Introduction
T-cell acute lymphoblastic leukemia (T-ALL)/T-cell
lymphoblastic lymphoma (T-LBL) is a rare and aggressive neoplasm
which originates from T-cell progenitors and may involve the bone
marrow (BM) and blood (for T-ALL) or the thymus, nodal and
extranodal sites (for T-TBL). T-ALL and T-LBL are similar in
morphology and are thus classified together as T-ALL/LBL by the
World Health Organization (WHO) (1).
With intensified chemotherapy, the prognosis of T-ALL/LBL has
markedly improved (2,3). Lymphoblasts in T-ALL/LBL typically
express terminal deoxynucleotidyltransferase (TDT) and variably
express T-lineage makers, including cluster of differentiation
(CD)la, CD2, CD3, CD4, CD5, CD7 and CD8. Immunophenotyping is
required to diagnose T-ALL/LBL.
Myeloid sarcoma is a rare extramedullary myeloid
neoplasm characterized as a tumor mass consisting of mature or
immature myeloid blasts and occurring at almost any anatomical site
other than the BM, including the skin, lymph nodes,
gastrointestinal tract, bone, soft tissue and testis (4). Myeloid sarcoma with clinical
characteristics is diagnosed by morphological observation,
immunophenotypical analysis, fluorescent in situ
hybridization (FISH) on tissue sections and/or conventional
karyotyping of BM or peripheral blood (PB) cells (4). In myeloid sarcoma, CD68 is the most
common type of expressed marker, followed by myeloperoxidase (MPO),
CD117, CD99, CD68/phosphoglucomutase-1, CD34 and TDT (4). In addition, chromosomal aberrations were
determined in ~50% of myeloid sarcoma cases, including monosomy 7,
trisomy 8 and mixed lineage leukemia-splitting; however, t(8;21)
was rarely observed (4). These
chromosomal aberrations allow for the distinction of myeloid
sarcoma from lymphomas, including LBL, and blastic plasmacytoid
dendritic cell neoplasms (5).
Leukemia/lymphoma presentation with myeloid sarcoma
is rare. In the present case report, a case of T-LBL with myeloid
sarcoma (with serous effusion as the main manifestation) in a
Chinese adult male patient is described.
Case report
A 33-year-old Chinese male was admitted to the
Department of Hematology, The Second Hospital of Hebei Medical
University (Shijiazhuang, China) on 4 July 2015, presenting with a
fever and having experienced wheezing and fatigue for the previous
7 days. The patient was a teacher and was otherwise healthy prior
to being admitted. Upon admission, superficial lymph node
enlargement in the right neck and bilateral axillar supraclavicular
fossa was identified. The patient's heart and lungs were normal,
without enlargement. Hematological analysis, using an automated
hematology analyzer (Sysmex, Kobe, Japan), identified that the
patient had a white blood cell count of 8.87×109
cells/l, a hemoglobin level of 13.5 g/dl and a platelet count of
438×109 cells/l. Serum levels of lactate dehydrogenase
and uric acid were at increased levels of 297 U/l and 264.8 mg/dl,
respectively. Additional laboratory findings were as follows:
C-reactive protein, 11 mg/dl; aspartate transaminase activity, 33
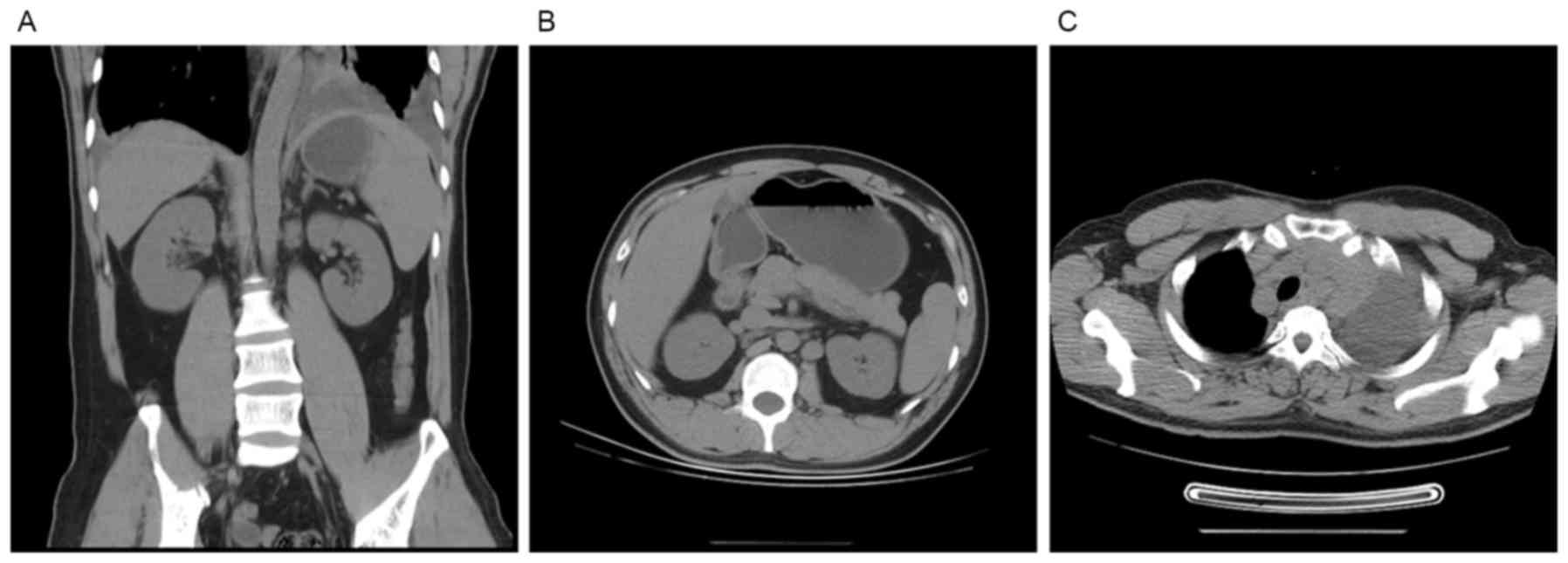
U/l; and fibrinogen, 0.82 g/l. A computed tomography (CT) scan,
using a 16-slice spiral CT (GE Healthcare, Chicago, IL, USA),
revealed an enhanced density of multiple nodules in the
retroperitoneal lymphonodus and in the mediastinum and abdomen
(Fig. 1), indicating multiple lymph
node enlargement which is characteristic of lymphoma.
Immunohistochemical staining for paired box (PAX)-5, MPO, CD20,
CD21, CD3, TDT, CD7, CD30 and Ki67 was performed on the lymph node,
using the following monoclonal antibodies: Rabbit anti-PAX-5 (cat.
no. TA322317, 1:50), rabbit anti-MPO (cat. no. TA349402, 1:100),
rabbit anti-CD20 (cat. no. TA353671, 1:50), rabbit anti-CD21 (cat.
no. TA327628, 1:100), rabbit anti-CD3 (cat. no. AP22689PU-N, 1:50),
rabbit anti-TDT (cat. no. TA327743, 1:100), rabbit anti-CD7 (cat.
no. TA353715, 1:100), rabbit anti-CD30 (cat. no. TA590389, 1:150)
and mouse anti-Ki67 (cat. no. TA803157, 1:150). All antibodies were
purchased from OriGene Technologies (Beijing, China).
Immunohistochemical staining was performed as follows: The slide
was rinsed twice for 5 min in TBS 0.025% Triton with gentle
agitation, then incubated in 0.3% H2O2 in TBS
for 15 min at room temperature. Subsequently, the slide was blocked
in 10% normal serum with 1% bovine serum albumin (BSA) in TBS for 2
h at room temperature. The primary antibody was then applied
diluted in TBS with 1% BSA to the slide and incubated overnight at
4°C. Subsequently, Rabbit Anti-Mouse IgG H&L horseradish
peroxidase conjugated (HRP; 1:1,000; ab6728; Abcam, Cambridge, UK)
or Goat anti-Rabbit IgG H&L HRP (1:1,000; ab6721; Abcam)
antibodies were added to the slide, diluted in TBS with 1% BSA and
incubated for 1 h at room temperature. The slide was then rinsed in
running tap water for 5 min, 2 times. The DAB color test was
performed using a DA1010 DAB kit (×20), according to the protocol
of the DA1010 DAB Color test kit (Beijing solaibotechnology Co.
Ltd., Beijing, China). Finally, following DAB coloring, the slide
was sealed and images captured using a LEICA DM 2000 light
microscope at magnifications, ×100 and ×400. Staining was
identified to be negative for the B-cell markers PAX-5, MPO and
CD20, but positive for the T-cell markers TDT, CD7, CD30 (sporadic
+), CD3 (partial +), Ki67 (50% +) and CD21 in the lymph node
(Fig. 2A). These results were
consistent with the immunophenotypical characteristics of T-LBL.
Hematoxylin and eosin staining revealed pathological changes
including viability, marked nuclear atypia and frequent
karyokinesis of cells in the lymph node (Fig. 2B).
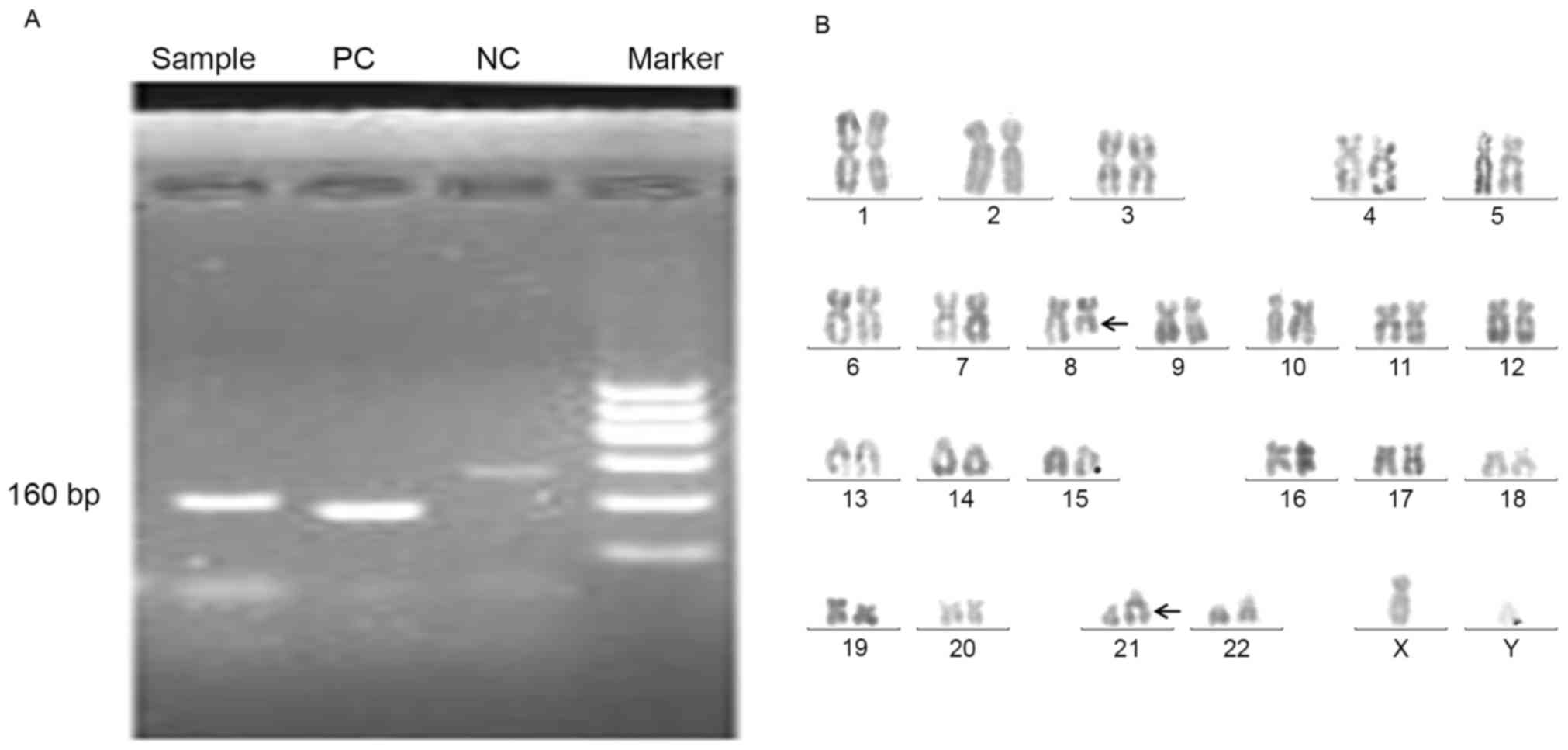
 | Figure 2.Immunohistological, histological,
karyotypic and flow cytometric analysis indicated the diagnosis of
T-LBL in a patient, without the involvement of the bone marrow (7
August 2015). (A) Immunohistological observation of lymph node
(magnification, ×200). (B) Histological analysis of lymph node
(magnification, ×200). (C) Karyotype analysis of the bone marrow
cells, using the R banding technique revealed the normal karyotype
(46,XY). PAX, paired box; MPO, myeloperoxidase; CD, cluster of
differentiation; TDT, terminal deoxynucleotidytransferase. |
A PB smear identified that neutrophilic
metamyelocytes were occasionally observed (~2.6%) in PB cells and a
BM smear revealed a limited proportion of myeloblasts (1%) and
promyelocytes (1%) among the nucleated BM cells. Karyotype
analysis, using the R banding technique (6), indicated a normal karyotype (46 XY) of
the BM cells (Fig. 2C). These results
revealed that the PB and BM were not involved. Flow cytometric
analysis, using a BD FACSCanto II™ instrument (BD Biosciences,
Franklin Lakes, NJ, USA) revealed that, among the nucleated BM
cells, the majority were positive for CD20 (93.3%) and CD22
(97.3%), and between 33 and 50% were positive for CD38 (35.3%),
CD79b (38.5%), CD20 (49.3%), CD24 (42.8%), κ (36.9%) and λ (30.9%).
In contrast, a limited number of nucleated BM cells were positive
for CD19 (1.5%) and CD5+CD23+ cells were
identified (2.5%; characteristic of B-cell lymphoma/leukemia),
which enabled a diagnosis of B-cell lymphoma to be excluded. CD103,
CD11c and CD25 were not determined in BM cells, thus excluding the
diagnosis of hairy cell leukemia, a type of B-cell lymphoma. In
addition, granulocyte series accounted for 33% of the nucleated BM
cells, thus excluding the presence of abnormal early myeloid cells.
The patient was diagnosed with mediastinal T-LBL, without evidence
of the involvement of the BM on 7 August 2015.
The patient received induction chemotherapy with
dexamethasone (15 mg/day on days 1–5), vincristine (1.4
mg/m2/day on day 1), daunorubicin (50
mg/m2/day on day 1), cyclophosphamide (750
mg/m2/day on day 1) and L-asparaginase (6,000
U/m2/day on days 1, 3, 5, 7, 9 and 11) and achieved
partial remission subsequently. After 2 weeks, the patient received
a course of dexamethasone (15 mg/day on day 1–5), vincristine (1.4
mg/m2/day on day 1), daunorubicin (50
mg/m2/day on day 1) and cyclophosphamide (750
mg/m2/day on day 1). On 17 November 2015, multiple
serous effusion (including pericardial effusion, pleural effusion
and ascites) was identified. Immunophenotypical analysis, using
flow cytometry, revealed that the blast cells accounted for 25% of
the nucleated BM cells, which were positive for T-LBL cell markers
(including CD33, CD34, CD38, HLA-DR, CD123, CD56, CD11c and CD15)
and negative for B-LBL cell markers (including MPO, CD117, CD16,
CD2, CD5, cytosolic CD3, CD7, CD64, CD14, CD19, CD10, CD20, CD22
and CD79a). These results confirmed the diagnosis of T-LBL in the
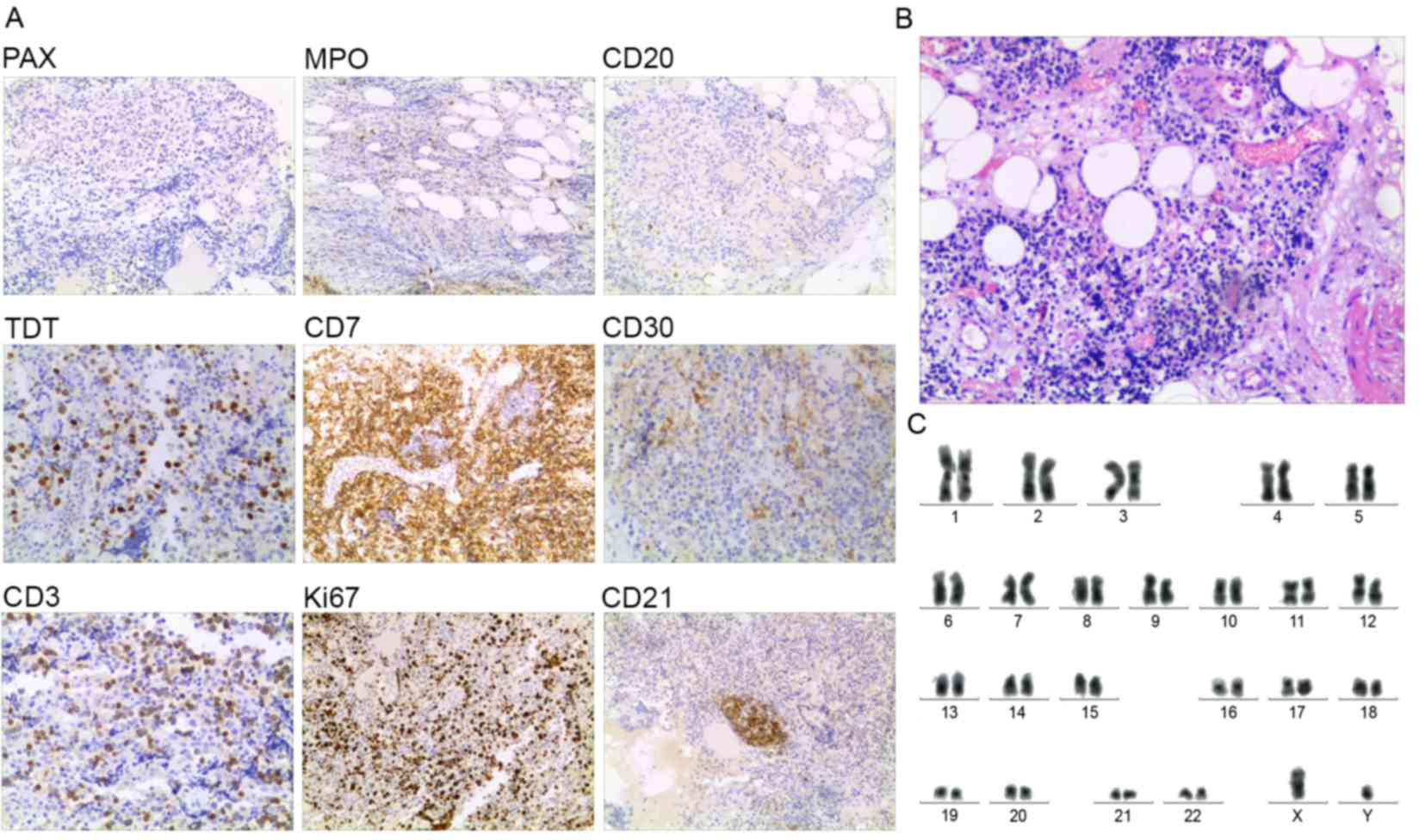
patient. The polymerase chain reaction (PCR) demonstrated that
acute myeloid leukemia 1-eight-twenty-one (AML1-ETO) fusion protein
was expressed in pericardial effusion cells (Fig. 3A), characteristic of the abnormal
clone of myeloid sarcoma (as a type of myeloid leukemia). The
following genes were not determined using FISH (DNA Technology,
Aarhus, Denmark): Breakpoint cluster region-Abelson murine leukemia
viral oncogene homolog 1; promyelocytic leukemia protein-retinoic
acid receptor α; myosin heavy chain 11-core-binding factor β
subunit; mixed-lineage leukemia 1; platelet-derived growth factor
receptor α (PDGFRA); platelet-derived growth factor receptor β
(PDGFRB); and fibroblast growth factor α1 (FGFA1). In addition,
aberrant karyotype t(8;21) was identified in pericardial effusion
cells (Fig. 3B). Therefore, the
patient was diagnosed with T-LBL complicated with extramedullary
myeloid sarcoma (in the pericardium) on 25 November 2015, according
to the 2008 WHO classification of Tumors of Haematopoietic and
Lymphoid Tissues (7).
The patient received a second course of induction
chemotherapy with cytarabine (1.0 g/m2/day, every 12 h
for 3 days) and mitoxantrone (8 mg/m2/day for 3 days),
and was expected to receive allogeneic hematopoietic stem cell
transplantation. However, since there were no matched donors
available and the patient was experiencing pericardial effusion,
following chemotherapy with mitoxantrone and cytarabine, the
patient was advised to receive local radiotherapy. On 28 December
2015, following three doses of local radiotherapy (50 cGy/dose),
the patient was required to stop radiation as the white blood cells
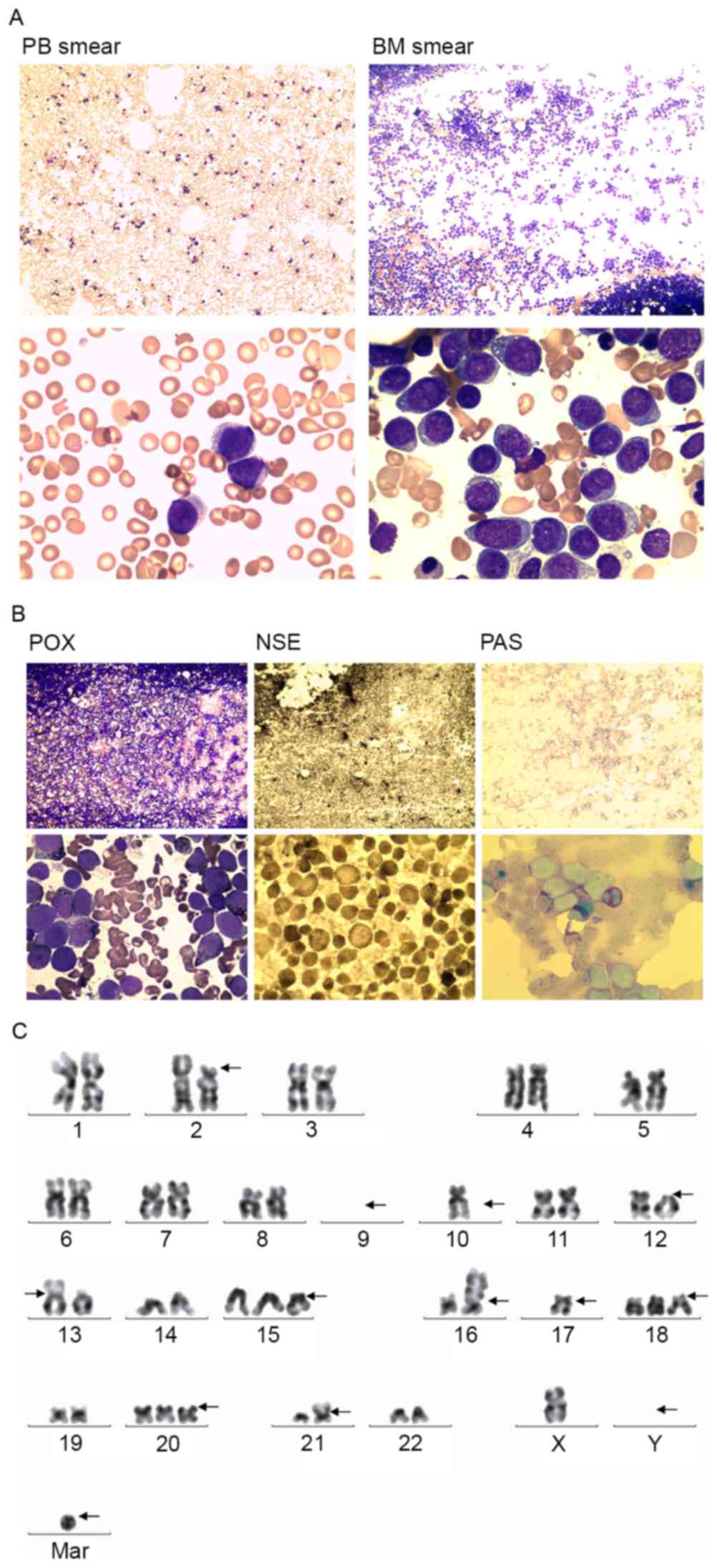
increased to 40.0×109 cells/l. BM and PB samples were
collected, and the BM and PB smears identified the proliferation of
primary myeloid cells (Fig. 4A) which
were positive for peroxidase, non-specific esterase and periodic
acid-Schiff staining (Fig. 4B).
Chromosomal analysis identified an abnormal karyotype of the BM
cells as follows: 42,X,-Y,del
(2)(p12),-9,-9,del(12)(p11),der(13;17)(q10;q10),add(16)(p13),add
(21)(p11)[3]/43,idem,+mar[8]/45,idem,-10,+15,+18,+20,+mar[4]
(Fig. 4C). On 30 December 2015, the
patient was diagnosed with T-LBL complicated with myeloid sarcoma,
which involved the BM. Furthermore, Second Generation
High-Throughput Sequencing was then carried out using a MiSeq
platform (Illumina Inc., San Diego, CA, USA) and the sequences
generated were run on Human Genome Version 19 using Illumina
bcl2fastq version 2.15 software (Illumina, Inc.). Three mutations
were identified in the pericardial effusion cells and the BM cells
as follows: Tet methylcytosine dioxygenase (TET) 2
NM_001127208:exon11:c.4789_4793del:p.F1597fs mutation; AML1
NM_001754:exon5:c.496_497insGGT TCG GAG TTG CGT GTC G:p.R166fs
mutation; and tumor suppressor protein (TP) 53
NM_000546:exon6:c.T581A:p.L194H mutation. The patient subsequently
received chemotherapy to control the disease. According to the
acute myeloid leukemia (myeloid sarcoma) therapeutic protocol for
T-LBL with multiple serous effusion (8), the patient was administered with
mitoxantrone (8 mg/m2/day for 3 days) and cytosine
arabinoside (100 mg/m2/day for 7 days), in combination
with L-asparaginase (6,000 U/m2/day on days 1, 3, 5, 7,
9 and 11). In addition, since mutations of the TET2, AML1 and TP53
genes were identified, the patient was administered decitabine (20
mg/m2/day, via an intravenous drip on days 1–5), a DNA
methylation inhibitor used to treat MDS/AML with TP53 and TET2 gene
mutations (9). Subsequently, the
multiple serous effusion was markedly decreased; however, myeloid
blast cells (33% of the total cells) were observed in a BM smear,
indicating that the patient had improved but did not achieve
remission. The patient was discharged on 31 January 2016 and ceased
treatment. The patient succumbed on 19 February 2016, at home.
The present case report was approved by the Ethics
Committee of the Second Hospital of Hebei Medical University
(Shijiazhuang, China) and informed consent was obtained from the
patient.
Discussion
The patient was diagnosed with T-LBL using CT scans
and pathological, cytological and phenotypical observations on 7
August 2015. However, the corresponding treatment regimen only
achieved partial remission, which may be because the treatment was
aimed at T-LBL only, despite the patient additionally exhibiting
myeloid sarcoma. Subsequently, on 25 November 2015, PCR, FISH and
karyotype analysis of the pericardial effusion cells were carried
out which identified myeloid sarcoma. Since PDGFRA, PDGFRB and
FGFA1, associated with myeloproliferative and lymphoid neoplasms
(10–12), were all negative in the pericardial
effusion cells, a diagnosis of myeloproliferative and lymphoid
neoplasms was excluded. Since the interval between the diagnosis of
T-LBL and that of myeloid sarcoma was <1 year (between August
and November 2015), the patient did not meet the characteristics of
T-acute myelocytic leukemia (13,14) and
was subsequently diagnosed with T-LBL complicated with myeloid
sarcoma, which is markedly rare.
A number of patients with T-LBL may subsequently
develop myeloid sarcoma (15);
however, T-ALL/LBL with myeloid sarcoma is rare and has only been
described in a limited number of cases (5,16). A
previous study identified a 14-year-old male who presented with
chronic myelogenous leukemia (CML) in the blast phase with
segregated extramedullary (nodal) myeloid sarcoma and T-LBL
(5). In addition, a 49-year-old
female patient with CML was identified to exhibit myeloid sarcoma
and T-LBL (16). In the present case
report, a 33-year-old Chinese male patient was described who
presented with primary T-LBL. The patient was diagnosed with
myeloid sarcoma during the treatment course (3 months following the
diagnosis of T-LBL) and, ~1 month subsequently, myeloid sarcoma
which involved the BM. It is not possible for myeloid sarcoma to
develop from T-LBL in such a short period; therefore, it is
hypothesized that myeloid sarcoma, in this patient, did not develop
from T-LBL, but was complicated with T-LBL during the pathogenesis.
To the best of our knowledge, this is the first case of T-LBL with
myeloid sarcoma in a Chinese adult male patient.
Typically, myeloid sarcoma occurs at almost any
anatomical site, with the exception of the BM (4). In the present case report, the patient
with T-LBL and myeloid sarcoma was identified to exhibit
involvement of the BM. The diagnosis of T-LBL excludes the
possibility of the involvement of the BM in T-LBL; therefore, the
involvement of the BM, observed 1 month after the primary detection
of myeloid sarcoma, may be attributable to myeloid sarcoma.
Involvement of the BM in the complicated myeloid sarcoma and the
aberrant karyotype t(8;21) in complicated myeloid sarcoma is rarely
observed.
Notably, three mutations were identified (TET2, AML1
and TP53 genes) in the pericardial effusion cells and BM cells
(TET2 NM_001127208:exon11:c.4789_4793del:p.F1597fs; AML1
NM_001754:exon5:c.496_497insGGTTCGGAGTTGCGTGTCG:p.R166fs; and TP53
NM_000546:exon6:c.T581A:p.L194H). It is hypothesized that mutations
of the TET2, AML1 and TP53 genes may participate in a common
pathway attributable to the pathogenesis of T-LBL and myeloid
sarcoma. Additional studies are required to explore the underlying
molecular mechanisms of the pathogenesis of T-LBL complicated with
myeloid sarcoma. The present case report described a rare case of
T-LBL complicated with myeloid sarcoma (with distinct BM
involvement and karyotype) in a Chinese adult male patient.
Acknowledgements
The present case report was supported by the Hebei
Provincial Natural Science Foundation of China (grant no.
H2016206577).
References
|
1
|
Steven H, Elias, Campo NancyLee, Harris,
et al: International Agency for Research on Cancer Lyon. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
1762008.
|
|
2
|
Thomas DA, O'Brien S, Cortes J, Giles FJ,
Faderl S, Verstovsek S, Ferrajoli A, Koller C, Beran M, Pierce S,
et al: Outcome with the hyper-CVAD regimens in lymphoblastic
lymphoma. Blood. 104:1624–1630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marks DI, Paietta EM, Moorman AV, Richards
SM, Buck G, DeWald G, Ferrando A, Fielding AK, Goldstone AH,
Ketterling RP, et al: T-cell acute lymphoblastic leukemia in
adults: Clinical features, immunophenotype, cytogenetics, and
outcome from the large randomized prospective trial (UKALL XII/ECOG
2993). Blood. 114:5136–5145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pileri SA, Ascani S, Cox MC, Campidelli C,
Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero
D, et al: Myeloid sarcoma: Clinico-pathologic, phenotypic and
cytogenetic analysis of 92 adult patients. Leukemia. 21:340–350.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Rutledge JC, Wu D, Fang M, Opheim
KE and Xu M: Chronic myelogenous leukemia presenting in blast phase
with nodal, bilineal myeloid sarcoma and T-lymphoblastic lymphoma
in a child. Pediatr Dev Pathol. 16:91–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verma RS and Lubs HA: A simple R banding
technic. Am J Hum Genet. 27:110–117. 1975.PubMed/NCBI
|
|
7
|
Borowitz MJ and Chan JKC: T lymphoblastic
leukaemia/lymphomaSwerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri
SA, Stein H, et al: WHO classification of tumours of haematopoietic
and lymphoid tissues. 4th edition. IARC Press; Lyon: pp. 176–178.
2008
|
|
8
|
Takahashi H, Koh K, Kato M, Kishimoto H,
Oguma E and Hanada R: Acute myeloid leukemia with mediastinal
myeloid sarcoma refractory to acute myeloid leukemia therapy but
responsive to L-asparaginase. Int J Hematol. 96:136–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bejar R, Lord A, Stevenson K, Bar-Natan M,
Pérez-Ladaga A, Zaneveld J, Wang H, Caughey B, Stojanov P, Getz G,
et al: TET2 mutations predict response to hypomethylating agents in
myelodysplastic syndrome patients. Blood. 124:2705–2712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bain BJ and Fletcher SH: Chronic
eosinophilic leukemias and the myeloproliferative variant of the
hypereosinophilic syndrome. Immunol Allergy Clin North Am.
27:377–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Metzgeroth G, Walz C, Score J, Siebert R,
Schnittger S, Haferlach C, Popp H, Haferlach T, Erben P, Mix J, et
al: Recurrent finding of the FIP1L1-PDGFRA fusion gene in
eosinophilia-associated acute myeloid leukemia and lymphoblastic
T-cell lymphoma. Leukemia. 21:1183–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tokita K, Maki K, Tadokoro J, Nakamura Y,
Arai Y, Sasaki K, Eguchi-Ishimae M, Eguchi M and Mitani K: Chronic
idiopathic myelofibrosis expressing a novel type of TEL-PDGFRB
chimaera responded to imatinib mesylate therapy. Leukemia.
21:190–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pedersen-Bjergaard J, Christiansen DH,
Desta F and Andersen MK: Alternative genetic pathways and
cooperating genetic abnormalities in the pathogenesis of
therapy-related myelodysplasia and acute myeloid leukemia.
Leukemia. 20:1943–1949. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith SM, Le Beau MM, Huo D, Karrison T,
Sobecks RM, Anastasi J, Vardiman JW, Rowley JD and Larson RA:
Clinical-cytogenetic associations in 306 patients with
therapy-related myelodysplasia and myeloid leukemia: The university
of Chicago series. Blood. 102:43–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abruzzo LV, Jaffe ES, Cotelingam JD,
Whang-Peng J, Del Duca V Jr and Medeiros LJ: T-cell lymphoblastic
lymphoma with eosinophilia associated with subsequent myeloid
malignancy. Am J Surg Pathol. 16:236–245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krishnan S, Sabai K, Chuah C and Tan SY:
Bilineal T lymphoblastic and myeloid blast transformation in
chronic myeloid leukemia with TP53 mutation-an uncommon
presentation in adults. Curr Oncol. 21:e147–e150. 2014. View Article : Google Scholar : PubMed/NCBI
|