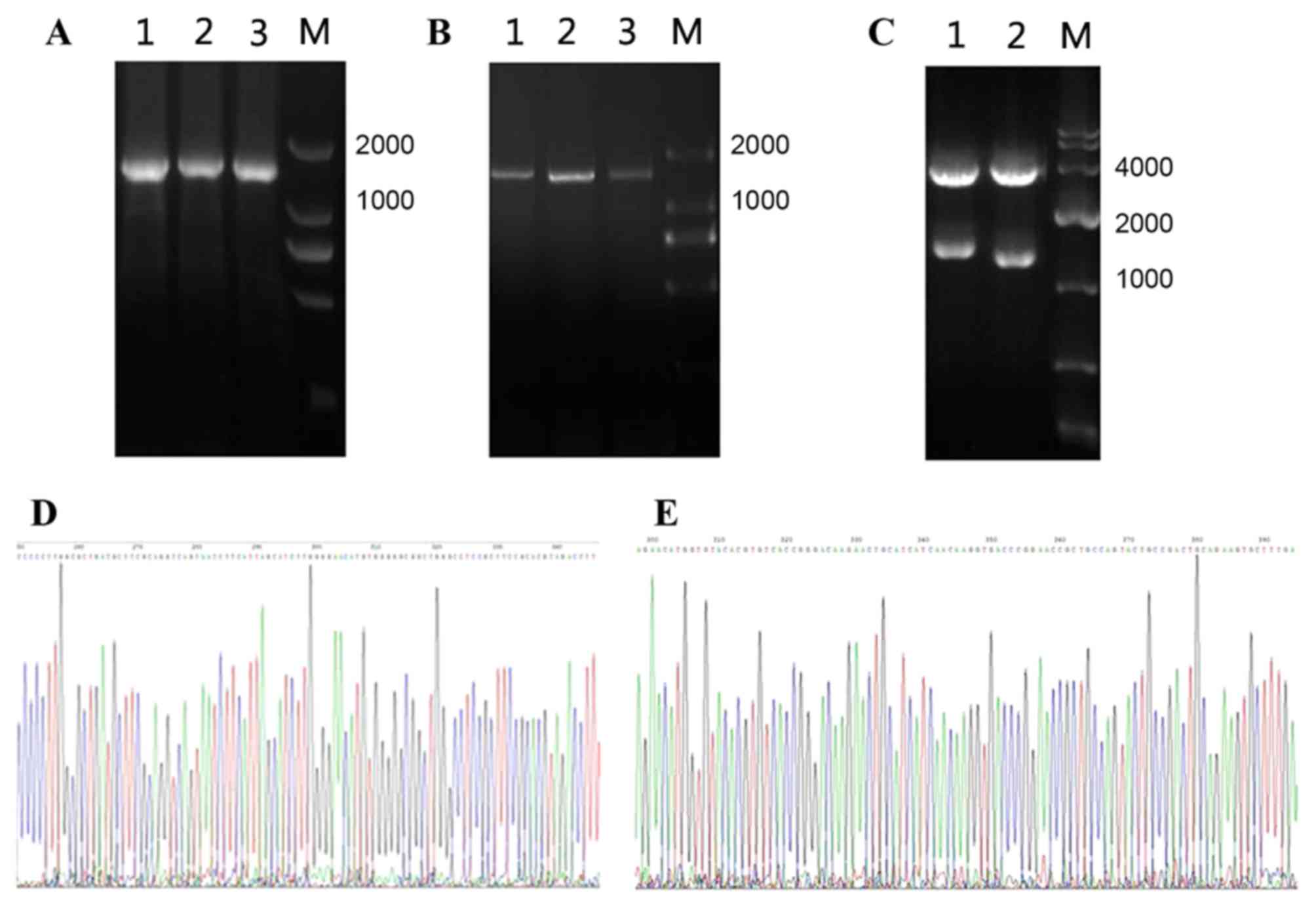
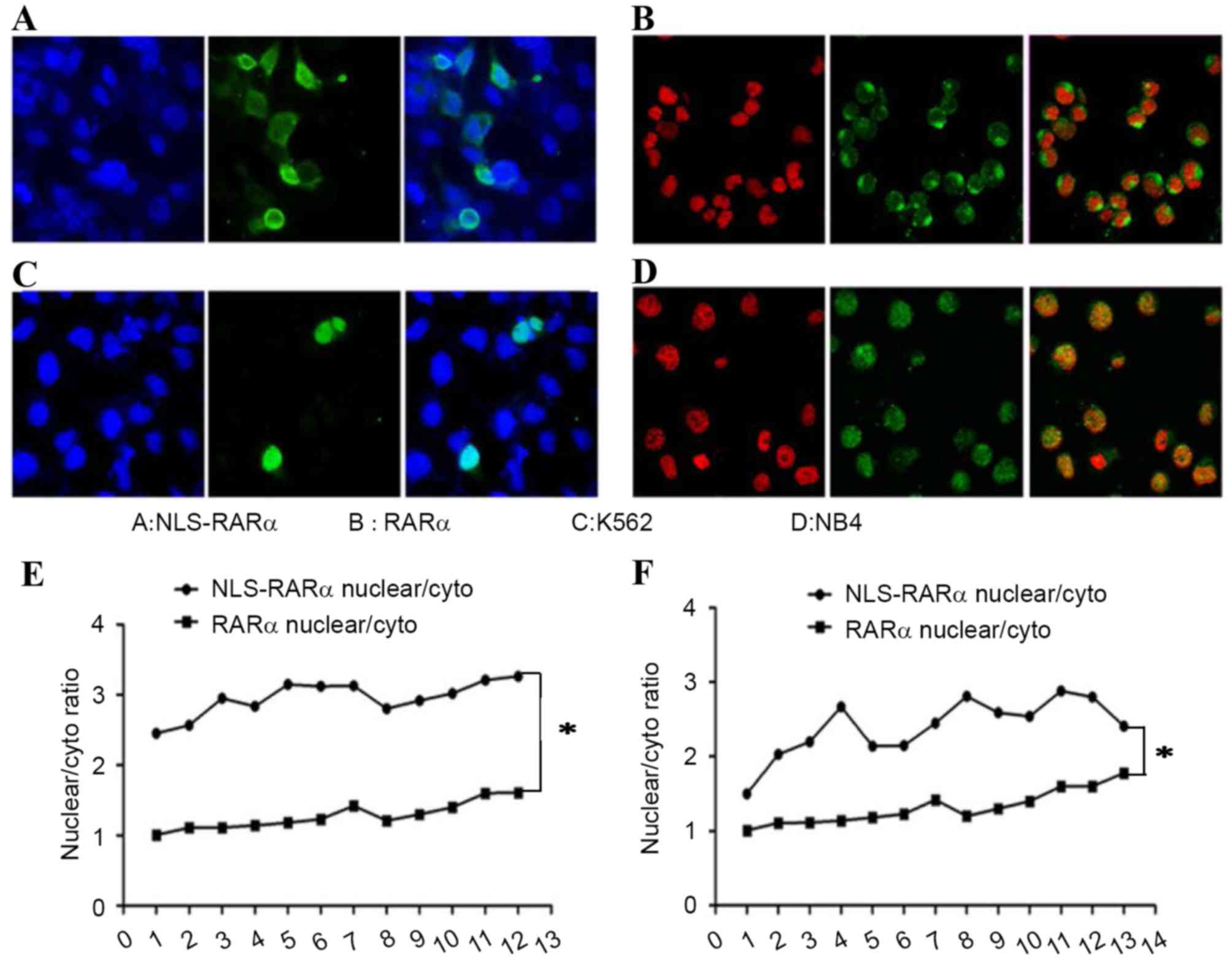
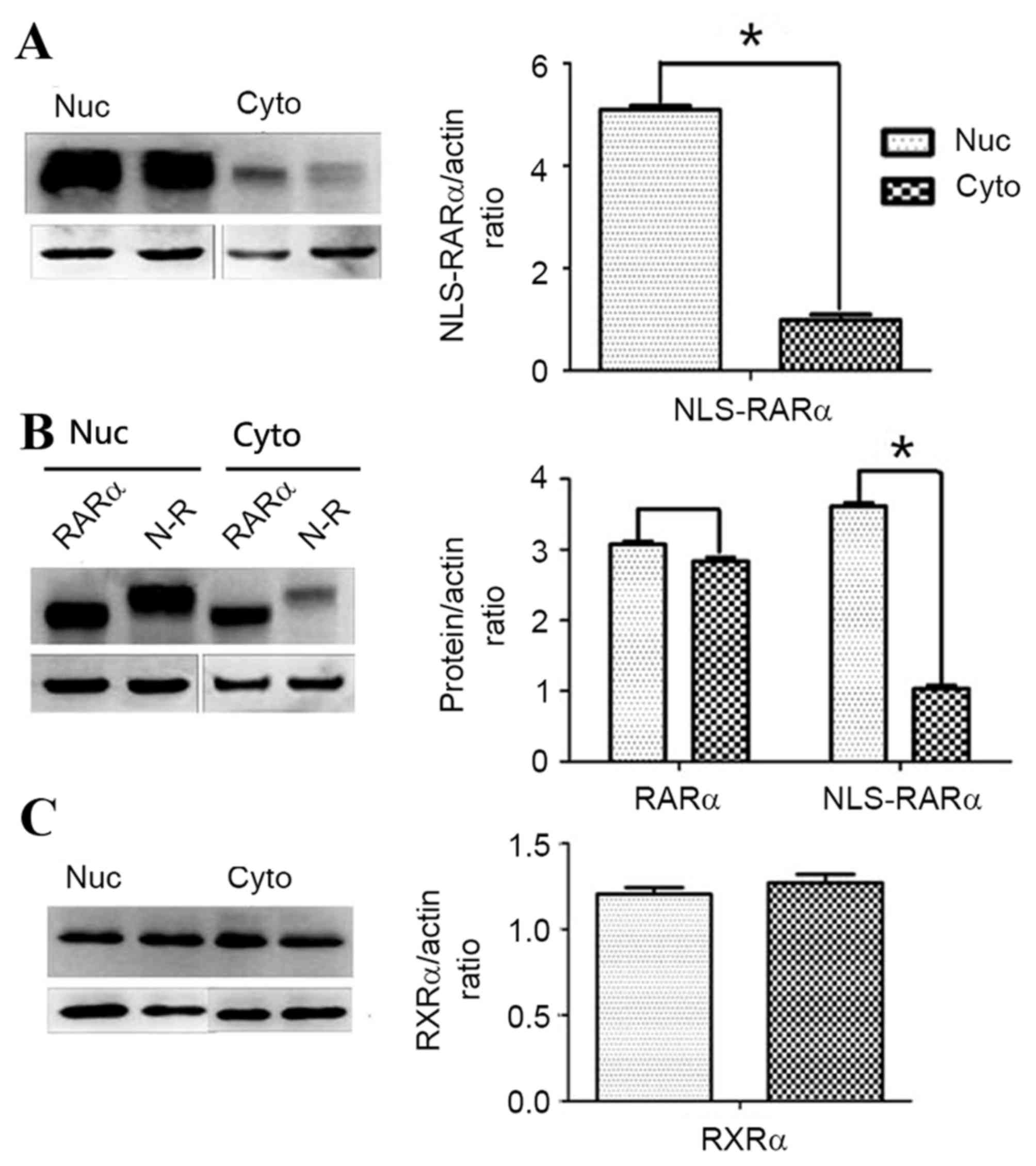
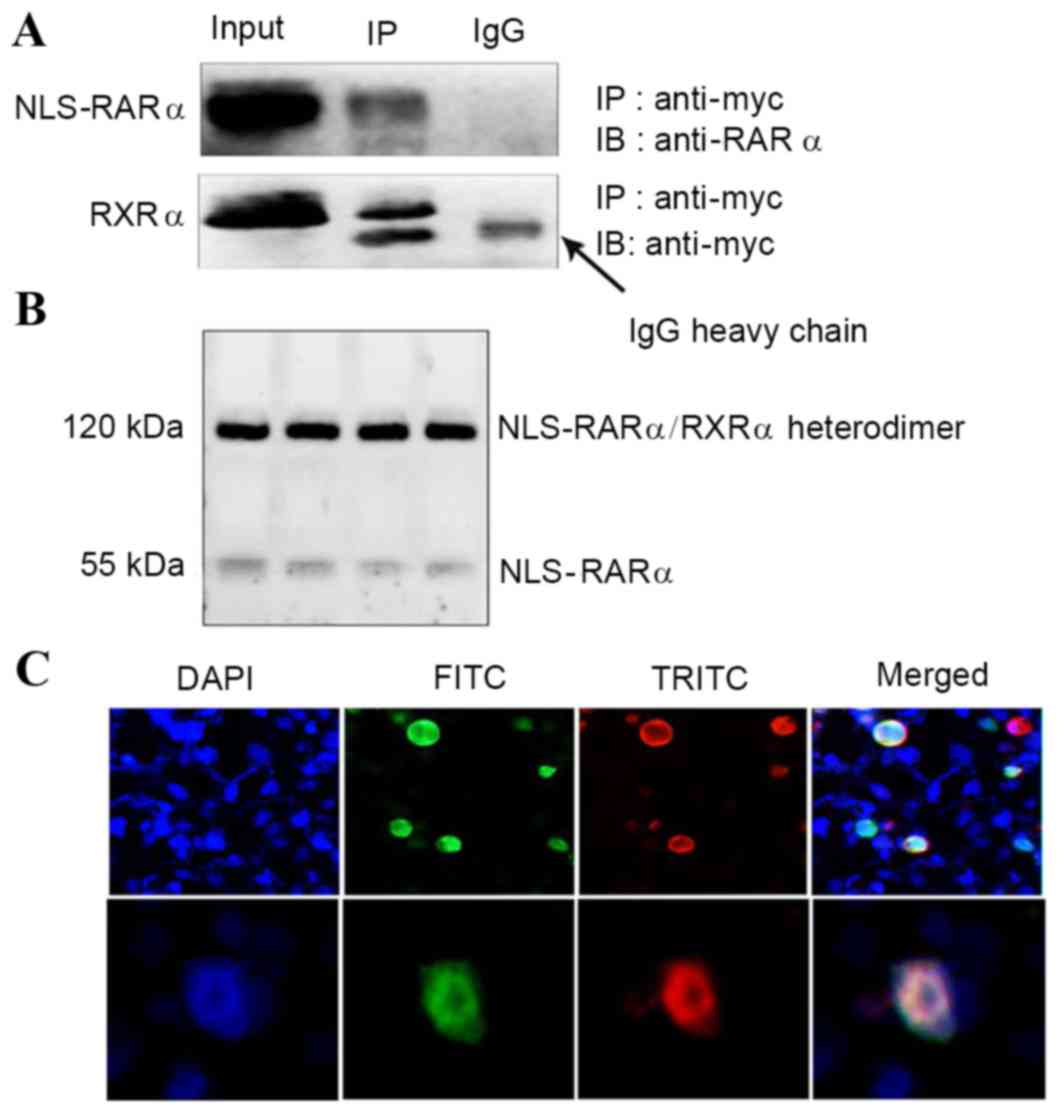
|
1
|
de Thé H, Le Bras M and
Lallemand-Breitenbach V: The cell biology of disease: Acute
promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol.
198:11–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zelent A, Guidez F, Melnick A, Waxman S
and Licht JD: Translocations of the RARalpha gene in acute
promyelocytic leukemia. Oncogene. 20:7186–7203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melnick A and Licht JD: Deconstructing a
disease: RARalpha, its fusion partners and their roles in the
pathogenesis of acute promyelocytic leukemia. Blood. 93:3167–3215.
1999.PubMed/NCBI
|
|
4
|
Chang KS, Stass SA, Chu DT, Deaven LL,
Trujillo JM and Freireich EJ: Characterization of a fusion cDNA
(RARA/myl) transcribed from the t(15;17) translocation breakpoint
in acute promyelocytic leukemia. Mol Cell Biol. 12:800–810. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou GB, Chen SJ and Chen Z: Acute
promyelocytic leukemia: A model of molecular target based therapy.
Hematology. 10 Suppl 1:S270–S280. 2005. View Article : Google Scholar
|
|
6
|
Raelson JV, Nervi C, Rosenauer A,
Benedetti L, Monczak Y, Pearson M, Pelicci PG and Miller WH Jr: The
PML/RAR alpha Oncoprotein is a direct molecular target of retinoic
acidin acute promyelocytic leukemia cells. Blood. 88:2826–2832.
1996.PubMed/NCBI
|
|
7
|
Zhou J, Pérès L, Honoré N, Nasr R, Zhu J
and de Thé H: Dimerization-induced corepressor binding and relaxed
DNA-binding specificity are critical for PML/RARA-induced
immortalization. Proc Natl Acad Sci USA. 103:pp. 9238–9243. 2006,
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamashev D, Vitoux D and De Thé H:
PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP
cross-talk in acute promyelocytic leukemia cell differentiation. J
Exp Med. 199:1163–1174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Nasr R, Pérès L, Riaucoux-Lormière
F, Honoré N, Berthier C, Kamashev D, Zhou J, Vitoux D, Lavau C and
de Thé H: RXR is an essential component of the oncogenic PML/RARA
complex in vivo. Cancer Cell. 12:23–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vitaliano-Prunier A, Halftermeyer J,
Ablain J, de Reynies A, Peres L, Le Bras M, Metzger D and de Thé H:
Clearance of PML/RARA-bound promoters suffice to initiate APL
differentiation. Blood. 124:3772–3780.. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ablain J and de The H: Revisiting the
differentiation paradigm in acute promyelocytic leukemia. Blood.
117:5795–5802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Qiu J, Chen G and Dong S:
Coiled-coil domain of PML is essential for the aberrant dynamics of
PML-RARalpha, resulting in sequestration and decreased mobility of
SMRT. Biochem Biophys Res Commun. 365:258–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Thé H and Chen Z: Acute promyelocytic
leukemia: Novel insights into the mechanisms of cure. Nat Rev
Cancer. 10:775–783. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ablain J and de Thé H: Retinoic acid
signaling in cancer: The parable of acute promyelocytic leukemia.
Int J Cancer. 135:2262–2272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lane AA and Ley TJ: Neutrophil elastase
cleaves PML-RARalpha and is important for the development of acute
promyelocytic leukemia in mice. 115:305–318. 2003.
|
|
16
|
Hayakawa F and Privalsky ML:
Phosphorylation of PML by mitogen-activated protein kinases plays a
key role in arsenic trioxide-mediated apoptosis. Cancer Cell.
5:389–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cokol M, Nair R and Rost B: Finding
nuclear localization signals. EMBO Rep. 1:411–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY,
Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, et
al: Arsenic trioxide controls the fate of the PML-RARalpha
oncoprotein by directly binding PML. Science. 328:240–243.. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu TT, Chen C, Chen SM, Xu Y, Wang Y, Chen
Z, Wang F, Xiao BK and Tao ZZ: Nuclear translocation of telomerase
reverse transcriptase is a critical process in lymphatic metastasis
of nasopharyngeal carcinoma. Oncol Lett. 9:265–269. 2015.PubMed/NCBI
|
|
20
|
Sánchez-Quesada C, López-Biedma A and
Gaforio JJ: The differential localization of a methyl group confers
a different anti-breast cancer activity to two triterpenes present
in olives. Food Funct. 6:249–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altucci L, Leibowitz MD, Ogilvie KM, de
Lera AR and Gronemeyer H: RAR and RXR modulation in cancer and
metabolic disease. Nat Rev Drug Discov. 6:793–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCulloch D, Brown C and Iland H: Retinoic
acid and arsenic trioxide in the treatment of acute promyelocytic
leukemia: Current perspectives. Onco Targets Ther. 10:1585–1601.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kakizuka A, Miller WH Jr, Umesono K,
Warrell RP Jr, Frankel SR, Murty VV, Dmitrovsky E and Evans RM:
Chromosomal translocation t(15;17) in human acute promyelocytic
leukemia fuses RAR alpha with a novel putative transcription
factor, PML. Cell. 66:663–674. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choudhry A and DeLoughery TG: Bleeding and
thrombosis in acute promyelocytic leukemia. Am J Hematol.
87:596–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, Gu LJ and Wang ZY: Use of all trans retinoic acid in the
treatment of acute promyelocytic leukemia. Blood. 72:567–572.
1988.PubMed/NCBI
|
|
26
|
Hu XX, Zhong L, Zhang X, Gao YM and Liu
BZ: NLS-RARα promotes proliferation and inhibits differentiation in
HL-60 cells. Int J Med Sci. 11:247–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Thé H, Lavau C, Marchio A, Chomienne C,
Degos L and Dejean A: The PML-RAR alpha fusion mRNA generated by
the t(15;17) translocation in acute promyelocytic leukemia encodes
a functionally altered RAR. Cell. 66:675–684. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zelent A, Guidez F, Melnick A, Waxman S
and Licht JD: Translocations of the RARalpha gene in acute
promyelocytic leukemia. Oncogene. 20:7186–7203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bastien J and Rochette-Egly C: Nuclear
retinoid receptors and the transcription of retinoid-target genes.
Gene. 328:1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Planelles L, Medema JP, Hahne M and
Hardenberg G: The expanding role of APRIL in cancer and immunity.
Curr Mol Med. 8:829–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delacroix L, Moutier E, Altobelli G,
Legras S, Poch O, Choukrallah MA, Bertin I, Jost B and Davidson I:
Cell-specific interaction of retinoic acid receptors with target
genes in mouse embryonic fibroblasts and embryonic stem cells. Mol
Cell Biol. 30:231–244. 2010. View Article : Google Scholar : PubMed/NCBI
|