Introduction
Immunotherapy has become an important alternative
option in oncology treatment, which represents a promising method
for patients due to its specificity for tumor cells and sustained
immunological memory that may safeguard against recurrences
(1). Application of tumor vaccines to
elicit antigen specific immune responses for cancer treatment is a
hot area of immunotherapy research. Inactivated whole tumor cells
providing the total array of antigens expressed by the individual
tumor are the most commonly utilized antigen types in tumor vaccine
research (2–4).
Currently, the known vaccine types utilized as whole
tumor cells include irradiated tumor cells, glutaraldehyde-fixed
tumor cells and tumor cell lysates (5–9). In
particular, along with the development of tumor adjuvant, the
efficiency of whole tumor cell vaccines has been markedly improved.
In a previous study, a whole hepatocellular carcinoma cell
lysate-based vaccine with diphtheria toxin and two tandem repeats
of mycobacterial HSP70 fragment 407–426 (M2) as adjuvant
exhibited modest antitumor effects in the preventive procedure
(10,11).
Although whole tumor cell vaccines have already been
widely studied, vaccine therapy has yielded suboptimal clinical
results in therapeutic procedures (12,13).
Furthermore, it is not clear which antigen form is more effective
in cancer vaccine preparation. Therefore in the present study,
tumor cell lysate (TCL) and glutaraldehyde-fixed tumor cells, two
commonly used antigen forms, were investigated to improve the
present vaccine strategy. Murine H22 hepatocellular carcinoma cell
lysate and glutaraldehyde-fixed H22 hepatocellular carcinoma cells
were conjugated with Freund's adjuvant to prepare vaccines, H22-TCL
and Fixed-H22-CELL, respectively. The antitumor efficacy of these
two vaccines was evaluated using a subcutaneous hepatocellular
carcinoma and an experimental metastasis model. Subsequently, the
elicited H22-specific antibodies were detected using the
enzyme-linked immunosorbent assay (ELISA) method. An MTT assay was
performed to determine the proliferation ability of lymphocytes
from immunized mice. Finally, histochemistry analysis was performed
to further visualize the necrosis in tumor tissues.
Materials and methods
Mice and cell lines
Male ICR mice, 3–4 weeks old (weighing 19–22 g),
were purchased from Changzhou Cavens Experimental Animal Co. Ltd
(Changzhou, Jiangsu, China). Mice were maintained at 20–26°C in
pathogen-free conditions at a relative humidity of 40–65% in a
12:12 h light-dark cycle and fed ad libitum. All procedures
in animal experiments were approved by the Animal Study Committee
of Binzhou Medical University (Yantai, Shandong, China). H22 murine
hepatocellular carcinoma cell line was purchased from The Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in RPMI-1640 (HyClone, GE Healthcare,
Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone,
GE Healthcare), 100 µg/ml streptomycin and 100 U/ml penicillin at
37°C with 5% CO2.
Vaccine preparation
H22 cells were collected from the tissue culture
flask and washed three times with PBS, then suspended in PBS so
that each 1 ml contained 1×107 H22 cells. A total of 100
µl cell suspension was lysed by five cycles of freezing in −20°C
for 30 min and thawing in 37°C for 10 min and 100 µl cell
suspension was fixed with 0.025% glutaraldehyde at room temperature
for 20 min, subsequently fixed cells were washed three times with
PBS. The H22 cell lysates and glutaraldehyde-fixed H22 cells were
mixed with Freund's adjuvant (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) (1:1). The mixed method was as follows (14), H22 tumor cell lysate was mixed with
Freund's adjuvant and a syringe was used to mix repeatedly and to
form stable water-in-oil emulsion, which was named H22-TCL.
Similarly, glutaraldehyde-fixed H22 cells were mixed with Freund's
adjuvant using the same method to prepare the Fixed-H22-CELL
vaccine.
Immunization protocols in tumor
models
A total of three groups of 18 male ICR mice (in
prophylactic, therapeutic or lung metastasis model, respectively)
were randomly divided into three further groups of six animals
each, which were treated with 100 µl PBS, H22-TCL or
Fixed-H22-CELL, respectively. Vaccines were administrated using
prophylactic or therapeutic strategies. For each immunization
strategy, mice were subcutaneously immunized with separate vaccines
or PBS in the left inguinal lymph node area.
In the prophylactic strategy, mice were immunized on
days −28, −21, −14 and −7. Subsequently, the tumor challenge
experiment was performed by subcutaneously injecting
1×106 H22 cells into the right flank on day 0. Sera were
collected weekly for immunoassay following initial
immunization.
In the therapeutic strategy, mice were injected with
1×106 H22 cells into the right flank on day 0 and then
immunized with separate vaccines on days 3, 10 and 17. Tumor volume
was evaluated every other day one week after the tumor challenge.
The tumor volume was determined using the formula: Volume
=0.52XY2, where ‘X’ is the larger diameter and ‘Y’ is
the smaller diameter. On day 21, all mice in each group were
sacrificed for tumor weight evaluation.
A further 18 male ICR mice were randomly divided
into three groups of six animals in each, and mice were immunized
on days −28, −21, −14 and −7 as described above, and then
intravenously injected in the tail with 5×105 H22 cells
on day 0 to establish a lung metastasis model (15). On day 21, all mice were sacrificed and
the lungs were removed, followed by perfusion with 2–3 ml of Indian
ink (Sangon Biotech Co., Ltd., Shanghai, China) using 22-gauge
gavage needles and subsequently the excised lungs were fixed with
10% formalin for 36 h at room temperature. Lung metastasis was
evaluated macroscopically by counting the metastatic nodules that
were clearly visible on the lung surface. For microscopic
observation, formalin-fixed lung tissues were stained with
hematoxylin-eosin (H&E) for 5 h at room temperature and the
examined by light microscopy (magnification, ×100).
ELISA analysis for serum anti-H22
antibody
A total of six mice of the three immunized groups in
the prophylactic strategy were administered with separate vaccines
every week for four consecutive weeks, and serum was collected
every week following initial immunization. The anti-H22 antibodies
present in the serum were evaluated using an ELISA, as described
previously (16). Briefly, 96-well
flat-bottomed ELISA plates were coated with 10 µg/well of whole H22
cell lysates protein. Sera diluted at 1:50, and horseradish
peroxidase-conjugated rabbit anti-mouse IgG (Beyotime Institute of
Biotechnology, Haimen, China) diluted at 1:100,000 were used at
37°C for 2 h. The enzyme reaction was developed using the
peroxidase substrate 3,3′, 5,5′-tetramethylbenzidine for 15–30 min
at 37°C, and quenched using H2SO4 (2 M) for 1
min at room temperature. The ELISA plate was read using a standard
ELISA reader at 450 nm. Each evaluation was performed in
triplicate.
T cell proliferation assay
Following one week after the final immunization of
the prophylactic strategy, splenocytes were isolated from
sacrificed mice of each immunized and PBS group. Firstly, spleens
were ground and passed through a 200 µm filter under sterile
conditions. Erythrocytes were lysed at room temperature using
Tris-NH4Cl for 5 min (pH 7.2). The splenocytes were
washed three times with PBS and resuspended in RMPI-1640
supplemented with 10% FBS. The spleen cells (2×105
cells/well) were incubated in triplicate in 96-well plates for 72 h
in the presence or absence of H22 cell lysate (100 µg/ml) at 37°C.
ConA (5 µg/ml) was used as a positive control. Cell proliferation
was analyzed by MTT assay. Following a 72-h incubation, the
supernatant in each well was discarded and 10 µl MTT (5 mg/ml) was
added to each well. Following incubation at 37°C for an additional
4 h, 100 µl dimethyl sulfoxide was pipetted to solubilize the
product for 10 min at room temperature. Subsequently, the 96-well
plate was evaluated using an ELISA reader at 570 nm. All assays
were performed in triplicate.
Histological evaluation of the
subcutaneous tumor tissues
Subcutaneous tumor tissues were fixed with 10%
formalin, embedded in paraffin and cut into 4 µm sections. H&E
staining was then performed on tissues sections for 5 h at room
temperature. All tissue sections were evaluated by light
microscopy, at magnification, ×100 for histological changes that
may be associated with the treatment.
Toxicity assessment
The treatment-associated toxicity was mainly
evaluated by analyzing the mice weight alterations. The tissues
(heart, liver, spleen, lung and kidney) were then fixed with 10%
formalin and embedded in paraffin. The 4 µm tissue sections were
stained with H&E for 5 h at room temperature, and evaluated by
light microscopy, at magnification, ×100.
Statistical analysis
Data are presented as the mean ± standard deviation.
Multiple comparisons were analyzed using one-way analysis of
variance with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Analysis was
performed in GraphPad Prism 6 (GraphPad Software, La Jolla, CA,
USA).
Results
H22-TCL vaccination induces
prophylactic anti-hepatocellular carcinoma effects
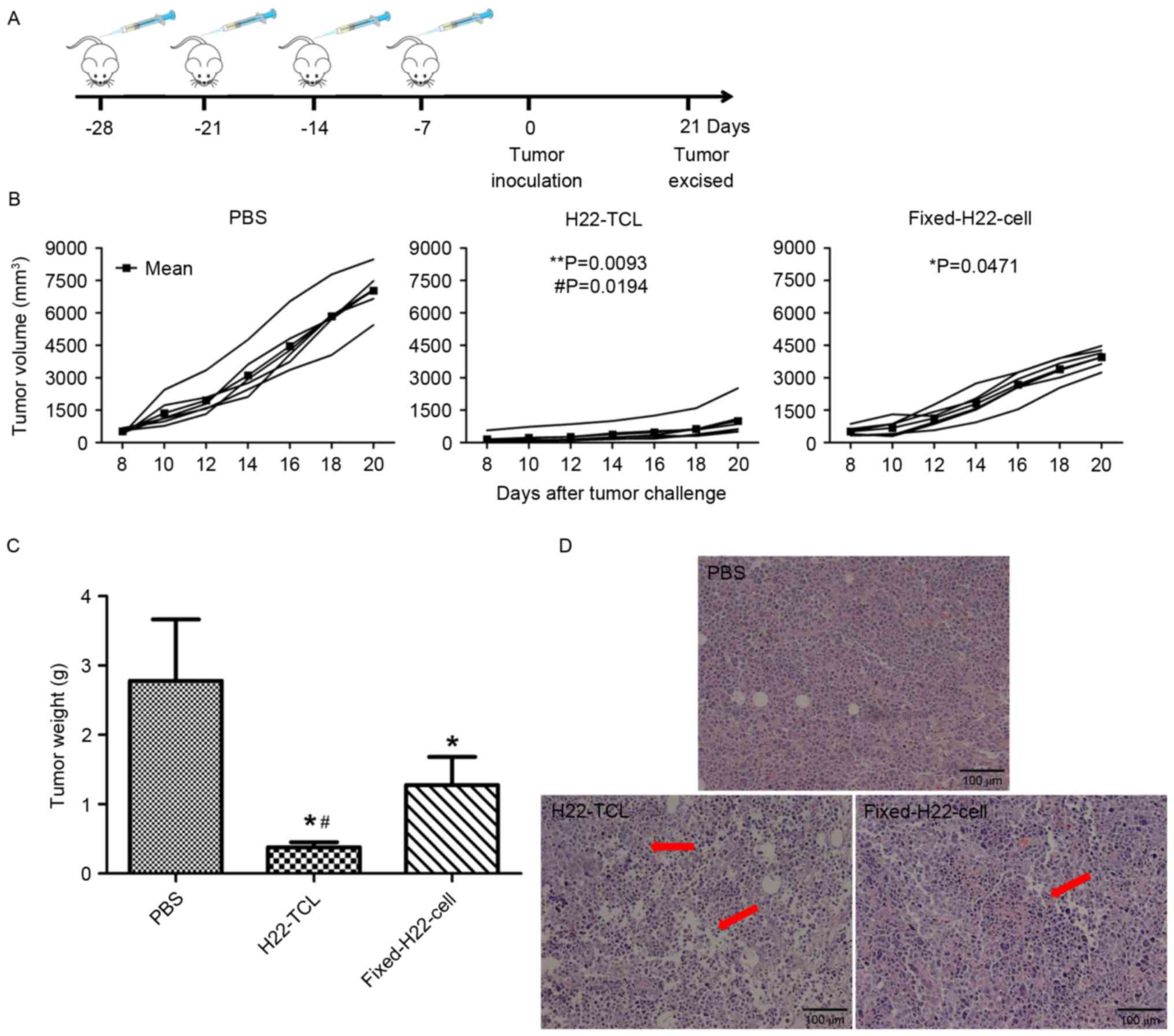
A vaccination protocol was designed as shown in
Fig. 1A to analyze the prophylactic
anti-hepatocellular carcinoma effect. As presented in Fig. 1B, PBS-treated mice developed rapidly
progressive disease following tumor inoculation. Conversely,
H22-bearing mice treated with Fixed-H22-CELL or H22-TCL exhibited
slower tumor growth, and the inhibition effect of H22-TCL was the
most significant. The excised tumor weight revealed similar
results, although the groups of mice immunized with Fixed-H22-CELL
and H22-TCL exhibited a significantly lower mean tumor weight
compared with the PBS group (Fig. 1C;
P<0.05). The tumor weight of mice immunized with H22-TCL was
significantly decreased compared with mice treated with
Fixed-H22-CELL (Fig. 1C; P<0.05).
The sections of excised tumors were stained with H&E and viewed
using a microscope, and the tumor sections from the H22-TCL and
Fixed-H22-CELL groups were detected with degeneration necrosis in
tumor cells and the tumor cell nucleus were dissolved. Conversely,
these phenomena were not present in tumor tissue sections from
PBS-treated mice (Fig. 1D).
H22-TCL vaccination induces
therapeutic anti-hepatocellular carcinoma effects
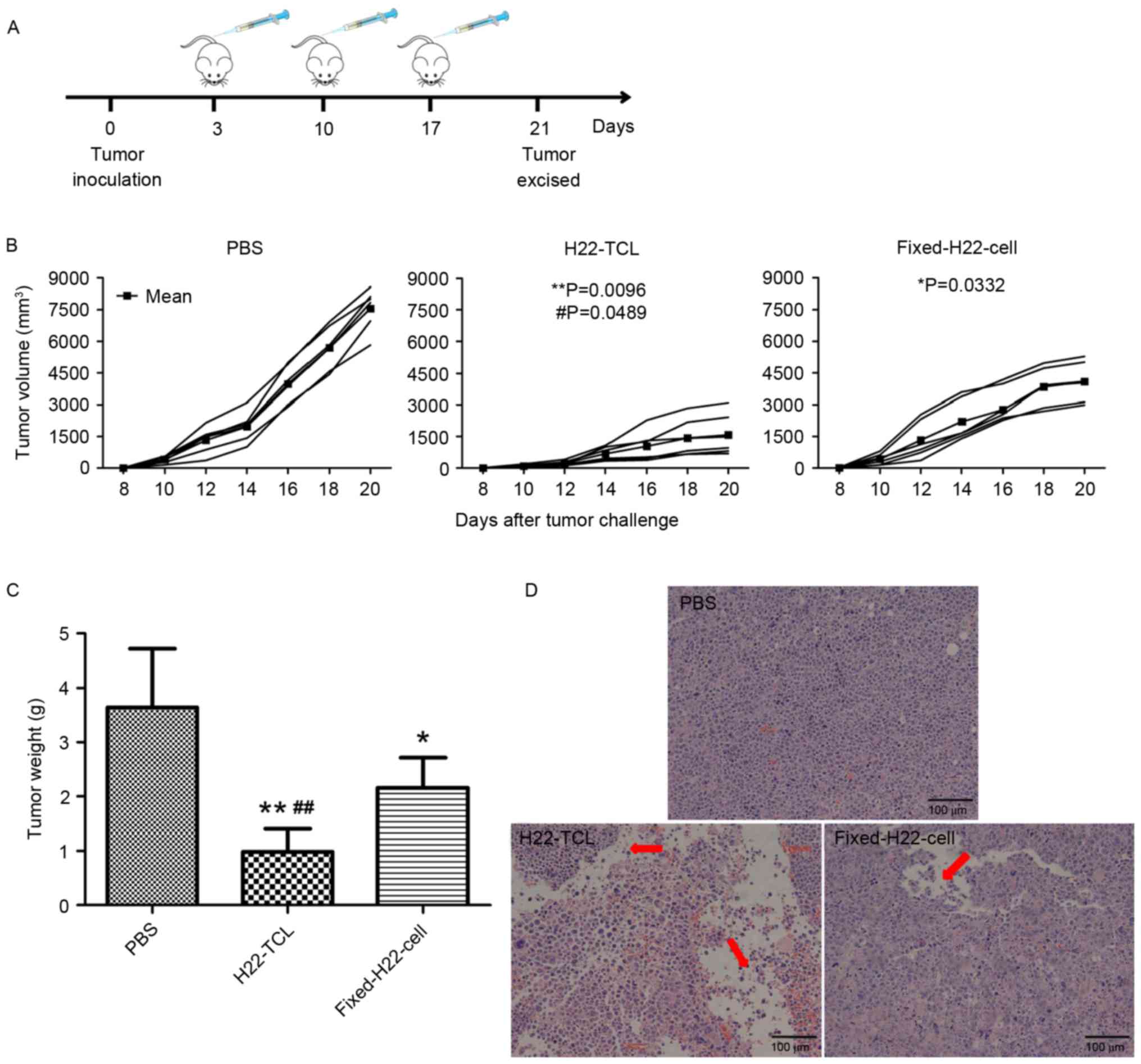
More relevant to the treatment of tumor is the
therapeutic potential, thus, a therapeutic vaccination protocol was
designed as shown in Fig. 2A. The
results in the therapeutic strategy were in accordance with that of
prophylactic strategy. In comparison with the PBS-treated group,
tumor growth was significantly inhibited in mice immunized with
H22-TCL or Fixed-H22-CELL (Fig. 2B).
Furthermore, the tumor growth of the H22-TCL group was
significantly slower compared with the Fixed-H22-CELL group
(P<0.05). The excised tumor weight revealed similar results, as
the group of mice immunized with H22-TCL exhibited the lowest mean
tumor weight among the three groups (Fig.
2C). H&E staining of the excised tumor tissues from the
H22-TCL group further indicated that H22-TCL immunization induced
the largest areas of inflammatory infiltrates and continuous
degenerative necrosis among the three experimental groups (Fig. 2D).
H22-TCL vaccination induces
prophylactic anti-metastasis effects
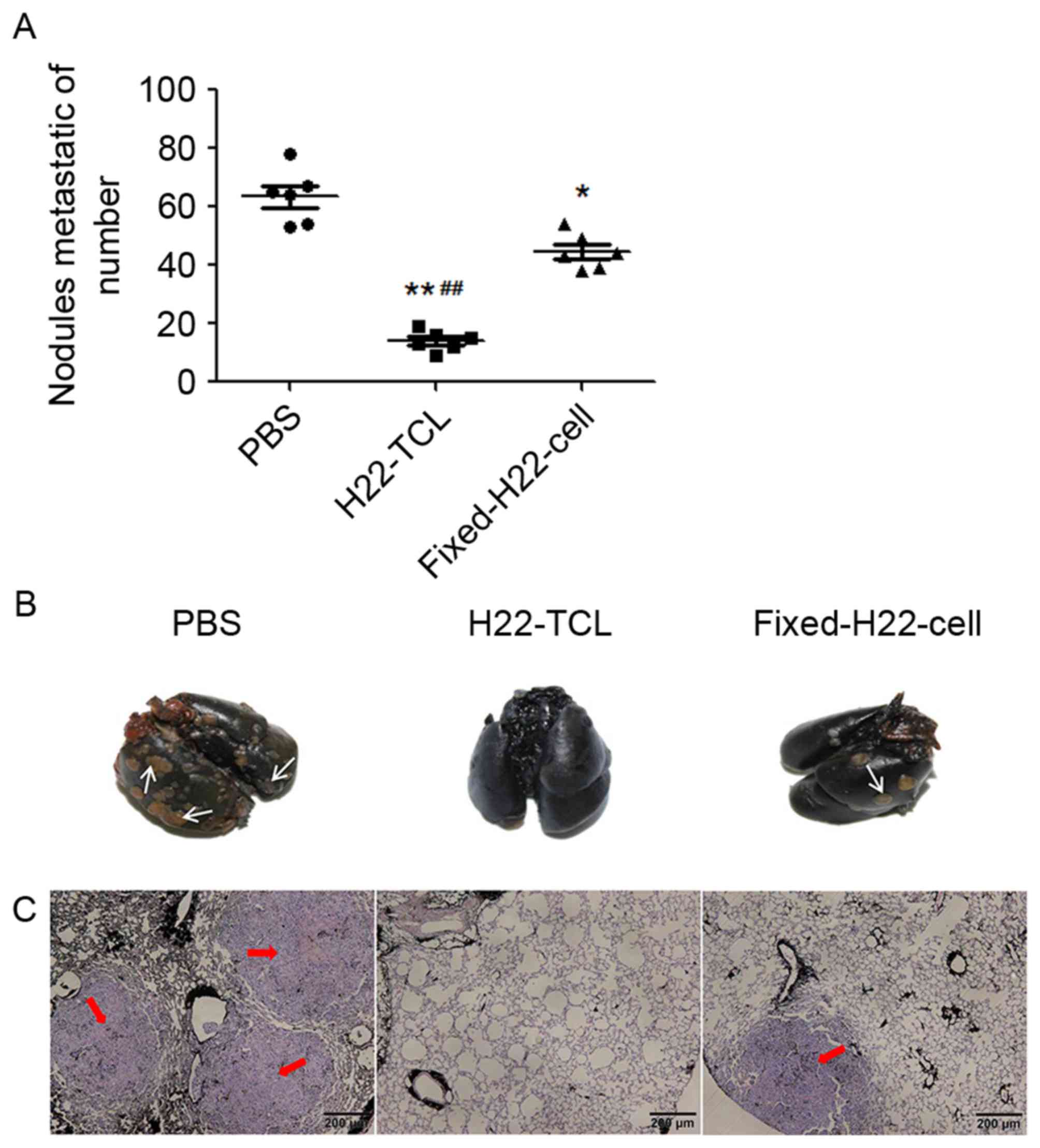
Subsequently, the present study investigated whether
the two vaccines could inhibit the growth of pulmonary metastasis.
The results are presented in Fig. 3.
In comparison with the PBS-treated mice, the number of metastatic
nodules following treatment with H22-TCL or Fixed-H22-CELL was
significantly reduced (Fig. 3A;
P<0.01 and P<0.05, respectively compared with the PBS group),
and the number of macroscopic metastatic nodules in the H22-TCL
group was the lowest (P<0.01 compared with the Fixed-H22-CELL
group). Furthermore, microscopic evaluation supported the
macroscopic findings, as the number of microscopic metastatic foci
in the H22-TCL group was the lowest (Fig.
3B and C).
H22-TCL vaccination induces a high
level of anti-H22 antibody production
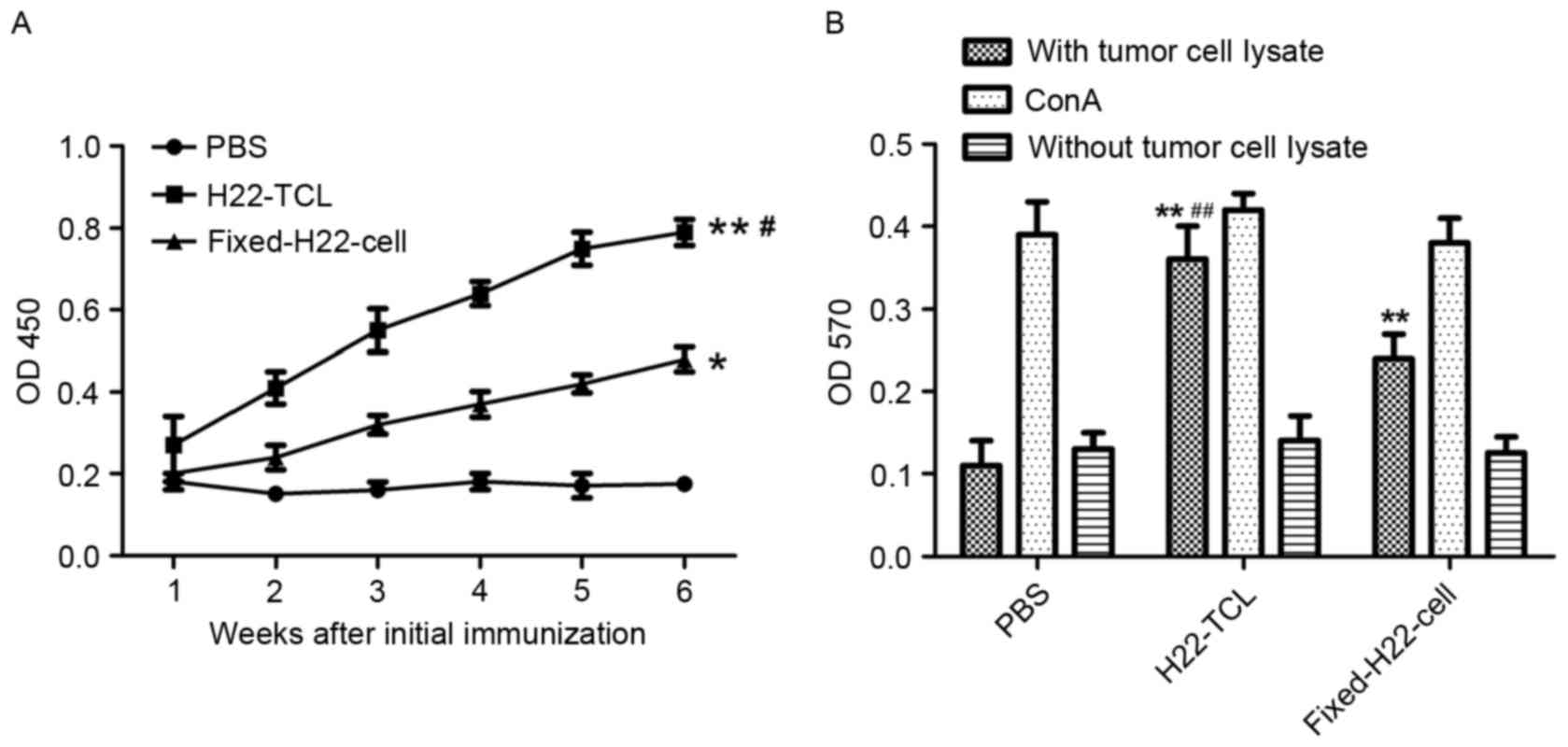
In order to investigate the humoral immune responses
elicited by various vaccines, ELISA was performed to determine the
expression level of anti-H22 antibodies in sera collected from
immunized mice. Compared with the PBS-treated group, H22 specific
IgG antibody responses were more evident in mice immunized with
H22-TCL or Fixed-H22-CELL (Fig 4A;
P<0.01 and P<0.05, respectively). In addition, the antibody
expression level of the H22-TCL group was significantly increased
compared with that of the Fixed-H22-CELL group (P<0.05).
H22-TCL vaccination induces a high T
lymphocyte proliferation activity
An MTT assay was performed to determine the
proliferation ability of lymphocytes from immunized mice. As
presented in Fig. 4B, compared with
the PBS group, a significant increase in the proliferation of
lymphocytes in the H22-TCL and Fixed-H22-CELL treatment groups was
revealed (P<0.01). In addition, the proliferation activity of
lymphocytes from mice immunized with H22-TCL was significantly
increased compared with that from mice immunized with
Fixed-H22-CELL (P<0.01).
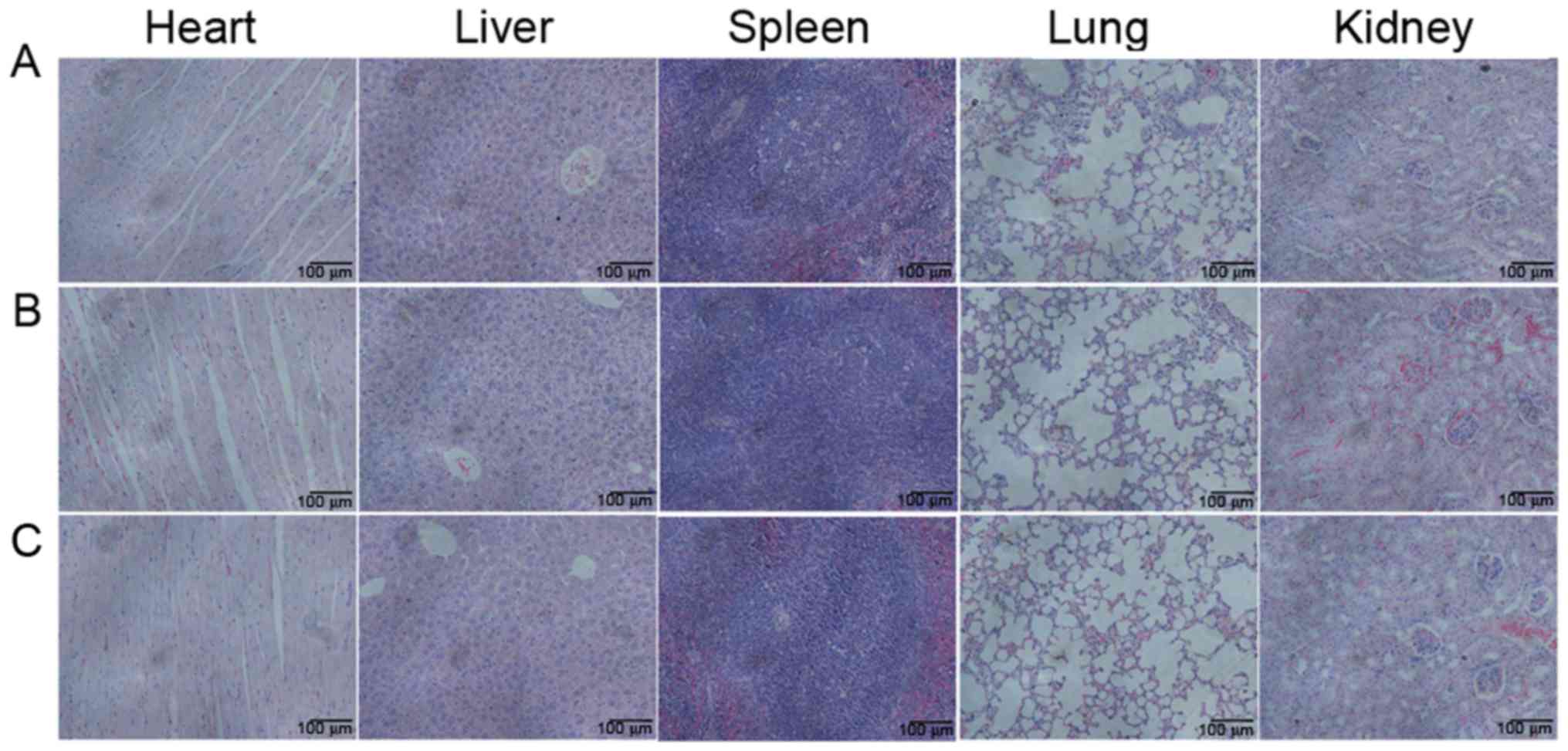
Toxicity observation
All mice appeared generally healthy, without any
noteworthy changes in appearance, fur, habits and body weight
following vaccine immunization. The effects of the vaccines on
normal tissues (heart, liver, spleen, lung and kidney) were further
examined. No pathologic changes were observed in the organs of the
immunized mice macroscopically (Fig.
5). Microscopic examination also revealed that H22-TCL and
Fixed-H22-CELL vaccines induced no damage in the organs excised
from immunized mice.
Discussion
Hepatocellular carcinoma is reported to be the
second leading cause of cancer-associated mortality worldwide, and
its incidence is rising (17).
However, notwithstanding great advances, no systemic
chemotherapeutic protocol has proved to be successful in HCC
treatment (18). Along with the
development of immunology and further understanding of the
mechanisms of tumorigenesis, tumor cell vaccines have become a new
cancer treatment research. Compared with traditional therapies,
tumor cell vaccines have the characteristic of strong specificity,
broad antitumor spectrum, low tolerance and have obtained success
in clinical trials (19,20).
Although cancer cell vaccines have been studied as a
promising cancer treatment strategy for decades, the antigen form,
which is the most effective in cancer vaccine preparation, has not
yet been clearly demonstrated. The antitumor efficiency of vaccines
prepared by tumor cell lysate and glutaraldehyde-fixed tumor cells,
two commonly used antigen forms (21–23), was
evaluated in the present study. The results of the present study
demonstrated that the tumor cell lysate-based vaccine induced
increased significant inhibition on tumor growth and metastasis
compared with the glutaraldehyde-fixed tumor cell-based vaccine,
which may be the result of the more evident antigen-specific
humoral and cellular immune responses. These results implied that
whole tumor cell lysate may be a more effective antigen form in
cancer vaccine preparation compared with glutaraldehyde-fixed tumor
cells, which would have clinical significance for cancer vaccine
preparation.
Recurrence and metastasis are typically the causes
of failure of multidisciplinary treatment for patients with HCC
(24,25). The present study evaluated the
anti-metastasis efficacy of the two vaccines using the tail venous
injection lung metastasis model. Consistent with the results of the
subcutaneous tumor model, the inhibition of metastasis by tumor
cell lysate-based vaccine was increased compared with the
glutaraldehyde-fixed tumor cell-based vaccine (Fig. 3). Microscopic evaluation of lung
tissues further demonstrated that the number of microscopic
metastatic foci in the tumor cell lysate-based vaccine group was
lowest. All the results revealed that the tumor cell lysate-based
vaccine may be used to decrease residual or metastatic tumor cells
for patients with HCC.
Taken together, the results from the present study
suggested that whole tumor cell lysate immunization may evoke a
stronger immune response compared with glutaraldehyde-fixed tumor
cells, which may result in a more significant inhibition on H22
hepatocellular carcinoma growth and metastasis. Collectively, these
results indicated that whole tumor cell lysate may be a more
effective antigen form in cancer cell vaccine preparation. The
findings of the present study may provide a rationale for the
further optimization of cancer cell vaccines and prompt further
studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81541158), Promotive
Research Fund for Young and Middle-aged Scientists of Shandong
Province (grant no. BS2014YY051), Shandong Provincial Natural
Science Foundation (grant no. ZR2015PH002), Medicine and Health
Science Technology Plan of Shandon Province (grant no. 2014WS0479)
and A Project of Shandong Province Higher Educational Science and
Technology Program (grant no. J15LM51).
References
|
1
|
Ray K: Liver cancer: The promise of new
approaches in the management of hepatocellular carcinoma-adding to
the toolbox? Nat Rev Gastroenterol Hepatol. 10:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong B, Dai G, Xu L, Zhang Y, Ling L, Sun
L and Lv J: Tumor cell lysate induces the immunosuppression and
apoptosis of mouse immunocytes. Mol Med Rep. 10:2827–2834. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grotz TE, Kottschade L, Pavey ES, Markovic
SN and Jakub JW: Adjuvant GM-CSF improves survival in high-risk
stage iiic melanoma: A single-center Study. Am J Clin Oncol.
37:467–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa
Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS and Onji M: Phase I/II
study of immunotherapy using tumor antigen-pulsed dendritic cells
in patients with hepatocellular carcinoma. Int J Oncol.
41:1601–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen IJ, Yen CF, Lin KJ, Lee CL, Soong YK,
Lai CH and Lin CT: Vaccination with OK-432 followed by TC-1 tumor
lysate leads to significant antitumor effects. Reprod Sci.
18:687–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang L and Ohno T: Protective antitumor
immunity induced by fixed tumor cells in combination with adjuvant
in a murine hepatoma model. Cancer Lett. 202:153–159. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Novaković S, Stegel V, Kopitar A, Ihan A
and Novaković BJ: Preventive and therapeutic antitumor effect of
tumor vaccine composed of CpG ODN class C and irradiated tumor
cells is triggered through the APCs and activation of CTLs.
Vaccine. 25:8241–8256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suckow MA, Rosen ED, Wolter WR, Sailes V,
Jeffrey R and Tenniswood M: Prevention of human PC-346C prostate
cancer growth in mice by a xenogeneic tissue vaccine. Cancer
Immunol Immunother. 56:1275–1283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin W, He Q, Hu Z, Chen Z, Qifeng M,
Zhichun S, Zhihui Q, Xiaoxia N, Li J and Gao J: A novel therapeutic
vaccine of GM-CSF/TNFalpha surface-modified RM-1 cells against the
orthotopic prostatic cancer. Vaccine. 28:4937–4944. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang ZY, Xing Y, Liu B, Lu L, Huang X, Ge
CY, Yao WJ, Xu ML, Gao ZQ, Cao RY, et al: Protective antitumor
immunity induced by tumor cell lysates conjugated with diphtheria
toxin and adjuvant epitope in mouse breast tumor models. Chin J
Cancer. 31:295–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Zhou L, Zhang P, Lu Y, Ge C, Yao W,
Xing Y, Xiao W, Dong Y, Wu J, et al: Enhanced antitumor efficacy by
combination treatment with a human umbilical vein endothelial cell
vaccine and a tumor cell lysate-based vaccine. Tumour Biol.
34:3173–3182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Copier J and Dalgleish A: Whole-cell
vaccines: A failure or a success waiting to happen? Curr Opin Mol
Ther. 12:14–20. 2010.PubMed/NCBI
|
|
13
|
Maki RG, Livingston PO, Lewis JJ, Janetzki
S, Klimstra D, Desantis D, Srivastava PK and Brennan MF: A phase I
pilot study of autologous heat shock protein vaccine HSPPC-96 in
patients with resected pancreatic adenocarcinoma. Dig Dis Sci.
52:1964–1972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernandez P, Petres S, Mécheri S, Gysin J
and Scherf A: Strain-transcendent immune response to recombinant
Var2CSA DBL5-ε domain block P. falciparum adhesion to
placenta-derived BeWo cells under flow conditions. PLoS One.
5:e125582010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blezinger P, Wang J, Gondo M, Quezada A,
Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R
and Min W: Systemic inhibition of tumor growth and tumor metastases
by intramuscular administration of the endostatin gene. Nat
Biotechnol. 17:343–348. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Xing Y, Zhou L, Yang X, Yao W, Xiao
W, Ge C, Ma Y, Yang J, Wu J, et al: Improved efficacy of
therapeutic vaccination with viable human umbilical vein
endothelial cells against murine melanoma by introduction of OK432
as adjuvant. Tumour Biol. 34:1399–1408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fowlkes V, Wilson CG, Carver W and
Goldsmith EC: Mechanical loading promotes mast cell degranulation
via RGD-integrin dependent pathways. J Biomech. 46:788–795. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozturk M and Oter S: Molecular approach to
treatment of hepatocellular carcinoma: New hope for therapeutic
targets. J Exp Integr Med. 1:83–84. 2011. View Article : Google Scholar
|
|
19
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elert E: Calling cells to arms. Nature.
504:S2–S3. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan X, Li W, Cui Y, Zhan Q, Zhang C, Yang
Z, Li X, Li S, Guan Q and Sun X: Specific cellular immune response
elicited by the necrotic tumor cell-stimulated macrophages. Int
Immunopharmacol. 27:171–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawahara M and Takaku H: Intradermal
immunization with combined baculovirus and tumor cell lysate
induces effective antitumor immunity in mice. Int J Oncol.
43:2023–2030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He A, Zhang W, Xu K, Wang J, Yang Y and
Chao X: Anti-tumor immune responses in immune-reconstituted mice
injected with a tumor vaccine. Med Oncol. 29:2261–2269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishii Y, Sakamoto T, Ito R and Yanaga K:
Anti-angiogenic therapy on hepatocellular carcinoma development and
progression. J Surg Res. 158:69–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y and Sun H: The tumor protection
effect of high-frequency administration of whole tumor cell vaccine
and enhanced efficacy by the protein component from Agrocybe
aegerita. Int J Clin Exp Med. 8:6914–6925. 2015.PubMed/NCBI
|