Introduction
Amino acid transport systems play an important role
in supplying amino acids to cells (1). To date, many amino acid transport
systems in mammals have been identified, and are classified into
several transporter families based on differences in substrate
selectivity and ion dependence (1,2). Among
these amino acid transporters, L-type amino acid transporter 1
(LAT1), which belongs to amino acid transport system L, is highly
expressed in tumor cell lines (3,4). LAT1 is
an Na+-independent amino acid transporter and transports
large branched-chain and aromatic neutral amino acids, including
the essential amino acids histidine, isoleucine, leucine,
phenylalanine, tryptophan, tyrosine, methionine and valine
(1,3,4). LAT1
forms heterodimeric complexes with the 4F2 cell-surface antigen
heavy chain (4F2hc) via a disulfide bond (3). The expression of LAT1 mRNA is restricted
to certain organs, such as the brain, spleen, placenta, and testis
(3–5).
Furthermore, it has been reported that LAT1 is highly expressed in
cancer tissue of various types of cancer, including oral squamous
cell carcinoma (6), esophageal
carcinoma (7), gastric cancer
(8), prostate cancer (9), non-small cell lung carcinoma (10), biliary tract cancer (11), pancreatic cancer (12), and breast cancer (13).
Although amino acids are essential nutrients due to
their roles as substrates of protein synthesis and in cellular ATP
generation, they also regulate translation, transcription, and cell
growth via mammalian target of rapamycin (mTOR) (14). Previous studies suggest that the
inhibition of mTOR by rapamycin can exert antitumor effects in
various types of tumors in vitro and in vivo
(15–17).
In the present study, we retrospectively examined
LAT1 expression in CRC, and demonstrated that LAT1 expression in
cancer tissues was increased when compared with that in
nonmalignant tissues. In addition, we showed that the biological
significance of upregulated LAT1 was associated with its
contribution to cellular proliferation via the regulation of the
mTOR pathway in vitro. We hypothesized that restriction of
the amount of amino acids available for uptake into cancer cells
through LAT1 could lead to similar antitumor effects. Taken
together, the findings indicate that LAT1 may be a promising
molecular target for the treatment of CRC in the future.
Materials and methods
Patients
This study was performed in accordance with ethical
guidelines for clinical research with the approval of our
institutional ethics committee. Informed consent was obtained from
the individuals included in the study.
Primary CRC specimens were obtained from 210
patients (123 male and 87 female) who underwent curative resection
at Fukushima Medical University between January 1990 and December
2007. The ages of the patients ranged from 24 to 89 years, with a
mean age of 66 years. At the time of primary tumor resection, the
carcinomas were staged according to the UICC classification: 7
cases were allocated to stage 0, 33 to stage I, 82 to stage II, 59
to stage III, and 29 to stage IV. Furthermore, colonic adenoma
specimens were obtained from 40 patients (36 male and 4 female) who
had undergone polypectomy at Fukushima Medical University between
March 2007 and April 2009. The ages of the colonic adenoma patients
ranged from 39 to 79 years, with a mean of 64.4 years.
Immunohistochemical staining
All specimens of CRC and colonic adenoma were fixed
in formalin and embedded in paraffin. Two serial sections
(thickness, 4 µm) were prepared: One was used for hematoxylin and
eosin (H&E) staining, and the other was used for
immunohistochemical staining of LAT1. Immunohistochemical staining
was performed on the paraffin-embedded sections using a polymer
peroxidase method. Briefly, deparaffinized and rehydrated sections
were treated with 0.3% hydrogen peroxide in methanol for 30 min to
block endogenous peroxidase activity, before sections were
autoclaved in 0.01 M pH 6.0 citrate buffer for 5 min, and cooled
for 30 min. After rinsing in PBS, the sections were incubated with
affinity-purified anti-LAT1 antibodies (mouse monoclonal; dilution,
1:200; Cosmo Bio Co., Tokyo, Japan) overnight at 4°C. A further
wash in PBS was followed by treatment with goat anti-mouse
immunoglobulin antibodies conjugated to a peroxidase-labeled
polymer (ENvision+kit; Dako Cytomation, Glostrup, Denmark) as the
secondary antibody for 30 min at room temperature. Staining was
visualized with diaminobenzidine (DAB), and was followed by
counterstaining with hematoxylin.
Evaluation of LAT1 expression
The evaluation of staining was performed according
to a previously described method with minor modifications (18). LAT1 expression of cells was considered
positive only if distinct cell staining was present. The proportion
of positively stained tumor cells was scored as follows: 1, ≤10% of
tumor cells stained; 2, 11–25% stained; 3, 26–50% stained; and 4,
≥51% stained. Staining scores of 3 and 4 were classified as high
expression of LAT1, whereas staining scores of 1 and 2 were
classified as low expression of LAT1.
Cell culture
We used the cultured human colon cancer cell lines,
including LoVo, SW48, SW620, and SW837, which were originally
obtained from the American Type Culture Collection (Rockville, MD,
USA) to examine LAT1 expression in primary and metastatic site of
colorectal cancer. SW48 and SW837 cells were derived from primary
colon and rectal cancer, respectively. LoVo and SW620 were derived
from metastatic site of lymph node. The characteristics of theses
cell lines are described on the following site: https://www.atcc.org/Products/Cells_and_Microorganisms/Cell_Lines/Human.aspx.
The cells were grown at 37°C in the presence of 5% CO2
in the recommended media. In the experiments, the amino acid
contents of the media were modified. These media were purchased
from Wako (Osaka, Japan). Fetal bovine serum (Nichirei Bioscience,
Tokyo, Japan) was added to all media at a concentration of 10%.
Protein extraction and western blot
analysis
Cells (1×106) were seeded in 10 cm plates
and allowed 1 day for attachment. Subsequently, the medium was
removed and exchanged with RPMI-1640 (control) or with a medium
containing 1/4 of the quantity of amino acids present in the
control (amino acid-restricted medium). The cells were cultured for
72 h and harvested. Cultured cells were washed with cold PBS and
lysed in RIPA buffer (1X TBS, 1% Nonidet P-40, 0.5% sodium
deoxycholate, 0.1% SDS, and 0.004% sodium azide) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) containing 10 µl PMSF
solution, 10 µl sodium orthovanadate solution, and 10 µl protease
inhibitor cocktail solution per ml of 1X RIPA lysis buffer. Lysates
were cleared by centrifugation at 15,000 rpm for 15 min at 4°C.
Protein concentration was determined by the Bradford assay using a
Quick Start™ Bradford Dye Reagent and Quick Start™ Bovine Serum
Albumin Standard set (Bio-Rad, Hercules, CA, USA). The protein
samples (15 µg) were then electrophoresed on a Novex 4–12%
Tris-Glycine Gel (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and the separated proteins were transferred to
nitrocellulose membranes (iBlot™ Gel Transfer System; Invitrogen;
Thermo Fisher Scientific, Inc.). The membranes were blocked with
Super Block Blocking Buffers (Thermo Fisher Scientific, Inc.) and
incubated with a 1:200 dilution of rabbit anti-LAT1 antibody (MBL,
Nagoya, Japan) for 2 h or with a 1:200 dilution of rabbit anti-mTOR
antibody (Cell Signaling Technology, Danvers, MA, USA) or mouse
monoclonal anti-β-actin antibody (Santa Cruz Biotechnology, Inc.)
for 1 h. The secondary antibodies were horseradish
peroxidase-conjugated goat anti-rabbit antibodies (Santa Cruz
Biotechnology, Inc.), applied at a 1:5,000 dilution for 30 min. The
membranes were incubated with Super Signal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.) and
scanned with a luminescent imaging analyzer LAS-4000 mini
(Fujifilm, Tokyo, Japan).
Cell proliferation assay
The cell proliferation assay was performed using a
Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). The cells were
seeded at a concentration of 5×103 cells/well in 96-well
plates. After allowing 1 day for attachment, the medium was removed
and exchanged with RPMI-1640 (control) or the medium in which the
amount of amino acids (histidine, isoleucine, leucine,
phenylalanine, tryptophan, tyrosine, methionine, and valine) were
adjusted to 1/4 of the amount of amino acids in the control medium.
After culturing for each 24 h from the start, cells were incubated
with 100 µl of 10% CCK-8 reagent for 4 h at 37°C. The color
reaction was measured at 450 nm with a Bio Rad ELISA reader
(Richmond, CA, USA).
Statistical analysis
Associations between categorical variables were
evaluated with the χ2 test, Fisher's exact test or the
Mann-Whitney U test. Differences at P<0.05 were considered
significant. A survival analysis was conducted using the
Kaplan-Meier method, and the log-rank test was used to determine
the significance of differences between the survival curves. The
period of overall survival (OS) was calculated from the time of
surgery to the date of death from any cause, and was censored at
the time of the last visit to our hospital or December 2014,
whichever came first. All statistical analyses were performed using
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). The cell
proliferation assays were performed in triplicate, and the results
were presented as the mean ± standard deviation; statistical
significance was analyzed with the Student's t-test for comparison
of the two groups, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical analysis of LAT1
in CRC and colonic adenoma
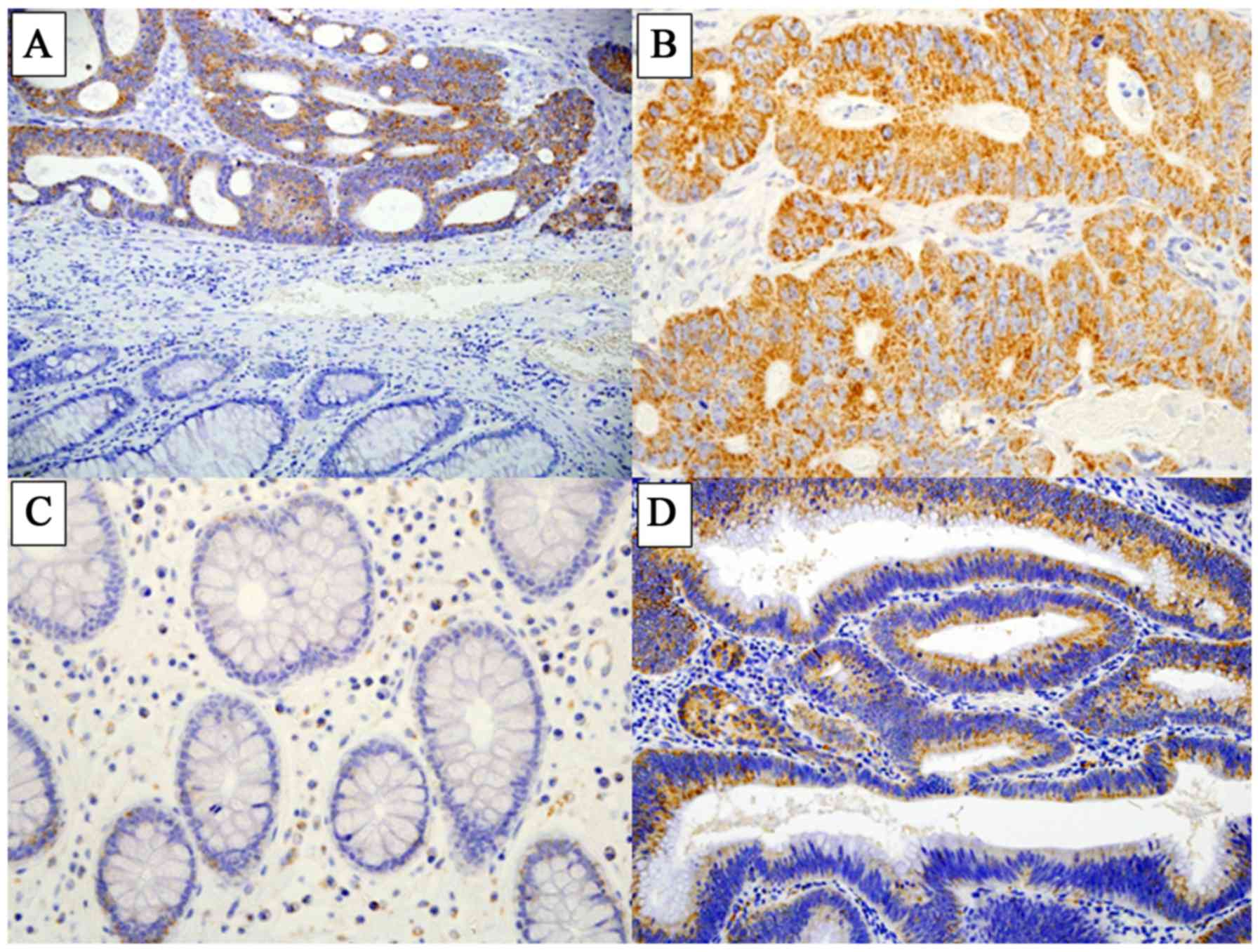
LAT1 expression was detected at high levels in CRC
tissues, while few normal epithelial cells in the cancer-adjacent
non-malignant tissue were positively stained for LAT1 (Fig. 1A-C). LAT1 was mostly expressed in the
cellular membrane and cytoplasm. Among the 210 CRC cases, a
staining score of 1 was recorded in 22 cases (10.5%), score 2 in 36
cases (17.1%), score 3 in 50 cases (23.8%), and score 4 in 102
cases (48.6%).
In the colonic adenoma specimens, LAT1 was expressed
in the membrane and cytoplasm of adenomatous cells (Fig. 1D). The distribution of LAT1 scores
among the 40 cases was as follows: Score 1, 19 cases (47.5%); score
2, 9 cases (22.5%); score 3, 7 cases (17.5%); and score 4, 5 cases
(12.5%).
High expression of LAT1, indicated by a staining
score of 3 or 4, was detected in 152 (72.4%) of the 210 CRC cases.
In the 40 colonic adenoma cases, 12 cases (30.0%) showed high
expression of LAT1. The incidence of high LAT1 expression was
significantly different between CRC and adenoma (P<0.001).
Associations between LAT1 expression
in CRC and clinicopathological factors and prognosis
High expression of LAT1 was significantly associated
with the depth of invasion (Tis and T1 vs. T2-4; P=0.048) and
venous invasion (P=0.027) in CRC tissues (Table 1). No significant associations could
be determined between the level of LAT1 expression and other
variables (patient sex or age, and tumor location, histology,
lymphatic invasion, lymph node metastasis and stage) (Table I).
 | Table I.Clinicopathologic correlation of LAT1
expression in CRC. |
Table I.
Clinicopathologic correlation of LAT1
expression in CRC.
|
| LAT1 expression |
|
|---|
|
|
|
|
|---|
| Characteristics | High expression
(n=152) (%) | Low expression (n=58)
(%) | P-value |
|---|
| Gender |
|
| 0.53 |
| Male | 87 (57.2) | 36 (62.1) |
|
|
Female | 65 (42.8) | 22 (37.9) |
|
| Age (years) |
|
| 0.89 |
|
<60 | 46 (30.3) | 17 (29.3) |
|
|
≥60 | 106 (69.7) | 41 (70.7) |
|
| Tumor location |
|
| 0.54 |
|
Right | 53 (34.9) | 16 (27.6) |
|
|
Left | 52 (34.2) | 24 (41.4) |
|
|
Rectum | 47 (30.9) | 18 (31.0) |
|
| Histology |
|
| 0.57 |
|
Well | 69 (45.4) | 26 (44.8) |
|
|
Moderate | 68 (44.7) | 24 (41.4) |
|
|
Poor | 5 (3.3) | 1 (1.7) |
|
|
Mucinous | 10 (6.6) | 7 (12.1) |
|
| Depth |
|
| 0.048 |
|
Tis | 2 (1.3) | 5 (8.6) | (Tis, T1 vs.
T2-4) |
| T1 | 10 (6.6) | 5 (8.6) |
|
| T2 | 17 (11.2) | 8 (13.8) |
|
| T3 | 110 (72.4) | 38 (65.5) |
|
| T4 | 13 (8.5) | 2 (3.5) |
|
| Lymphatic
invasion |
|
| 0.066 |
|
Absent | 27 (17.8) | 17 (29.3) |
|
|
Present | 125 (82.2) | 41 (70.7) |
|
| Venous
invasion |
|
| 0.027 |
|
Absent | 24 (15.8) | 17 (29.3) |
|
|
Present | 128 (84.2) | 41 (70.7) |
|
| Lymph node
metastasis |
|
| 0.42 |
|
Negative | 89 (58.6) | 35 (60.3) |
|
|
Positive | 63 (41.4) | 19 (32.8) |
|
|
Unknown | 0 (0.0) | 4 (6.9) |
|
| UICC stage |
|
| 0.21 |
| 0 | 2 (1.3) | 5 (8.6) |
|
| I | 22 (14.5) | 11 (19.0) |
|
| II | 63 (41.4) | 18 (31.0) |
|
|
III | 43 (28.3) | 16 (27.6) |
|
| IV | 22 (14.5) | 7 (12.1) |
|
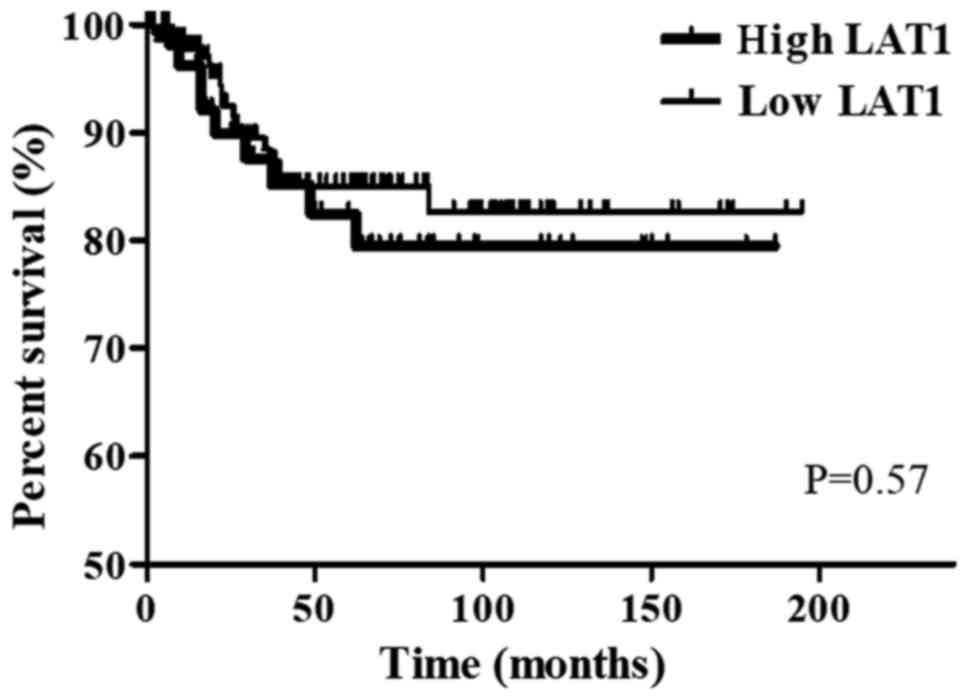
Regarding OS in CRC patients, no significant
difference was observed between patients with high expression of
LAT1 (n=152) and those with low expression of LAT1 (n=58) (P=0.57)
(Fig. 2).
Expression of LAT1 protein in CRC cell
lines and cell proliferation under limited availability of
essential amino acids
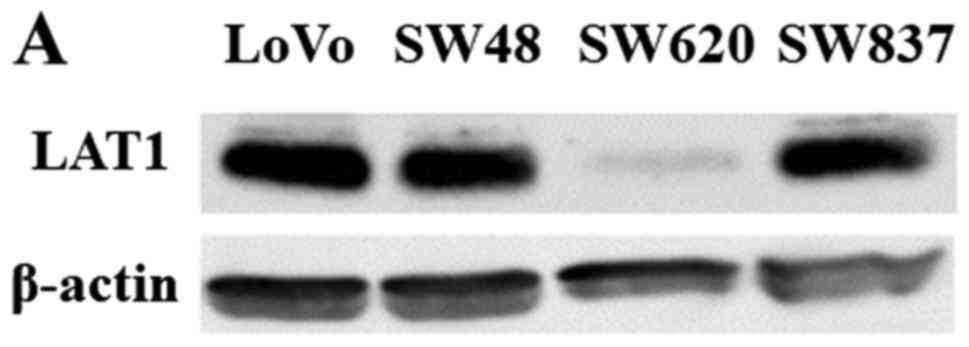
LAT1 expression was detected as a band of ~38 kDa in
all four CRC cell lines (LoVo, SW48, SW620, and SW837) (Fig. 3A). The level of LAT1 expression varied
depending on the cell line: LAT1 expression in SW620 cells was
relatively weak in comparison with the other CRC cell lines
(Fig. 3A).
Based on the aforementioned LAT1 expression data
from the CRC cell lines, LoVo cells, which exhibited a relatively
high level of LAT1 expression, and SW620 cells, which showed a
relatively low level of LAT1 expression, were used for the cell
proliferation assays with control medium or amino acid-restricted
medium. The rate of LoVo cell proliferation in the amino
acid-restricted medium was significantly lower than that in the
control medium, while the SW620 cell proliferation rate did not
change significantly (Fig. 3B).
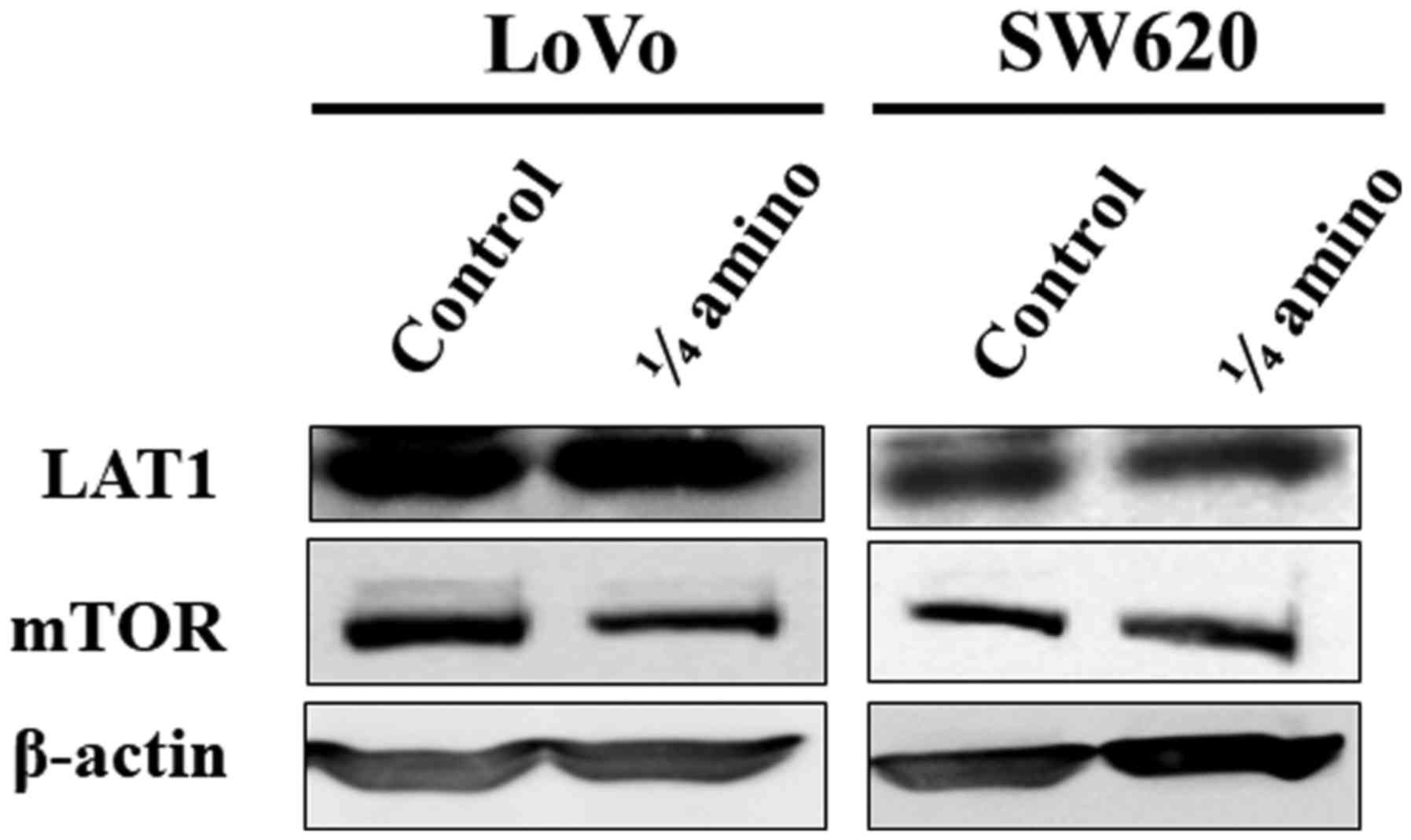
LAT1 and mTOR expression under limited
availability of essential amino acids
To elucidate the mechanism underlying the growth
suppression of CRC cell lines by restriction of amino acids, the
expression levels of LAT1 and mTOR were examined in LoVo and SW620
cells cultured in the control medium or the amino acid-restricted
medium for 72 h. LAT1 expression was not altered between the two
groups in either of the cell lines. The limited availability of
essential amino acids reduced the level of mTOR expression in LoVo
cells, whereas no such alteration of mTOR expression was observed
in the SW620 cells (Fig. 4).
Discussion
Previous studies demonstrated that LAT1 was highly
expressed in cancer tissue of various cancer types, including oral
squamous cell cancer (6), esophageal
cancer (7), gastric cancer (8), prostate cancer (9), non-small cell lung cancer (10), biliary tract cancer (11), pancreatic cancer (12), and breast cancer (13), using an immunohistochemical staining
method, as in the present study. However, there have been few
reports concerning LAT1 expression in CRC. In previous studies,
high levels of LAT1 expression were detected in 35% of colon cancer
cases (16/45 patients) (19) and 50%
of rectal cancer cases (22/44 patients) (20). In the present study, we investigated
the frequency of high LAT1 expression in 210 CRC patients, and
found that LAT1 expression was highly expressed in >70% of
cases. Increased LAT1 expression was frequently observed in the
membrane and cytoplasm of cancer cells, while no LAT1 expression
was detected in the normal mucosa. Regarding other types of cancer,
the frequency of high LAT1 expression has been detected in 93 (59%)
of 157 esophageal squamous cell carcinoma patients (7), 36 (41.4%) of 87 gastric cancer patients
(8), 13 (24%) of 54 prostate cancer
patients (9), 163 (51%) of 321 non
small lung cancer patients (10), 89
(64%) of 139 biliary tract cancer patients (11), 51 (52.6%) in 97 pancreatic cancer
patients (12), and 56 (43.4%) of 129
breast cancer patients (13). Thus,
compared with other cancer types, the frequency of high LAT1
expression was relatively high in the cases of CRC examined in the
present study. In addition to CRC, we found that LAT1 was also
highly expressed in 30% of 40 cases of colonic adenoma examined.
These findings suggested that LAT1 expression is elevated in the
early/precursor stages of colon carcinogenesis, and may be
associated with cellular proliferation. In addition, LAT1
expression was further enhanced in CRC tissues, indicating its
possible involvement in the acquisition of malignant potential.
In the present study, high LAT1 expression was
significantly associated with the depth of invasion and venous
invasion, and showed no associations with any of the other
clinicopathological factors investigated. No data regarding the
association between the LAT1 expression in CRC and
clinicopathological factors were reported in previous studies
(19,20). Previous reports (7,8,10) indicated that LAT1 expression was
associated with depth of invasion, lymph node metastasis, and
venous invasion in esophageal squamous cell carcinoma, and with
lymph node metastasis in gastric carcinoma and non-small cell lung
cancer. Moreover, high LAT1 expression was reported to be
associated with poor prognosis in several types of cancer (7,8,10–13). In
the present study, no relationship between high LAT1 expression and
lymph node metastasis, which might influence the prognosis, was
identified in CRC. Additionally, no difference in prognosis between
CRC patients with high LAT1 expression and those with low LAT1
expression was observed with regard to OS. Although the present
study did not examine the Ki-67 labeling index, which has been
widely used as a proliferation marker, several reports (8,10–12,19) have
suggested that LAT1 expression is significantly associated with the
Ki-67 labeling index in various cancers. Conflicting results
(21–23) have been reported regarding the
associations between the Ki-67 labeling index and clinical outcomes
in CRC patients. Allegra et al (22), in a study of CRC patients who
underwent curative resection between November 1977 and December
1990, reported that patients with high Ki-67 expression had
significantly improved disease-free survival and OS rates than
those with low levels of Ki-67. High LAT1 expression was
consistently associated with cellular proliferation, which may
result in the possibility of enhanced chemosensitivity. In the
present study, we investigated LAT1 expression in patients with
stage III or IV CRC who received 5FU-based monotherapy. Further
investigation will be required to conclude the association between
LAT1 expression and the prognosis of CRC patients treated with
newer anticancer drugs, including oxaliplatin and irinotecan.
While amino acids that enter cancer cells through
LAT1 (including leucine, isoleucine, phenylalanine, methionine,
tyrosine, histidine, tryptophan, and valine) are essential
nutrients since they are used as substrates for intracellular
protein synthesis and ATP generation, they also regulate
translation, transcription, and cell growth through mTOR (14). We have shown that the restriction of
the availability of these eight different amino acids can suppress
the viability of LoVo cells, which express a relatively high level
of LAT1 protein; however, in SW620 cells, which show a relatively
low protein expression level of LAT1, the cell viability did not
differ between the control and amino acid-restricted media.
Knockdown of LAT1 expression using an siRNA method may be valuable
in further elucidating the function of LAT1 overexpression in
cancer cells. In fact, a previous report revealed that the
downregulation of LAT1 expression inhibited the growth, migration
and invasion of gastric cancer cells (24). In the current study, we also
demonstrated another LAT1-dependent method of cell growth
suppression: A restriction of the availability of amino acids could
suppress cell proliferation in the cancer cells with high-level
LAT1 expression, whereas it did not affect cell growth in the
cancer cells with low LAT1 expression, which may have a similar
expression level to that in normal cells. In a previous study, the
concentration of those eight amino acids in CRC tissue was
approximately two times higher than that in colonic normal tissue
(25). In normal tissues, LAT2 and
other transporters facilitate the uptake of the amino acids
(26). Therefore, the restriction of
those amino acids may be selectively effective for the starvation
of cancer cells with high LAT1 expression. Furthermore, we found
that the restriction of the amino acids could suppress mTOR
expression without alteration of LAT1 expression in LoVo cells.
LAT1 is linked to mTOR via the intake of leucine, which stimulates
the mTOR pathway and thereby affects cellular proliferation
(27). Although previous studies
(28,29) have demonstrated that essential amino
acids activate the mTOR pathway, the exact mechanisms regulating
mTOR expression remain unknown. However, the reason that the
restriction of essential amino acids could inhibit cellular
proliferation in cancer cells with a high level of LAT1 expression
may be explained by the suppression of mTOR expression. Further
experiments, including knock-down or overexpression of LAT1 and the
use of rapamycin, will elucidate the role of the LAT1/mTOR
pathway.
In the present study, we found that LAT1 expression
was enhanced in approximately 70% of CRC and 30% of colonic adenoma
tissues. Recently, LAT1 inhibitor compounds have been developed and
have demonstrated growth inhibitory effects on HT-29 cells, which
are derived from colon cancer, in in vivo and in
vitro experiments (30).
Furthermore, positron emission tomography (PET) has been attempted
in lung cancer diagnosis using
L-[3-18F]-α-methyltyrosine [(18F) FMT], an
amino acid tracer for PET, since the uptake of [18F] FMT
correlates with LAT1 expression (31). Accumulation of data, including the
current results, will be useful for improving the diagnosis and
treatment of CRC.
References
|
1
|
Christensen HN: Role of amino acid
transport and countertransport in nutrition and metabolism. Physiol
Rev. 70:43–77. 1990.PubMed/NCBI
|
|
2
|
Palacín M, Estévez R, Bertran J and
Zorzano A: Molecular biology of mammalian plasma membrane amino
acid transporters. Physiol Rev. 78:969–1054. 1998.PubMed/NCBI
|
|
3
|
Kanai Y, Segawa H, Miyamoto Ki, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanagida O, Kanai Y, Chairoungdua A, Kim
DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, et
al: Human L-type amino acid transporter 1 (LAT1): Characterization
of function and expression in tumor cell lines. Biochim Biophys
Acta. 1514:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prasad PD, Wang H, Huang W, Kekuda R,
Rajan DP, Leibach FH and Ganapathy V: Human LAT1, a subunit of
system L amino acid transporter: Molecular cloning and transport
function. Biochem Biophys Res Commun. 255:283–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DK, Ahn SG, Park JC, Kanai Y, Endou H
and Yoon JH: Expression of L-type amino acid transporter 1 (LAT1)
and 4F2 heavy chain (4F2hc) in oral squamous cell carcinoma and its
precursor lesions. Anticancer Res. 24:1671–1675. 2004.PubMed/NCBI
|
|
7
|
Honjo H, Kaira K, Miyazaki T, Yokobori T,
Kanai Y, Nagamori S, Oyama T, Asao T and Kuwano H:
Clinicopathological significance of LAT1 and ASCT2 in patients with
surgically resected esophageal squamous cell carcinoma. J Surg
Oncol. 113:381–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichinoe M, Mikami T, Yoshida T, Igawa I,
Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H and Okayasu I: High
expression of L-type amino-acid transporter 1 (LAT1) in gastric
carcinomas: Comparison with non-cancerous lesions. Pathol Int.
61:281–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Segawa A, Nagamori S, Kanai Y, Masawa N
and Oyama T: L-type amino acid transporter 1 expression is highly
correlated with gleason score in prostate cancer. Mol Clin Oncol.
1:274–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaira K, Oriuchi N, Imai H, Shimizu K,
Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, et
al: Prognostic significance of L-type amino acid transporter 1
expression in resectable stage I–III nonsmall cell lung cancer. Br
J Cancer. 98:742–748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaira K, Sunose Y, Ohshima Y, Ishioka NS,
Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, et
al: Clinical significance of L-type amino acid transporter 1
expression as a prognostic marker and potential of new targeting
therapy in biliary tract cancer. BMC Cancer. 13:4822013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaira K, Sunose Y, Arakawa K, Ogawa T,
Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, et
al: Prognostic significance of L-type amino-acid transporter 1
expression in surgically resected pancreatic cancer. Br J Cancer.
107:632–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furuya M, Horiguchi J, Nakajima H, Kanai Y
and Oyama T: Correlation of L-type amino acid transporter 1 and
CD98 expression with triple negative breast cancer prognosis.
Cancer Sci. 103:382–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Bihan S, Marsaud V, Mercier-Bodard C,
Baulieu EE, Mader S, White JH and Renoir JM: Calcium/calmodulin
kinase inhibitors and immunosuppressant macrolides rapamycin and
FK506 inhibit progestin-and glucocorticosteroid receptor-mediated
transcription in human breast cancer T47D cells. Mol Endocrinol.
12:986–1001. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J, Adam RM, Santiestevan E and Freeman
MR: The phosphatidylinositol 3′-kinase pathway is a dominant growth
factor-activated cell survival pathway in LNCaP human prostate
carcinoma cells. Cancer Res. 59:2891–2897. 1999.PubMed/NCBI
|
|
17
|
Luan FL, Hojo M, Maluccio M, Yamaji K and
Suthanthiran M: Rapamycin blocks tumor progression: Unlinking
immunosuppression from antitumor efficacy. Transplantation.
73:1565–1572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaira K, Oriuchi N, Imai H, Shimizu K,
Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H, et
al: L-type amino acid transporter 1 (LAT1) is frequently expressed
in thymic carcinomas but is absent in thymomas. J Surg Oncol.
99:433–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaira K, Oriuchi N, Imai H, Shimizu K,
Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, et
al: L-type amino acid transporter 1 and CD98 expression in primary
and metastatic sites of human neoplasms. Cancer Sci. 99:2380–2386.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebara T, Kaira K, Saito J, Shioya M, Asao
T, Takahashi T, Sakurai H, Kanai Y, Kuwano H and Nakano T: L-type
amino-acid transporter 1 expression predicts the response to
preoperative hyperthermo-chemoradiotherapy for advanced rectal
cancer. Anticancer Res. 30:4223–4227. 2010.PubMed/NCBI
|
|
21
|
Martins SF, Amorim R, Mota SC, Costa L,
Pardal F, Rodrigues M and Longatto-Filho A: Ki-67 expression in crc
lymph node metastasis does not predict survival. Biomed Res Int.
2015:1316852015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allegra CJ, Paik S, Colangelo LH, Parr AL,
Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N and Wieand HS:
Prognostic value of thymidylate synthase, Ki-67, and p53 in
patients with Dukes' B and C colon cancer: A national cancer
institute-national surgical adjuvant breast and bowel project
collaborative study. J Clin Oncol. 21:241–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jansson A and Sun XF: Ki-67 expression in
relation to clinicopathological variables and prognosis in
colorectal adenocarcinomas. APMIS. 105:730–734. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi L, Luo W, Huang W, Huang S and Huang
G: Downregulation of L-type amino acid transporter 1 expression
inhibits the growth, migration and invasion of gastric cancer
cells. Oncol Lett. 6:106–112. 2013.PubMed/NCBI
|
|
25
|
Hirayama A, Kami K, Sugimoto M, Sugawara
M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et
al: Quantitative metabolome profiling of colon and stomach cancer
microenvironment by capillary electrophoresis time-of-flight mass
spectrometry. Cancer Res. 69:4918–4925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Segawa H, Fukasawa Y, Miyamoto K, Takeda
E, Endou H and Kanai Y: Identification and functional
characterization of a Na+-independent neutral amino acid
transporter with broad substrate selectivity. J Biol Chem.
274:19745–19751. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicklin P, Bergman P, Zhang B,
Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson
C, et al: Bidirectional transport of amino acids regulates mTOR and
autophagy. Cell. 136:521–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hara K, Yonezawa K, Weng QP, Kozlowski MT,
Belham C and Avruch J: Amino acid sufficiency and mTOR regulate p70
S6 kinase and eIF-4E BP1 through a common effector mechanism. J
Biol Chem. 273:14484–14494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Campbell LE, Miller CM and Proud
CG: Amino acid availability regulates p70 S6 kinase and multiple
translation factors. Biochem J. 334:261–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oda K, Hosoda N, Endo H, Saito K,
Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y and
Endou H: L-type amino acid transporter 1 inhibitors inhibit tumor
cell growth. Cancer Sci. 101:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaira K, Oriuchi N, Otani Y, Shimizu K,
Tanaka S, Imai H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, et
al: Fluorine-18-alpha-methyltyrosine positron emission tomography
for diagnosis and staging of lung cancer: A clinicopathologic
study. Clin Cancer Res. 13:6369–6378. 2007. View Article : Google Scholar : PubMed/NCBI
|