Introduction
Telomeres were discovered and named by Muller in
1938, and comprise of a repetitive DNA sequence (5′-TTAGGG-3′)
(1,2).
Telomeres are protein-DNA complexes at the ends of eukaryotic
chromosomes, which aid in completing the replication of chromosome
ends, preventing chromosomes from fusion and chromosome
reorganization and degradation (3–5). A
previous study reported that telomeres may be involved in gene
expression regulation, thus modulating the cell replication process
and aging (6). Telomeres are also
associated with numerous proteins, including telomeric repeat
binding factor (TERF/TRF)1, TRF2, TERF1 interacting nuclear factor
2 (TIN2), protection of telomere 1 (POT1), tripeptidyl peptidase 1
(TPP1) and TERF2 interacting protein. Furthermore,
telomere-associated proteins possess functions that maintain the
integrity of the chromosome tail and regulate the telomere
extension process (7). Telomerase is
a ribonucleoprotein complex, which consists of telomerase RNA, a
reverse transcriptase subunit (telomerase reverse transcriptase,
hTERT) and associated proteins (8,9). Its
activation is essential to the continuous proliferation of cells
and is involved in malignant tumor proliferation (10,11).
Human POT1 is a housekeeping gene containing a total
of 22 exons, which is expressed extensively in human tissues and
cells, and its translation begins in the sixth exon. POT1 is
located on chromosome 7 (7q31.33) and has a total length of ~120
kb, the cDNA sequence length is ~2631 bp and the coding region is
located between bp 24 and 1928 (12).
Previous studies have established that POT1-TPP1 in combination
with a single-stranded telomeric DNA enhances telomerase activity
(13,14). In addition, hPOT1 is able to convey
the relevant information regarding telomere length that TRF1-TRF2
contains, to the telomeric DNA ends and telomerase, subsequently
telomerase is activated, guaranteeing stability of the telomere
length (12,15). Veldman et al (16) reported that HeLa telomere stability
was decreased significantly following the inhibition of hPOT1
function via RNA interference knockdown, ultimately leading to cell
senescence and apoptosis. Armbruster et al (17) demonstrated that mutations to the DAT
domain of hTERT restored telomerase activity and extended telomere
length following increased expression of POT1 in
telomerase-positive cells. However, in cells with low POT1
expression in telomerase-negative cells, telomere length was not
affected. This suggests that POT1 has a telomerase-dependent
regulatory function on telomere length (17). POT1 regulates telomerase activity via
the collaboration with other DNA-binding proteins and as part of
telomere protein complexes in mammalian cells. When POT1 expression
is increased or decreased, it affects the conformation of the
telomere complex, relieving the steric effect and enabling telomere
elongation due to POT1 binding with single-stranded telomeric DNA
(18). Therefore, it is evident that
POT1 exhibits an influence on telomerase activity and the
maintenance of telomere length.
In the present study, the transcription activity of
the POT1 promoter in 4 tumor cell lines was determined by
constructing its sequences with different lengths of the luciferase
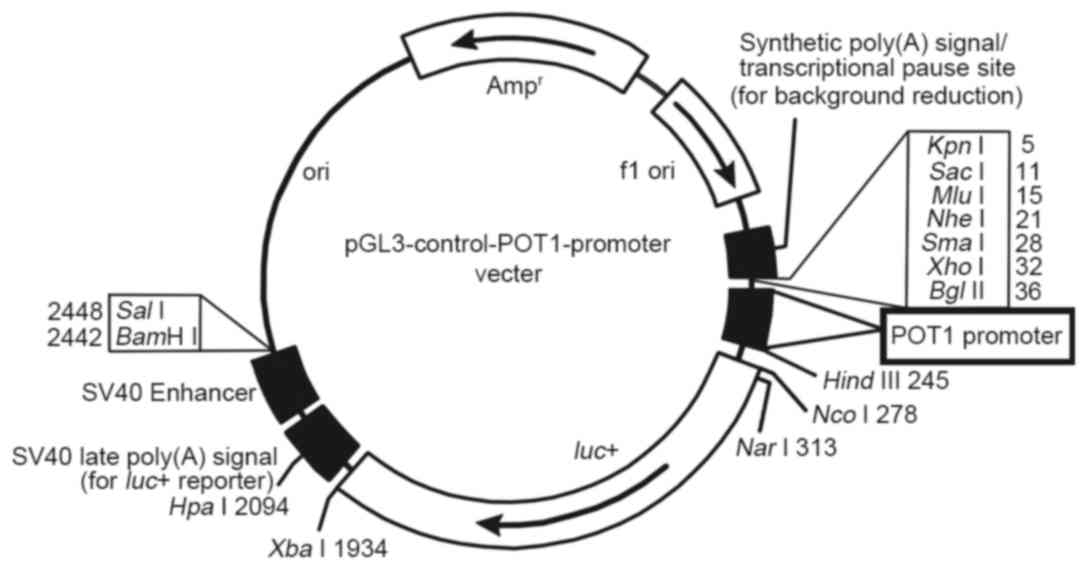
reporter gene carrier (Fig. 1). In
addition, the regulatory mechanism of the POT1 promoter was
preliminarily investigated through evaluating its correlation with
POT1, telomerase and telomere length.
Materials and methods
Cell culture
The A549, HeLa, H460 and HepG2 cancer cells were
provided by the laboratory of the Guangdong Medical College
Institute (Guangdong, China), and were stored in the laboratory of
Guangdong Medical College Institute. For all experiments, the four
types of cancer cells were plated in Dulbecco's modified Eagle
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 4.5 g/l D-glucose and 10% fetal calf serum (Zhejiang
Tianhang Biological Technology Co., Ltd., Hangzhou, China). All
cells were cultured at 37°C in a humidified environment containing
5% CO2.
Plasmid construction
POT1-promoter-1 (−160 to +40) and POT1-promoter-2
(−370 to +90) clone segment primers (Table I) were synthesized by Beijing Genomics
Institute (Shenzhen, China). Both primer pairs contained the
BglII (5′-GCAGATCT-3′) and HindIII (5′-GCAAGCTT-3′)
endonuclease sites. The genomic DNA was extracted from A549 cells
using the MiniBest Universal Genomic DNA Extraction kit (version
5.0; Takara Biotechnology Co., Ltd., Dalian, China), according to
the manufacturer's protocol. Then the target fragment was amplified
through polymerase chain reaction (PCR) using 2X Taq PCR MasterMix
(Tiangen Biotech Co., Ltd., Beijing, China), and recovered using
the MiniBest DNA Fragment Purification kit (version 3.0; Takara
Biotechnology Co., Ltd.), according to the manufacturer's protocol.
The thermocycler conditions for the PCR were as follows: 3 min at
94°C; 30 cycles of 94°C for 30 sec, 66°C for 30 sec and 72°C for 1
min; and 72°C for 5 min. Subsequently, the pGL3-control plasmid
(Promega Corporation, Madison, WI, USA) and PCR products containing
the desired sequence were double-digested with BglII and
HindIII, and annealed together using T4 DNA ligase (Takara
Biotechnology Co., Ltd.) at 16°C overnight. The ligation products
were transformed into chemocompetent Escherichia coli DH5α
cells (Tiangen Biotech Co., Ltd., Beijing, China) maintained in LB
medium (Shanghai Kehua Bio-Engineering Co., Ltd., Shanghai, China)
and bacterial culture medium containing ampicillin (Tiangen Biotech
Co., Ltd., Beijing, China) to select for the recombinant
plasmid-positive colonies. Identification of recombinants with the
desired PCR products were confirmed using 1% agarose
electrophoresis and DNA sequencing. The recombinant plasmids were
termed as POT1-promoter-1 (pGL3-Control-POT1-promoter-1) and
POT1-promoter-2 (pGL3-Control-POT1-promoter-2).
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Primer | DNA sequence
(5′-3′) |
|---|
| POT1, sense |
5′-TGTTTCCGTGTTGATGATGTG-3′ |
| POT1,
antisense |
5′-TGGCACCTTTGGACCTCTAC-3′ |
| TERT, sense |
5′-CAAGCTGTTTGCGGGGATTC-3′ |
| TERT,
antisense |
5′-TGGCACCTTTGGACCTCTAC-3′ |
| GAPDH, sense |
5′-GGAGTCTGGGAAGGGTTG-3′ |
| GAPDH,
antisense |
5′-CAGTTTGGCTTGCTGGTC-3′ |
| tel, sense |
5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′ |
| tel, antisense |
5′-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA-3′ |
| β-globin,
sense |
5′-GCTTCTGACACAACTGTGTTCACTAGC-3′ |
| β-globin,
antisense |
5′-CACCAACTTCATCCACGTTCACC-3′ |
| POT1-promoter-1,
sense |
5′-GCAGATCTCCCGCTTCCCCTAAGCTTGCCTCCC-3′ |
| POT1-promoter-1,
antisense |
5′-GCAAGCTTGGTTCACACACTGATGGCGCCTGGA-3′ |
| POT1-promoter-2,
sense |
5′-GCAGATCTGCAAGACTCAATGGTGGCA-3′ |
| POT1-promoter-2,
antisense |
5′-GCAAGCTTGGGCATAGTCGCTTGTTCT-3′ |
Transfection and dual-luciferase
report assays
A total of 0.5–2×105 A549, HeLa, H460 or
HepG2 cancer cells/well were seeded in 24-well plates. After 24 h
incubation at 37°C, 1.5 µg of POT1-promoter-1, POT1-promoter-2,
pGL3-basic and pGL3-control were mixed with 0.03 µg pRL-TK (all
Promega Corporation) in 150 µl Opti-MEM I® Reduced Serum
(Thermo Fisher Scientific, Inc.); the mixtures were separately
co-transfected with Firefly luciferase (Fluc)-Renilla luciferase
(Rluc) into tumor cells using Lipofectamine™ 2000
(Thermo Fisher Scientific, Inc.). After another 24 h of incubation
at 37°C, luciferase activity was measured using the
Dual-Luciferase® Reporter Assay system (Promega
Corporation). Briefly, the culture medium was removed and the cells
were washed with cold 1X PBS. Then 1X passive lysis buffer (PLB;
100 µl; Promega Corporation) was added to the 24-well plates. The
culture plates were agitated at a low speed for 20 min at room
temperature and transferred to clean 1.5 ml centrifuge tubes (in
special cases, a cell scraper or pipette was used to repeatedly
beat the samples to ensure complete lysis of the samples).
Subsequently, Luciferase Assay reagent II (100 µl/well) and cell
lysate (20 µl/well) were added to 96-well plates, and mixed evenly.
The activity of Fluc was detected using a microplate reader with
1–2 sec delay and 5–10 sec reading. Finally, the activity of Rluc
was detected following the addition of 100 µl
Stop&Glo® reagent (Promega Corporation) for
normalization.
RNA extraction and reverse
transcription (RT)
Total RNA was extracted from each non-transfected
sample using TRIzol reagent (Thermo Fisher Scientific, Inc.) and RT
was performed according to the FastQuant RT kit protocol (Tiangen
Biotech Co., Ltd.). The isolated RNA was used as a template for
reverse transcription using the following protocol: Each 20 µl
reaction contained 5X gDNA buffer, 10X Fast RT buffer, FQ-RT Primer
mix, RT Enzyme mix, RNase-free ddH2O and 1 µg of total
RNA. Briefly, the RNA, gDNA buffer and RNase-free ddH2O
were incubated at 42°C for 3 min, and then immediately placed on
ice. The solutions described above were mixed and incubated at 42°C
for 15 min followed by incubation at 95°C for 3 min. The reaction
solutions were stored at −20°C for use in subsequent
experiments.
Quantitative (q)PCR for POT1 and
hTERT
Primer sequences of POT1, TERT and GAPDH are listed
in Table I. The total volume of 20 µl
of qPCR contained 0.25 µM each of forward and reverse primers, 10
µl SYBR® Premix Ex Taq™ (Takara Biotechnology
Co., Ltd.), 4 µl cDNA and 5 µl nuclease-free ddH2O.
Three replicates were performed. The thermocycler conditions were
as follows: 5 min at 95°C; 40 cycles of 95°C for 15 sec and 60°C
for 30 sec; 95°C for 5 sec; and 60°C for 1 min. The reaction was
performed using an ABI Prism® 7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The results
were analyzed using the 2−ΔΔCq method (19) to compare the transcriptional levels of
POT1 and hTERT in each sample to the non-compound-treated control.
ΔCq=Cq(POT1 or hTERT)-Cq(GAPDH), ΔΔCq=ΔCq (target genes in the
sample to be tested)-ΔCq (target gene in the control sample). The
relative amount of sample template was 2−ΔΔCq.
DNA extraction and qPCR for telomere
length
DNA from all non-transfected tumor cells were
extracted using the Takara MiniBest Universal Genomic DNA
Extraction kit (Version 5.0) according to the manufacturer's
protocol. The total PCR volume of 20 µl contained 36 ng DNA
template, 0.25 µM forward and reverse primers each [Table I; tel (1) and β-globin (20)], 10 µl 2X SYBR® Premix Ex
Taq and nuclease-free ddH2O. Three replications were
performed. The thermocycling conditions maintained were as follows:
5 min at 95°C; 40 cycles of 95°C for 15 sec and 60°C for 32 sec;
95°C for 5 sec; and 60°C for 1 min. For the present study, telomere
(T) and single copy gene (S; reference gene, β-globin) PCRs were
performed in separate 96-well plates. The T/S ratio was
~2ΔCq. ΔCq=Cq(Telomere)-Cq(β-globin). The relative T/S
ratio (T/S of one sample relative to the T/S of another sample) was
2−(ΔCq1-ΔCq2)=2−ΔΔCq. ΔCq1 was the T/S ratio
of each sample, ΔCq2 was the T/S ratio of control DNA. The mean of
the relative T/S ratio is proportional to telomere length as
previously reported (21,22).
Analysis of the association between
POT1 promoter and POT1, hTERT, and telomere length
The association between the POT1 promoter relative
activity and POT1 relative expression, hTERT relative expression
and the relative of telomere length was performed using partial
correlation coefficients and linear regression.
Statistical analysis
All data are representative of three independent
experiments and expressed as the mean ± standard error of the mean.
The five different groups being compared in the dual-luciferase
report assays were as follows: Blank, non-transfected cells;
positive, pGL3-Control-transfected cells; negative,
pGL3-Basic-transfected cells; tested, cells transfected with
POT1-promoter-1 or POT1-promoter-2. The qPCR tests were performed
on the four non-transfected tumor cell lines. Statistical
significance was assessed using a two-tailed t-test, linear
regression and partial correlation coefficient analysis with SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Recombinant plasmid construction of
pGL3-Control-POT1-promoter-1
The luciferase reporter gene carrier is demonstrated
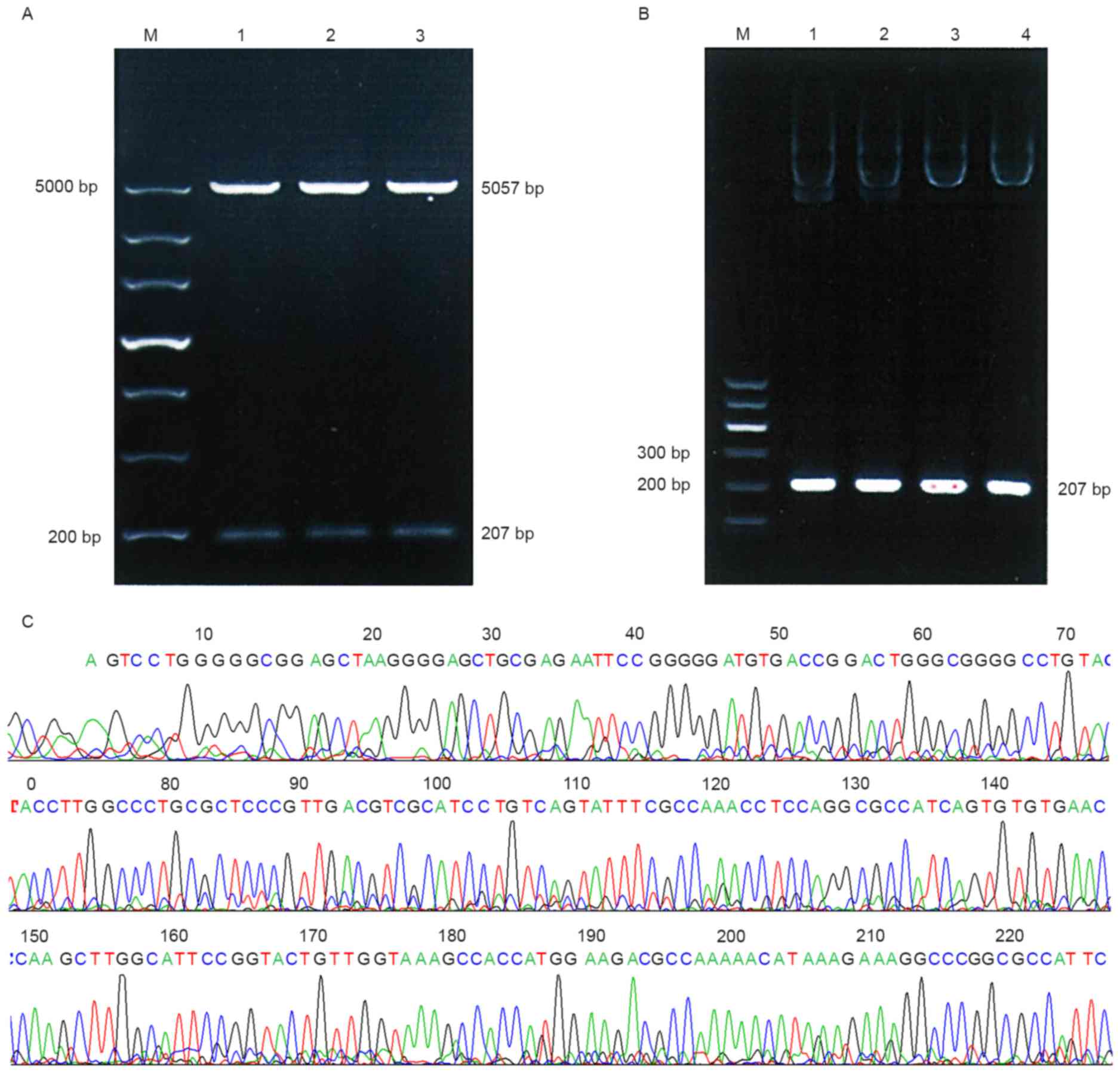
in Fig. 1. As presented in Fig. 2A, POT1-promoter-1 was identified using
BglII and HindIII digestion, the PCR product of the
recombinant plasmids (Fig. 2B) and
sequencing results (Fig. 2C) indicate
that the desired gene was successfully inserted into the plasmid
vector pGL3-Control. These results suggest that no base mutation
had occurred in the two fragments.
Recombinant plasmid construction of
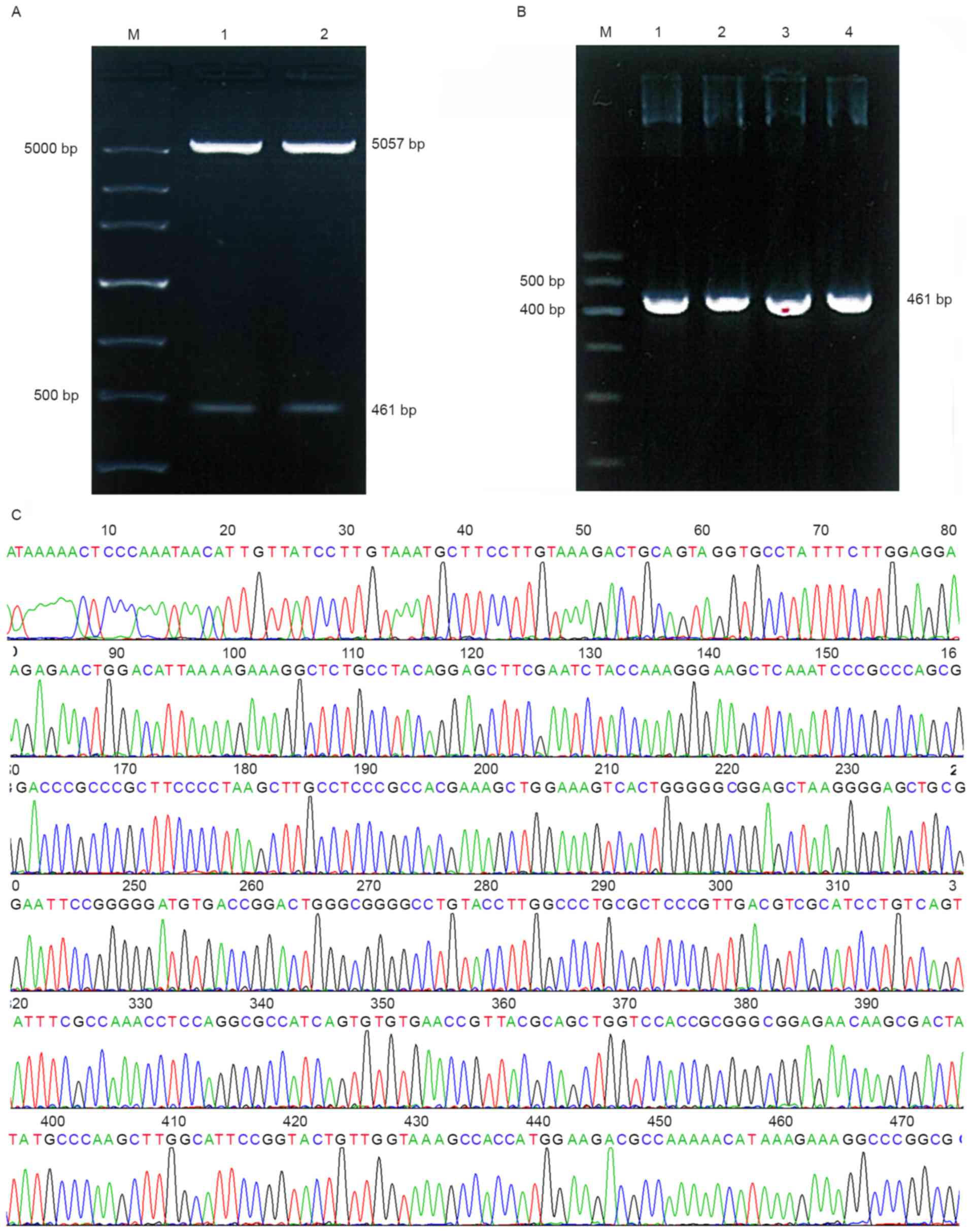
pGL3-Control-POT1-promoter-2
As presented in Fig.
3A, two bands were observed following double BglII and
HindIII digestion. The PCR product of the recombinant
plasmids (Fig. 3B) and sequencing
results (Fig. 3C) indicate that the
desired gene was successfully inserted into pGL3-Control. These
results suggest that there were no base mutations in the two
fragments.
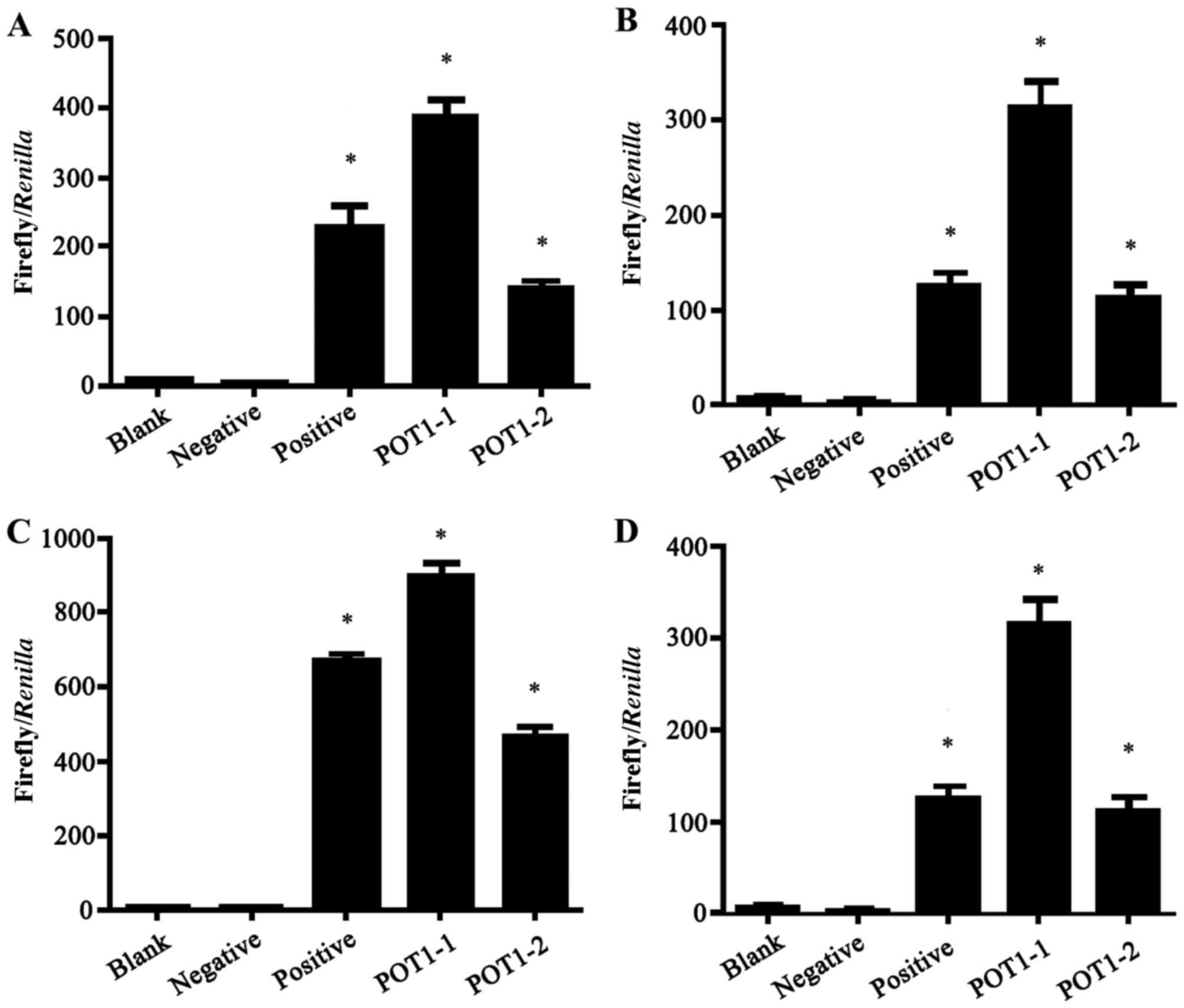
Results of the dual luciferase
reporter gene assay for all four tumor cell lines
Fluc and Rluc vectors were mixed in the ratios 1:1,
10:1, 20:1, 50:1, and 100:1. Following transfection for 12, 24 or
36 h, the highest transfection efficiency was identified using a
50:1 ratio for 24 h. The mixture of Fluc-Rluc (50:1) and the
plasmid was co-transfected into the four different types of tumor
cells. The transcription activity of the POT1 promoter was then
detected using the Dual-Luciferase Reporter assay system 24 h. As
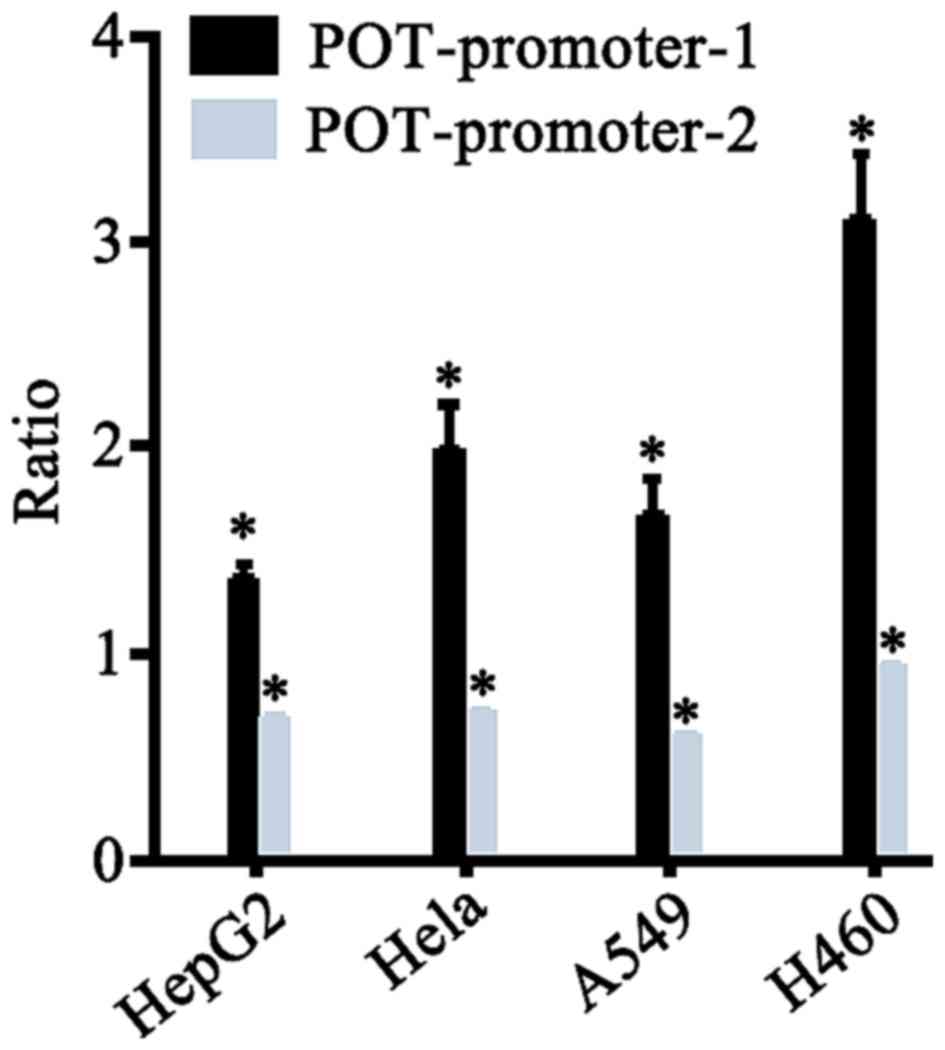
presented in Fig. 4, the
transcription activity was highest in the POT1-promoter-1 group in
all four tumor cell lines. The luciferase activity in the positive,
POT1-promoter-1 and POT1-promoter-2 groups were significantly
increased compared with the negative group (P<0.001; Fig. 4).
Comparison between the transcription
activity of POT1 promoter-1 and −2 reporter genes in different
tumor cell lines
pGL3-Control with strong SV40 promoter activity was
used as a reference, the ratio between POT1 and SV40 promoter
activity provided as an output value of transcription activity. The
transcription activities between the POT1 promoter-1 and −2
reporter genes in different tumor cell lines were compared
(x- ±s, n=3). As presented in Fig.
5 and Table II, POT1-promoter-1
exhibited significantly higher transcription activity compared with
that of POT1-promoter-2 in all four cancer cell lines. Therefore,
the following experiments were performed using POT1-promoter-1.
 | Table II.The relative activity of the two
POT1-promoter regions in four human tumor cell lines. |
Table II.
The relative activity of the two
POT1-promoter regions in four human tumor cell lines.
| Cell line |
POT1-promoter-1/SV40 promoter, % |
POT1-promoter-2/SV40 promoter, % |
|---|
| HepG2 |
1.34±0.08a |
0.68±0.02a |
| HeLa |
1.97±0.24a |
0.71±0.06a |
| A549 |
1.65±0.59a |
0.59±0.01a |
| H460 |
3.08±0.33a |
0.92±0.02a |
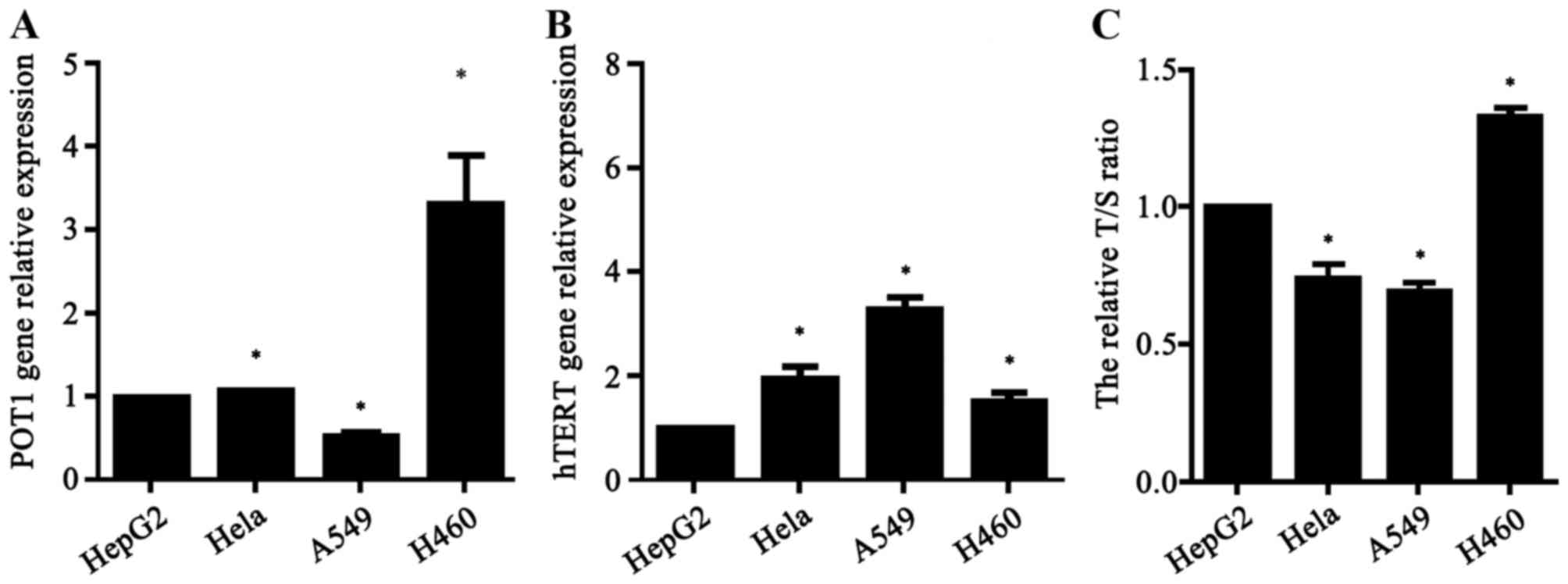
qPCR
The POT1 expression, hTERT expression and telomere
length of all non-transfected tumor cells were determined using
qPCR analysis. All values were normalized to GAPDH and are
expressed relative to the HepG2 cell group, which were selected
arbitrarily and used as a control group for base comparison. The
relative activity of telomerase was indirectly demonstrated by
detecting the relative expression of hTERT. The relative T/S ratio
was used to reflect telomere length. POT1 expression (Fig. 6A), telomerase activity (Fig. 6B) and telomere length (Fig. 6C) were significantly different across
the four types of tumor cells. The highest relative expression of
POT1 was in H460 cells, and the lowest in A549 cells. The highest
relative activity of telomerase was in A549 and the lowest in HepG2
cells. The relative telomere length was longest in H460 cells and
shortest in A549 cells.
Correlation between the POT1 promoter
activity and POT1 expression, hTERT expression, telomere
length
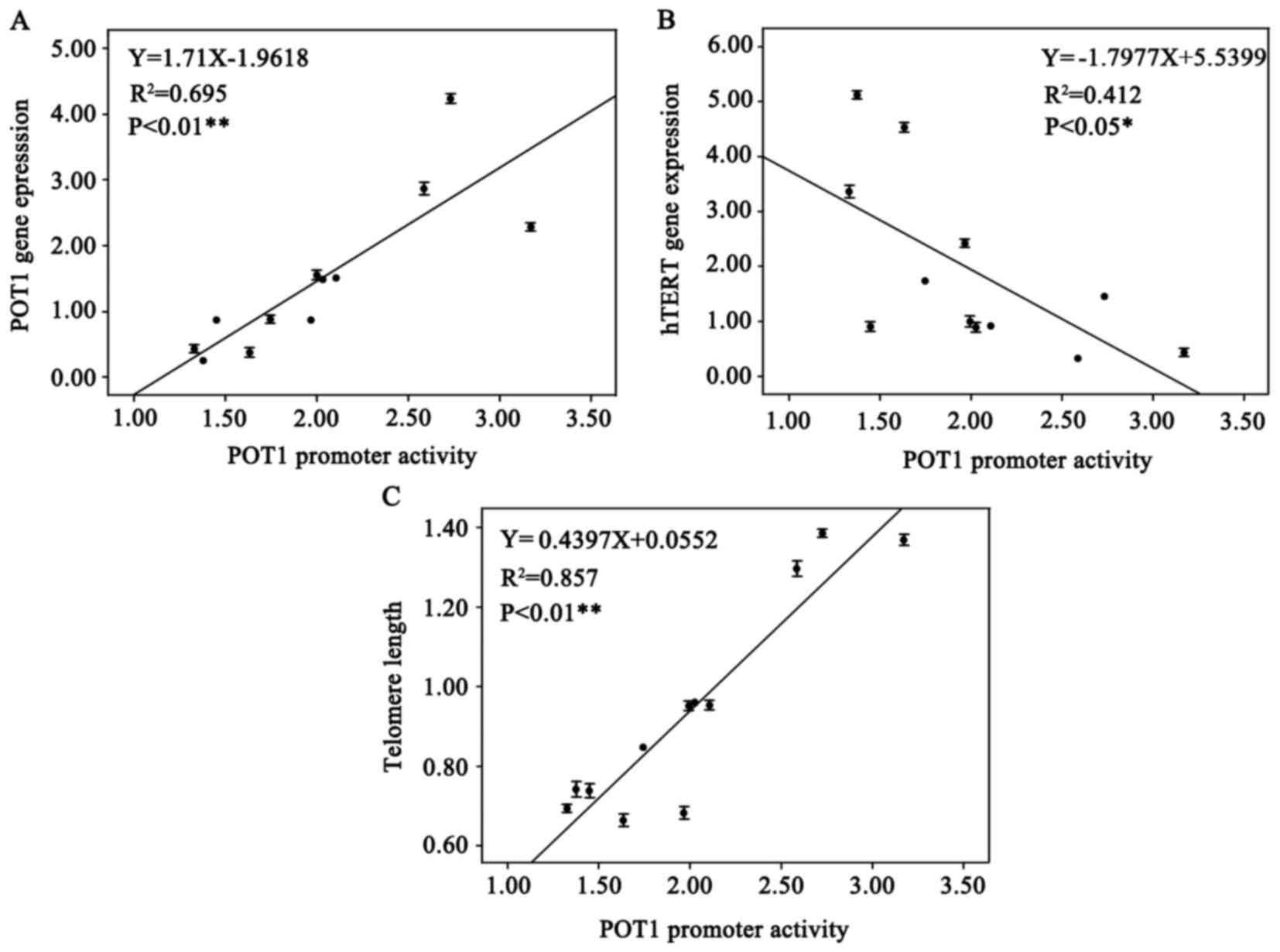
An analysis of the partial correlation coefficients
between the POT1 promoter activity and the three aforementioned
factors was performed. Luciferase activity of POT1-promoter-1 was
used as a covariate. There was a significant positive correlation
between the POT1 promoter activity and POT1 expression (partial
correlation coefficient, 0.839) and telomere length (partial
correlation coefficient, 0.792) (both P<0.05; Table III and IV), while a significant negative
correlation was identified between POT1 promoter activity and hTERT
expression (partial correlation coefficient, −0.700; P<0.05;
Table V). Furthermore, Fig. 7 demonstrates a marked correlation
between POT1 promoter activity and POT1 and hTERT expression and
telomere length by linear regression analysis (Fig. 7A and C, P<0.01; Fig. 7B, P<0.05), which are similar to the
results of the partial correlation analysis.
 | Table III.Partial correlation coefficients
between POT1 promoter activity and POT1 expression. |
Table III.
Partial correlation coefficients
between POT1 promoter activity and POT1 expression.
| Control
variables | POT1 promoter
activity | POT1
expression |
|---|
| POT1 promoter
activity |
|
|
|
Correlation | 1.000 | 0.839 |
|
P-value |
| 0.001a |
| df | 0 | 9 |
| POT1
expression |
|
|
|
Correlation | 0.839 | 1.000 |
|
P-value | 0.001a |
|
| df | 9 | 0 |
 | Table IV.Partial correlation coefficients
between POT1 promoter activity and telomere length. |
Table IV.
Partial correlation coefficients
between POT1 promoter activity and telomere length.
| Control
variables | POT1 promoter
activity | Telomere
length |
|---|
| POT1 promoter
activity |
|
|
|
Correlation | 1.000 | 0.792 |
|
P-value |
| 0.004a |
| df | 0 | 9 |
| Telomere
length |
|
|
|
Correlation | 0.792 | 1.000 |
|
P-value | 0.004a |
|
| df | 9 | 0 |
 | Table V.Partial correlation coefficients
between POT1 promoter activity and hTERT expression. |
Table V.
Partial correlation coefficients
between POT1 promoter activity and hTERT expression.
| Control
variables | POT1 promoter
activity | hTERT
expression |
|---|
| POT1 promoter
activity |
|
|
|
Correlation | 1.000 | −0.700 |
|
P-value |
| 0.016a |
| df | 0 | 9 |
| hTERT
expression |
|
|
|
Correlation | −0.700 | 1.000 |
|
P-value | 0.016a |
|
| df | 9 | 0 |
Discussion
Reporter genes are genes with a readily measurable
phenotype that may be distinguished in a background of endogenous
proteins (23). Reporter gene
technology is widely used to monitor the cellular events associated
with signal transduction and gene expression (23). The principal advantage of reporter
gene technology is its high sensitivity, reliability, convenience
and adaptability to large-scale measurements (23). In the present study, two different
fragments of the active promoter region of POT1 were obtained
through cloning. Luciferase reporter gene vectors that contained
different sequence lengths of the POT1 promoter were successfully
constructed using DNA recombinant technology. The results of the
double enzyme digestion PCR analysis and base sequencing
demonstrated that the vector was successfully constructed.
Construction of the POT1 promoter reporter gene vector is an
effective method for investigating the transcriptional activity of
the POT1 promoter and is able to provide a foundation for further
studies on the transcriptional regulation mechanism of the POT1
promoter.
The luciferase reporter gene assay is one of the
most predominantly used methods for identifying the interaction
between a promoter and the genome (24). The dual-luciferase reporter assay
system uses a promoter-luciferase-based structure and is used for
transient transfection. Tiffen et al (25) confirmed that the expression and
associated light-emitting activity of the luciferase gene does not
affect the growth of tumor cells. In the present study,
POT1-promoter-1 and −2 were separately transfected into four
different types of tumor cell lines (HepG2, HeLa, A549 and H460).
The transcriptional activity of the POT1-promoter-1 was
significantly increased compared with that of POT1-promoter-2. This
indicates that compared with POT1-promoter-2 (−370 to +90 bp),
POT1-promoter-1 (−160 to +40 bp) may be associated with more
positive or negative regulatory elements enabling for participation
in POT1 transcription regulation. The confirmation of the POT1
promoter transcriptional activity provided a foundation for further
investigation into the underlying POT1 regulatory mechanism.
In the previous 20 years, alterations in telomere
length have been established as an important biomarker for cancer
and studies into telomere length have aided in developing novel
cancer therapies (26,27). The maintenance of telomere length is a
necessary condition for the continuous division and immortalization
of normal and malignant cells (28).
Normally, with each round of cell division small fragments of
telomeric DNA are lost and the DNA is shortened eventually leading
to cell aging or apoptosis (29). Xin
et al (30) suggested that the
binding of the POT1-TPP1 complex may recruit telomerase, producing
a positive regulatory effect on the extension of telomeres. Izgi
et al (31) demonstrated that
colorectal cancer cells express a 4.33-fold increase in hTERT
compared with normal cells. In addition, a positive correlation
between telomerase activity and hPOT1 expression was identified
(31). However, certain studies have
revealed that POT1 may exhibit a negative regulatory effect, which
is able to suppress telomerase duplication and the extension of
telomeric DNA: In 2008, Churikov and Price (32) demonstrated that the loss of chicken
POT1 resulted in exceptionally rapid telomere growth. Furthermore,
another study revealed that compared with those cells that
overexpressed hTERT alone, telomere length was extended when hPOT1
expression was suppressed and hTERT was overexpressed
simultaneously in human skin fibroblasts (33). Furthermore, a negative correlation
between the expression levels of telomere-associated proteins
(TRF1, TRF2, POT1 and TIN2) and telomere content has been
identified in breast cancer cells (34). In addition, telomere shortening has a
role in suppressing tumor formation: Zimmermann et al
(35) demonstrated the ability for
tumor formation was significantly reduced in mice which had both a
telomere gene (a dominant-negative mutant of hTERT) and a tumor
inhibition gene (P53) knockout, compared with mice with only the
tumor inhibition gene knockout. One hypothesis as to explain the
observed effects includes the shortening of telomeres, promoting
apoptosis or the obstruction of cell cycle progression (35). These results indicated that POT1 has
numerous functions in the maintenance of telomere length and
telomerase.
Although important results have been demonstrated by
numerous previous studies, there are also limitations to the
current conclusions. The underlying regulation mechanism of the
POT1 promoter and telomere remain to be elucidated. Therefore, it
is important to clarify the association among the POT1 promoter,
POT1, telomerase and telomere length. In the present study,
alterations in the relative expression of POT1 and hTERT, as well
as in the telomere length were detected in the non-transfected
tumor cells (A549, H460, HeLa, and HepG2) using qPCR. The
co-transfection luciferase assay and qPCR results demonstrated that
there was a significant negative correlation between the POT1
promoter activity and hTERT expression. A potential explanation for
this observation is the expression of hTERT was affected primarily
by regulatory elements of the hTERT promoter. However, a
significantly positive correlation was identified between the
activity of the POT1 promoter, and the expression of POT1 or
telomere length. Therefore, this indicates that POT1 promoter
activity and POT1, as well as telomere length, may become
potentially useful biomarkers for cancer in the future.
Furthermore, these results preliminarily demonstrate that the POT1
promoter may be involved in the regulation of telomere length.
In conclusion, the results of the present study have
identified a potential association between the POT1 promoter
activity, POT1 and telomere length. Further experiments using
numerous human tumor types are required for elucidating the
underlying regulatory mechanism of the POT1 promoter and identified
useful tumor biomarkers.
Acknowledgements
The present study was supported by the First Science
and Technology Program of Guangdong province (grant no.
2008B030301023) and the Science and Technology Program of Higher
Learning Institutions in Dongguan (grant nos. 200910815264 and
2012108102016).
References
|
1
|
Lau LM, Dagg RA, Henson JD, Au AY, Royds
JA and Reddel RR: Detection of alternative lengthening of telomeres
by telomere quantitative PCR. Nucleic Acids Res. 41:e342013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumann P, Podell E and Cech TR: Human
Pot1 (protection of telomeres) protein: Cytolocalization, gene
structure, and alternative splicing. Mol Cell Biol. 22:8079–8087.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blasco MA, Lee HW, Hande MP, Samper E,
Lansdorp PM, DePinho RA and Greider CW: Telomere shortening and
tumor formation by mouse cells lacking telomerase RNA. Cell.
91:25–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Steensel B, Smogorzewska A and de
Lange T: TRF2 protects human telomeres from end-to-end fusions.
Cell. 92:401–413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HW, Blasco MA, Gottlieb GJ, Horner JW
II, Greider CW and DePinho RA: Essential role of mouse telomerase
in highly proliferative organs. Nature. 392:569–574. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen BN, Elmore LW and Holt SE:
Mechanism of dominant-negative telomerase function. Cell Cycle.
8:3227–3233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozturk S, Sozen B and Demir N: Telomere
length and telomerase activity during oocyte maturation and early
embryo development in mammalian species. Mol Hum Reprod. 20:15–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyerson M, Counter CM, Eaton EN, Ellisen
LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ,
Liu Q, et al: hEST2, the putative human telomerase catalytic
subunit gene, is up-regulated in tumor cells and during
immortalization. Cell. 90:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura TM, Morin GB, Chapman KB,
Weinrich SL, Andrews WH, Lingner J, Harley CB and Cech TR:
Telomerase catalytic subunit homologs from fission yeast and human.
Science. 277:955–959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Sullivan JN, Bronner MP, Brentnall TA,
Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH,
Crispin DA, et al: Chromosomal instability in ulcerative colitis is
related to telomere shortening. Nat Genet. 32:280–284. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plentz RR, Schlegelberger B, Flemming P,
Gebel M, Kreipe H, Manns MP, Rudolph KL and Wilkens L: Telomere
shortening correlates with increasing aneuploidy of chromosome 8 in
human hepatocellular carcinoma. Hepatology. 42:522–526. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loayza D and De Lange T: POT1 as a
terminal transducer of TRF1 telomere length control. Nature.
423:1013–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Podell ER, Zaug AJ, Yang Y, Baciu
P, Cech TR and Lei M: The POT1-TPP1 telomere complex is a
telomerase processivity factor. Nature. 445:506–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Latrick CM and Cech TR: POT1-TPP1 enhances
telomerase processivity by slowing primer dissociation and aiding
translocation. EMBO J. 29:924–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumann P and Cech TR: Pot1, the putative
telomere end-binding protein in fission yeast and humans. Science.
292:1171–1175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veldman T, Etheridge KT and Counter CM:
Loss of hPot1 function leads to telomere instability and a cut-like
phenotype. Curr Biol. 14:2264–2270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armbruster BN, Linardic CM, Veldman T,
Bansal NP, Downie DL and Counter CM: Rescue of an hTERT mutant
defective in telomere elongation by fusion with hPot1. Mol Cell
Biol. 24:3552–3561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colgin LM, Baran K, Baumann P, Cech TR and
Reddel RR: Human POT1 facilitates telomere elongation by
telomerase. Curr Biol. 13:942–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prather AA, Puterman E, Lin J, O'Donovan
A, Krauss J, Tomiyama AJ, Epel ES and Blackburn EH: Shorter
leukocyte telomere length in midlife women with poor sleep quality.
J Aging Res. 2011:7213902011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zee RY, Michaud SE, Germer S and Ridker
PM: Association of shorter mean telomere length with risk of
incident myocardial infarction: A prospective, nested case-control
approach. Clin Chim Acta. 403:139–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lapham K, Kvale MN, Lin J, Connell S,
Croen LA, Dispensa BP, Fang L, Hesselson S, Hoffmann TJ, Iribarren
C, et al: Automated assay of telomere length measurement and
informatics for 100,000 subjects in the genetic epidemiology
research on adult health and aging (GERA) cohort. Genetics.
200:1061–1072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naylor LH: Reporter gene technology: The
future looks bright. Biochem Pharmacol. 58:749–757. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miraglia LJ, King FJ and Damoiseaux R:
Seeing the light: Luminescent reporter gene assays. Comb Chem High
Throughput Screen. 14:648–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiffen JC, Bailey CG, Ng C, Rasko JE and
Holst J: Luciferase expression and bioluminescence does not affect
tumor cell growth in vitro or in vivo. Mol Cancer. 9:2992010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wentzensen IM, Mirabello L, Pfeiffer RM
and Savage SA: The association of telomere length and cancer: a
meta-analysis. Cancer Epidemiol Biomarkers Prev. 20:1238–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang CL, Chen XH, Li L, Zhou Y, Wang C
and Hou S: The association between telomere length and cancer
prognosis: Evidence from a meta-analysis. PLoS One.
10:e01331742015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilson E and Londoño-Vallejo JA: Telomere
length profiles in humans: All ends are not equal. Cell Cycle.
6:2486–2494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ju Z and Rudolph Lenhard K: Telomere
dysfunction and stem cell ageing. Biochimie. 90:24–32. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin H, Liu D, Wan M, Safari A, Kim H, Sun
W, O'Connor MS and Songyang Z: TPP1 is a homologue of ciliate
TEBP-beta and interacts with POT1 to recruit telomerase. Nature.
445:559–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Izgi A, Gunal A, Yalcin S and Gunduz U:
Telomere 1 (POT1) gene expression and its association with
telomerase activity in colorectal tumor samples with different
pathological features. Biomed Pharmacother. 68:841–846. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Churikov D and Price CM: Pot1 and cell
cycle progression cooperate in telomere length regulation. Nat
Struct Mol Biol. 15:79–84. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Possemato R, Timmons JC, Bauerlein EL,
Wada N, Baldwin A, Masutomi K and Hahn WC: Suppression of hPOT1 in
diploid human cells results in an hTERT-dependent alteration of
telomere length dynamics. Mol Cancer Res. 6:1582–1593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Butler KS, Hines WC, Heaphy CM and
Griffith JK: Coordinate regulation between expression levels of
telomere-binding proteins and telomere length in breast carcinomas.
Cancer Med. 1:165–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zimmermann S, Biniossek ML, Maurer C,
Münzer P, Pantic M, Veelken H and Martens UM: Proteomic profiling
in distinct cellular compartments of tumor cells reveals
p53-dependent upregulation of S100A6 upon induction of telomere
dysfunction. Proteomics. 9:5175–5187. 2009. View Article : Google Scholar : PubMed/NCBI
|