-induced apoptosis in HCC cells
(15). Hence, the association between
MUC1 and JAK/STAT3 signaling in the regulation of HCC cell
radiosensitivity was also assessed.
Materials and methods
Cell culture
The human HCC SMMC-7721 cell line was obtained from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and cultured in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml penicillin
and 100 µg/ml streptomycin at 37°C in a humidified atmosphere with
5% CO2.
Plasmid construct and short hairpin
RNAs (shRNAs)
A full-length human MUC1 cDNA (OriGene Technologies,
Inc., Rockville, MD, USA) was amplified by polymerase chain
reaction (PCR) using Taq polymerase (Promega Corporation, Madison,
WI, USA). The PCR primers are as follows: Forward,
5′-ATGACACCGGGCACCCAGTCT-3′ and reverse,
5′-GCTACAAGTTGGCAGAAGTG-3′. Thermocycler conditions were as
follows: Initial denaturation at 95°C for 10 min, then 32 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 45 sec and
extension at 72°C for 60 sec. PCR products were subcloned into the
mammalian expression vector pcDNA3.1(+) (Invitrogen; Thermo Fisher
Scientific, Inc.). The identity of the pcDNA3.1/MUC1 plasmid was
confirmed by DNA sequencing. STAT3 shRNA, MUC1 shRNA and negative
control shRNA were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Cell transfection
For overexpression of MUC1, cells were transfected
with the pcDNA3.1/MUC1 plasmid (0.4 µg) or empty vector (0.4 µg)
using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Transfected cells were selected for 2 weeks in the presence of 0.8
mg/ml of G418 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). For
the knockdown experiments, MUC1-overexpressing cells were
transfected with control shRNA, STAT3 shRNA or MUC1 shRNA (1 µg of
each shRNA) using the Lipofectamine 2000 transfection reagent.
Cells were harvested for gene expression analysis or exposure to
irradiation 24 h after transfection.
Irradiation treatment
Cells (5×106) plated in 60-mm tissue
culture dishes were cultured until ~60% confluency was achieved and
then irradiated with 2–10 Gy of X-rays at a dose rate of 0.7 Gy/min
using an X-ray generator (Precision X-Ray, Inc., North Branford,
CT, USA). Following treatment for 1, 3, 6, 10, 16, and 20 h, cells
were collected for further analyses. Non-irradiated cells were used
as controls.
Clonogenic survival assay
For the clonogenic assays, cells were exposed to
different does of X-rays as aforementioned. Immediately following
irradiation, the cells were trypsinized, counted and reseeded onto
6-well plates at a density of 800 cells/well. The cells were
cultured for 14 days and stained with 0.05% crystal violet at room
temperature for 30 min (Sigma-Aldrich; Merck KGaA). The number of
colonies consisting of ≥50 cells was counted. The clonogenic
survival curves were constructed from three independent
experiments.
Reverse transcription quantitative PCR
(RT-qPCR) analysis
MUC1 mRNA levels were determined using RT-qPCR
analysis, as described previously (16). Briefly, total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed to first-strand cDNA using a PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). PCR
amplifications were performed using an ABI 7900 TaqMan Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primer sequences for MUC1 were as follows: Forward,
5′-TCAGCTTCTACTCTGGTGCACAA-3′ and reverse,
5′-ATTGAGAATGGAGTGCTCTTGCT-3′. PCR amplification of human GAPDH was
performed in parallel with the primers: forward,
5′-CGACCACTTTGTCAAGCTCA-3′and reverse, 5′-AGGGGTCTACATGGCAACTG-3′.
The relative MUC1 mRNA expression was determined according to the
2−ΔΔCq method following normalization against GAPDH
transcripts (17).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (Thermo Fisher Scientific, Inc.) containing a Complete
Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN,
USA) and centrifuged at 12,000 × g for 15 min at 4°C. Total protein
concentrations were quantified using a Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) The supernatants containing total
cellular protein were subjected to 10 or 12% SDS-PAGE.
Subsequently, the proteins were transferred to nitrocellulose
membranes. Membranes were blocked with 5% fat-free milk in
Tris-buffered saline (pH 7.4) with 0.1% Tween 20 (TBST) for 30 min
at room temperature and incubated with the primary antibodies
listed below overnight at 4°C. After washing with TBST, membranes
were incubated with horseradish peroxidase-conjugated goat
anti-mouse (cat. no., sc-2005; 1:4,000 dilution) or goat
anti-rabbit IgG (cat. no., sc-2004; 1:4,000 dilution; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature, and developed
using a enhanced chemiluminescence kit (GE Healthcare, Chicago, IL,
USA). Protein signals were quantified via densitometric analysis
using Quantity One software (version 4.6.2; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The primary antibodies (1:500 dilution)
used are as follows: Mouse monoclonal anti-MUC1 (cat. no. 4538);
mouse monoclonal anti-phospho-STAT3 (cat. no. 4113); mouse
monoclonal anti-STAT3 (cat. no. 9139); rabbit monoclonal
anti-β-actin (cat. no. 4970); rabbit polyclonal anti-phospho-JAK2
(cat. no. 3771); rabbit polyclonal anti-JAK2 (cat. no. 3230) (all
from Cell Signaling Technology, Inc., Danvers, MA, USA); rabbit
polyclonal anti-cleaved caspase-3 (cat. no. ab2302); rabbit
monoclonal anti-cleaved poly (ADP-ribose) polymerase (PARP; cat.
no. ab32064); mouse monoclonal anti-induced myeloid leukemia cell
differentiation protein Mcl-1 (Mcl-1; cat. no. ab114016); and
rabbit monoclonal anti-BCL2 like 1 (Bcl-xL; cat. no. ab32370) (all
from Abcam, Cambridge, MA, USA).
Apoptosis detection using flow
cytometry
Apoptosis was measured using the Annexin V-FITC
Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's protocol. Briefly, cells were washed with
Annexin V-binding buffer and incubated in binding buffer containing
Annexin V-FITC (25 µg/ml) and propidium iodide (PI; 25 µg/ml) for
10 min in the dark at room temperature. Stained cells were analyzed
using a FacsCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA) was
employed to perform statistical analyses. Differences among
multiple groups were analyzed by one-way analysis of variance
followed by the Tukey's test. P<0.05 was considered to indicate
a statistically significant difference.
Results
MUC1 is upregulated in HCC cells
following irradiation
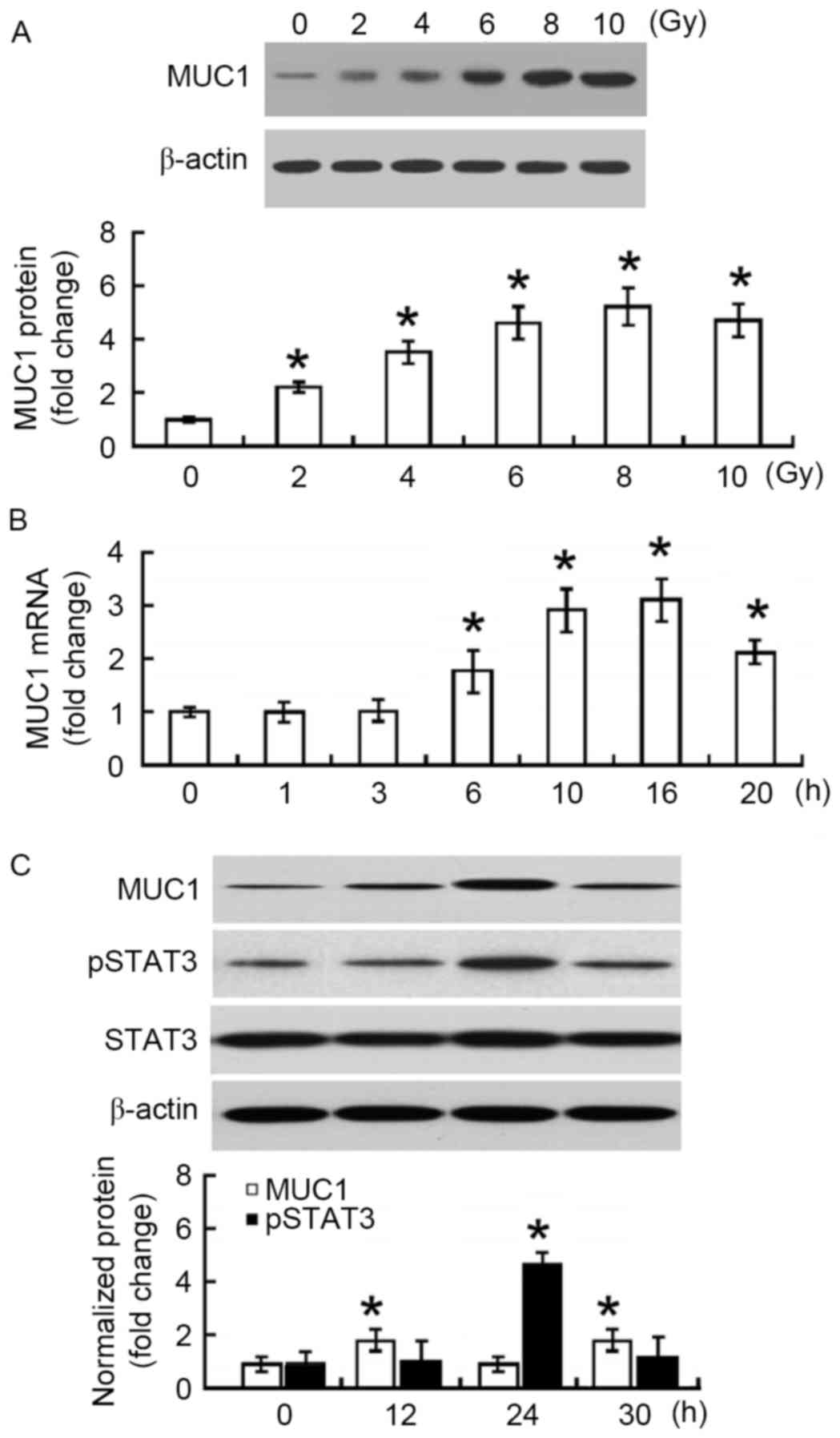
First, the changes in MUC1 protein expression in
irradiated SMMC-7721 cells were measured. At 24 h after
irradiation, a 2–5-fold increase in the MUC1 protein level was
observed when compared with non-irradiated SMMC-7721 cells
(P<0.05; Fig. 1A). The increase
was in a dose-dependent manner, reaching a peak at 8 Gy. To confirm
the upregulation of MUC1 in response to irradiation, the changes in
MUC1 mRNA abundance were examined. Time-course studies demonstrated
that MUC1 mRNA levels increased and peaked at 16 h following 8 Gy
irradiation (Fig. 1B). MUC1 protein
levels reached a peak at 24 h after irradiation and then declined
significantly at 30 h (Fig. 1C).
Furthermore, phosphorylated STAT3 protein exhibited similar
expression changes following irradiation (Fig. 1C).
MUC1 overexpression decreases the
sensitivity of HCC cells to radiation
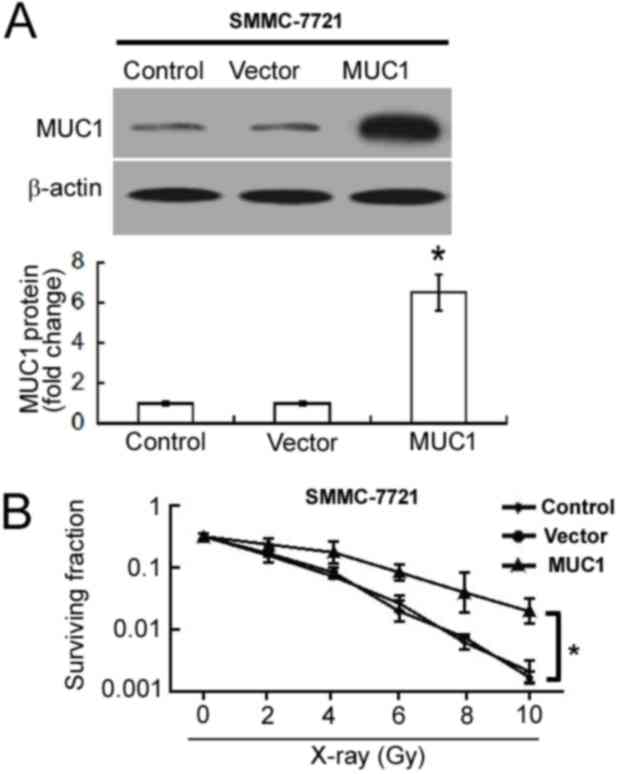
To determine the influence of MUC1 overexpression on
cellular radiosensitivity, MUC1 was overexpressed in SMMC-7721
cells. Western blot analysis confirmed the upregulation of MUC1 in
pcDNA3.1/MUC1-transfected cells compared with empty
vector-transfected cells (Fig. 2A).
Overexpression of MUC1 significantly increased the clonogenic
survival of SMMC-7721 cells following irradiation when compared
with empty vector-transfected cells (P<0.05; Fig. 2B).
MUC1 overexpression inhibits
irradiation-induced apoptosis
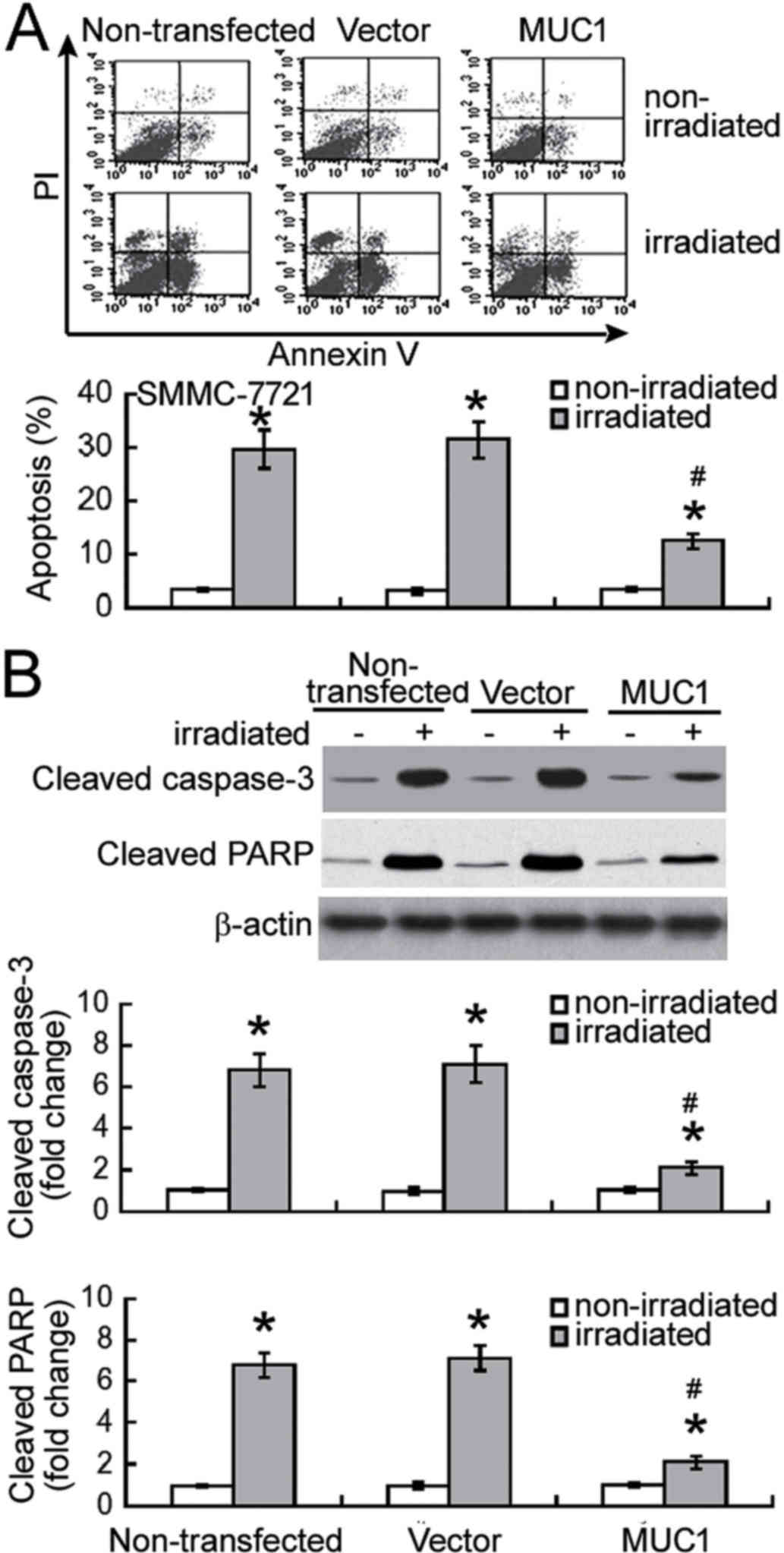
Next, the effect of MUC1 overexpression on
radiation-induced apoptosis was examined using Annexin-V/PI
staining analysis. Exposure to 8 Gy irradiation resulted in
significant apoptosis in non-transfected and vector-transfected
SMMC-7721 cells when compared with their corresponding
non-irradiated controls (P<0.05; Fig.
3A). Notably, enforced expression of MUC1 significantly
inhibited the irradiation-induced apoptosis by >60% (P<0.05).
Irradiation-induced apoptotic response was confirmed in SMMC-7721
cells by measuring the activation of caspase-3 and PARP. It was
observed that the levels of active cleaved caspase-3 and PARP were
significantly increased in non-transfected and vector-transfected
cells following irradiation (P<0.05; Fig. 3B). Furthermore, overexpression of MUC1
significantly attenuated the activation of caspase-3 and PARP in
response to irradiation exposure (P<0.05).
MUC1 inhibits irradiation-induced
apoptosis via activation of JAK2/STAT3
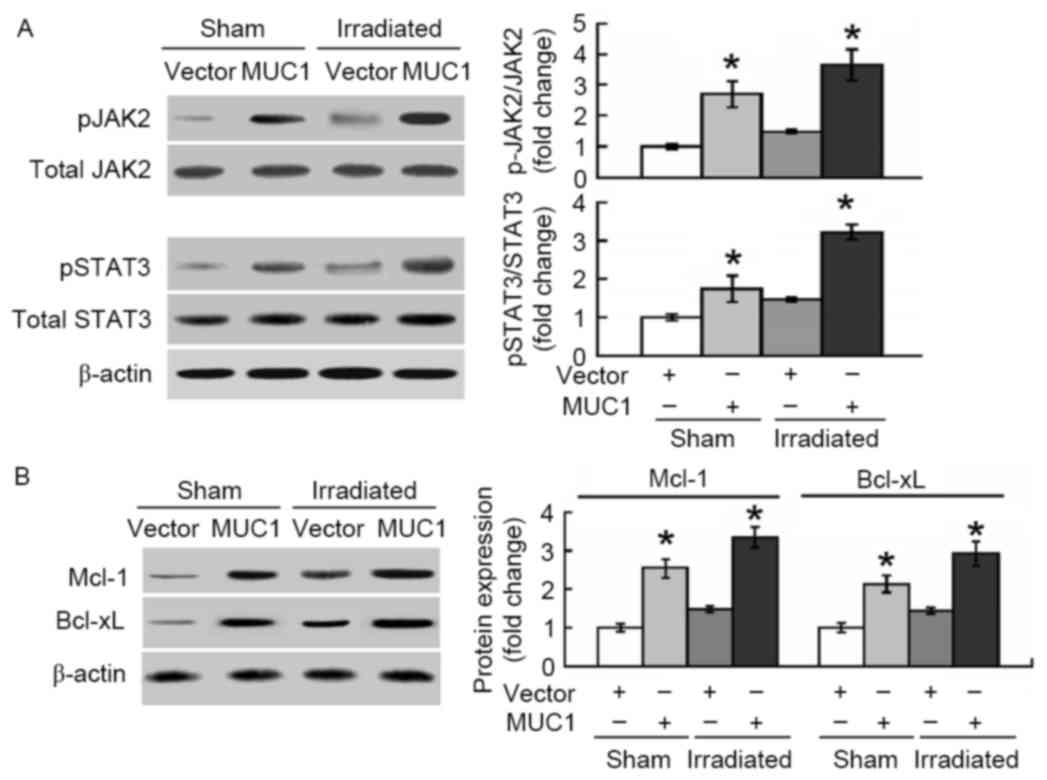
The molecular pathways involved in MUC1-mediated
protection against apoptosis induced by irradiation were then
explored. As presented in Fig. 4A,
overexpression of MUC1 significantly promoted the phosphorylation
of JAK2 and STAT3 in SMMC-7721 cells with or without irradiation
treatment. In addition, the anti-apoptotic proteins Mcl-1 and
Bcl-xL, downstream targets of STAT3, were induced by MUC1
overexpression (Fig. 4B).
To confirm the involvement of the STAT3 signaling
pathway in the function of MUC1, MUC1-overexpressing cells were
transfected with control or STAT3 shRNA prior to irradiation.
Notably, the depletion of STAT3 restored the apoptotic response of
MUC1-overexpressing cells following irradiation (Fig. 5A). Similarly, delivery of MUC1 shRNA
resensitized MUC1-overexpressing cells to irradiation-induced
apoptosis (Fig. 5A). At the molecular
level, transfection with STAT3- or MUC1-targeting shRNA
significantly reduced the protein levels of Mcl-1 and Bcl-xL in
MUC1 stably transfected SMMC-7721 cells (Fig. 5B).
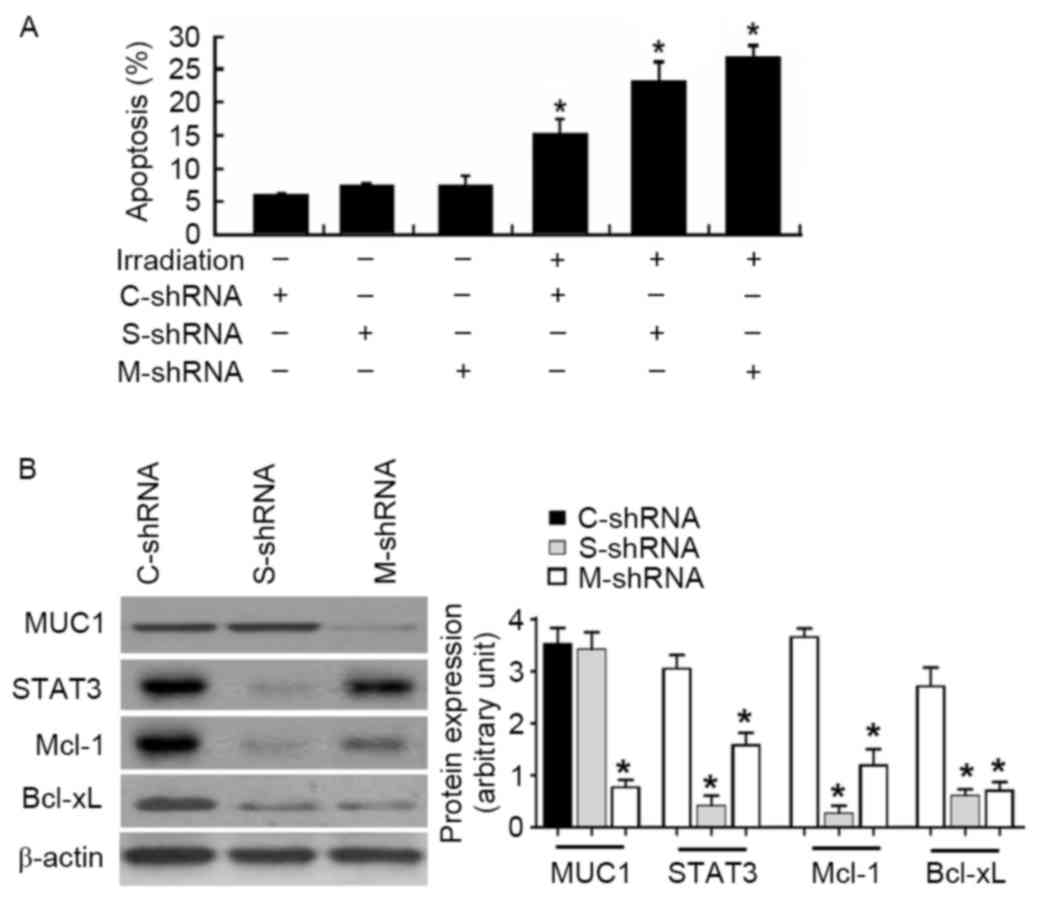 | Figure 5.Knockdown of STAT3 or MUC1 enhances
irradiation-induced apoptosis in MUC1 stably transfected SMMC-7721
cells. (A) Cells were transiently transfected with C-shRNA, S-shRNA
or M-shRNA prior to irradiation exposure. Apoptotis analysis was
determined by Annexin-V/propidium iodide staining. Values are
results from representative experiments performed in triplicates.
(B) Western blot analysis of indicated proteins in MUC1 stably
transfected SMMC-7721 cells retransfected with C-shRNA, S-shRNA or
M-shRNA. Left, representative western blots. Right, quantitative
analysis of protein expression from three independent experiments.
Data are expressed as the mean ± standard deviation. *P<0.05 vs.
C-shRNA-transfected cells. MUC1, Mucin 1; STAT3, signal transducer
and activator of transcription 3; Mcl-1, induced myeloid leukemia
cell differentiation protein Mcl-1; Bcl-xL, BCL2 like 1; shRNA,
short hairpin RNA; C-shRNA, control-shRNA; S, STAT3-shRNA; M-shRNA,
MUC1 shRNA. |
Discussion
The data of the present study demonstrated that
exposure to irradiation resulted in a transient increase in the
mRNA and protein levels of MUC1 in HCC cells, suggesting its
possible role as a stress-responsive survival factor. In support of
this hypothesis, Chen et al (18) reported that MUC1 protects human colon
cancer HCT116 cells from apoptosis under genotoxic stress induced
by cisplatin. Similarly, Wei et al (19) demonstrated that MUC1 suppresses the
p53-dependent apoptotic response to DNA damage. Under conditions of
nutrient deprivation and hypoxia, MUC1 has been revealed to
increase the cancer cell survival rate (20,21).
However, the adaptive induction of MUC1 in the present study did
not appear to be sufficient to prevent apoptotic death in
irradiated HCC cells, as significant apoptosis was detected at 48 h
after irradiation. To confirm the role of MUC1 in the regulation of
cellular response to irradiation, MUC1 was stably overexpressed in
HCC cells and the clonogenic survival rate following irradiation
exposure was examined. Of note, enforced expression of MUC1
significantly elevated the clonogenic survival of
irradiation-treated HCC cells when compared with transfection of
empty vector. Taken together, these results suggest that
maintenance of high-level MUC1 protein is required to protect HCC
cells from irradiation-induced apoptosis.
Induction of apoptosis is an important mechanism for
killing cancer cells via radiation exposure (22). MUC1 has demonstrated the ability to
modulate the apoptotic response in different cellular contexts. For
instance, inhibition of MUC1 significantly induced apoptosis in
pancreatic cancer cells (23) and
increased the sensitivity of lung cancer cells to anticancer
drug-induced apoptosis (24).
Upregulation of MUC1 has been identified to render human bronchial
epithelial cells more resistant to apoptosis following exposure to
nickel acetate (25). Hence, the
effect of MUC1 on radiation-induced apoptosis in HCC cells was
further assessed in the current study. Notably, it was demonstrated
that MUC1 overexpression significantly attenuated apoptotic
response in irradiated HCC cells, as determined using Annexin-V/PI
staining. Furthermore, irradiation-induced activation of caspase-3,
a major mediator of apoptosis, was significantly compromised by
MUC1 overexpression. These results collectively indicated that MUC1
overexpression confers protection against radiation-induced
apoptosis in HCC cells. However, one of the major limitations of
the present study was the use of only one HCC cell line
(SMMC-7721).
The JAK2/STAT3 signaling pathway is commonly
associated with the emergence of apoptosis resistance in cancer
cells, thus representing a potential target for anticancer therapy
(26,27). The data in the current study revealed
that MUC1 overexpression significantly enhanced the phosphorylation
of JAK2 and STAT3 in irradiated HCC cells. Furthermore, the
downstream anti-apoptotic proteins Mcl-1 and Bcl-xL were also
induced by MUC1 overexpression. To check the possibility that MUC1
regulates the radiosensitivity of HCC cells through alteration of
the JAK2/STAT3 signaling pathway, MUC1 and STAT3 shRNA were
coexpressed prior to radiation treatment. Similar to the knockdown
of MUC1, depletion of STAT3 reversed the protective effect of MUC1
against irradiation-induced apoptosis in HCC cells. Taken together,
evidence was provided that MUC1-mediated radioresistance in HCC
cells is partially ascribed to activation of the JAK2/STAT3
signaling pathway. Despite these findings, the involvement of other
signaling pathways in the action of MUC1 cannot be excluded.
Indeed, MUC1 has been documented to inhibit cisplatin-induced
apoptosis of colon cancer cells via activation of JNK
mitogen-activated protein kinase (MAPK) signaling (18). Modulation of
extracellular-signal-regulated kinase 1/2, MAPK and Akt
serine/threonine kinase signaling has also been reported to
contribute to the oncogenic roles of MUC1 in pancreatic cancer
cells (23).
In conclusion, to the best of our knowledge, the
results of the present study have presented the first evidence for
the implication of MUC1 overexpression in radioresistance of HCC
cells. The results suggested that MUC1-mediated protection against
irradiation-induced apoptosis is associated with activation of the
JAK2/STAT3 signaling pathway, and induction of anti-apoptotic
proteins Mcl-1 and Bcl-xL. Further studies are warranted to explore
the significance of targeting MUC1 in improving radiotherapy in
animal models of HCC.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 31:1245–1255. 2012. View Article : Google Scholar
|
|
3
|
Schlachterman A, Craft WW Jr, Hilgenfeldt
E, Mitra A and Cabrera R: Current and future treatments for
hepatocellular carcinoma. World J Gastroenterol. 21:8478–8491.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalogeridi MA, Zygogianni A, Kyrgias G,
Kouvaris J, Chatziioannou S, Kelekis N and Kouloulias V: Role of
radiotherapy in the management of hepatocellular carcinoma: A
systematic review. World J Hepatol. 7:101–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee IJ and Seong J: Radiotherapeutic
strategies in the management of hepatocellular carcinoma. Oncology.
81 Suppl 1:S123–S133. 2011. View Article : Google Scholar
|
|
6
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitamoto S, Yokoyama S, Higashi M, Yamada
N, Takao S and Yonezawa S: MUC1 enhances hypoxia-driven
angiogenesis through the regulation of multiple proangiogenic
factors. Oncogene. 32:4614–4621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronnier C, Bruyère E, Lahdaoui F,
Jonckheere N, Perrais M, Leteurtre E, Piessen G, Mariette C and Van
Seuningen I: The MUC1 mucin regulates the tumorigenic properties of
human esophageal adenocarcinomatous cells. Biochim Biophys Acta.
1843:2432–2437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Liu G, Li Q, Wang F, Xie F, Zhai
R, Guo Y, Chen T, Zhang N, Ni W, et al: Mucin1 promotes the
migration and invasion of hepatocellular carcinoma cells via
JNK-mediated phosphorylation of Smad2 at the C-terminal and linker
regions. Oncotarget. 6:19264–19278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Q, Piyush T, Chen C, Hollingsworth
MA, Hilkens J, Rhodes JM and Yu LG: MUC1 extracellular domain
confers resistance of epithelial cancer cells to anoikis. Cell
Death Dis. 5:e14382014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohr AM, Bailey JM, Lewallen ME, Liu X,
Radhakrishnan P, Yu F, Tapprich W and Hollingsworth MA: MUC1
regulates expression of multiple microRNAs involved in pancreatic
tumor progression, including the miR-200c/141 cluster. PLoS One.
8:e733062013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nath S, Daneshvar K, Roy LD, Grover P,
Kidiyoor A, Mosley L, Sahraei M and Mukherjee P: MUC1 induces drug
resistance in pancreatic cancer cells via upregulation of multidrug
resistance genes. Oncogenesis. 2:e512013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kharbanda A, Rajabi H, Jin C, Raina D and
Kufe D: Oncogenic MUC1-C promotes tamoxifen resistance in human
breast cancer. Mol Cancer Res. 11:714–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou B, Chen H, Wei D, Kuang Y, Zhao X, Li
G, Xie J and Chen P: A novel miR-219-SMC4-JAK2/Stat3 regulatory
pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res.
33:552014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CY, Lin CS, Tai WT, Hsieh CY, Shiau
CW, Cheng AL and Chen KF: Sorafenib enhances radiation-induced
apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J
Radiat Oncol Biol Phys. 86:456–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Dinwiddie DL, Harrod KS, Jiang Y and
Kim KC: Anti-inflammatory effect of MUC1 during respiratory
syncytial virus infection of lung epithelial cells in vitro. Am J
Physiol Lung Cell Mol Physiol. 298:L558–L563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Li D, Ren J, Li C and Xiao ZX:
MUC1 activates JNK1 and inhibits apoptosis under genotoxic stress.
Biochem Biophys Res Commun. 440:179–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei X, Xu H and Kufe D: Human MUC1
oncoprotein regulates p53-responsive gene transcription in the
genotoxic stress response. Cancer Cell. 7:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin L, Kharbanda S and Kufe D: Mucin 1
oncoprotein blocks hypoxia-inducible factor 1alpha activation in a
survival response to hypoxia. J Biol Chem. 282:257–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin L, Kharbanda S and Kufe D: MUC1
oncoprotein promotes autophagy in a survival response to glucose
deprivation. Int J Oncol. 34:1691–1699. 2009.PubMed/NCBI
|
|
22
|
Balcer-Kubiczek EK: Apoptosis in radiation
therapy: A double-edged sword. Exp Oncol. 34:277–285.
2012.PubMed/NCBI
|
|
23
|
Tréhoux S, Duchêne B, Jonckheere N and Van
Seuningen I: The MUC1 oncomucin regulates pancreatic cancer cell
biological properties and chemoresistance. Implication of p42-44
MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophys Res Commun.
456:757–762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Wells A, Padilla MT, Kato K, Kim KC
and Lin Y: A signaling pathway consisting of miR-551b, catalase and
MUC1 contributes to acquired apoptosis resistance and
chemoresistance. Carcinogenesis. 35:2457–2466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castorina A and Giunta S: Mucin 1 (MUC1)
signalling contributes to increase the resistance to cell death in
human bronchial epithelial cells exposed to nickel acetate.
Biometals. 27:1149–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Tekle C, Chen YW, Kristian A, Zhao
Y, Zhou M, Liu Z, Ding Y, Wang B, Mælandsmo GM, et al: B7-H3
silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3
phosphorylation. Mol Cancer Ther. 10:960–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DH, Sung KS, Bartlett DL, Kwon YT and
Lee YJ: HSP90 inhibitor NVP-AUY922 enhances TRAIL-induced apoptosis
by suppressing the JAK2-STAT3-Mcl-1 signal transduction pathway in
colorectal cancer cells. Cell Signal. 27:293–305. 2015. View Article : Google Scholar : PubMed/NCBI
|