Introduction
Acute promyelocytic leukemia (APL), classified as M3
in the French-American-British classification system (1), is a subtype of acute myelocytic leukemia
(AML). APL represents between 7 and 27% of all AML types worldwide;
however, in China this proportion increases to between 12 and 23%
(2). Compared with other AML types,
APL is associated with an increased risk of hemorrhage and an
increased mortality rate (3).
Currently, systematical chemotherapies based on
all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO)
are the predominant treatment methods (4); however, single and combination drug
therapies have many disadvantages, including early drug resistance,
early recurrence and adverse side effects (5). Thus, the treatment outcome and prognosis
of APL remain poor.
The functions and therapeutic roles for microRNAs
(miRNAs/miRs) in APL have been reported (6,7). miRNAs
are small (between 19 and 25 nucleotides) non-coding RNA molecules
that negatively regulate gene expression by interacting with the 3′
untranslated region of targeted mRNA, eventually leading to
translational suppression and/or degradation of the mRNA (8). Previous studies have demonstrated that
miRNAs serve important roles in the generation and progression of a
number of human solid or hematological neoplasms via regulation of
cell growth, differentiation, invasion and other pathological
processes (9). In patients with AML,
miR-96 was identified to be downregulated and associated with
leukemic burden, in addition to recurrence-free survival and
overall survival (10). In addition,
miR-125b was demonstrated to be highly expressed in pediatric APL
compared with other subtypes of AML and was associated with
treatment response and relapse in patients with pediatric APL
(11). The underlying molecular
mechanisms of this effect may be that miR-125b downregulates the
expression of the tumor suppressor Bcl-2-antagonist/killer 1 to
promote leukemic cell proliferation and inhibit cell apoptosis
(11). miR-218 was demonstrated to be
an important tumor suppressor in a number of types of cancer; in
hepatocellular carcinoma, miR-218 has been demonstrated to be
downregulated, and associated with increased tumor size (12). In vitro, overexpression of
miR-218 repressed cell proliferation and induced apoptosis in HepG2
and SMMC-7721 cells (12).
B-cell-specific Moloney murine leukemia virus
insertion site-1 (BMI-1) is located in human chromosome 10p11.23.
Liu et al (13) demonstrated
that BMI-1 was able to suppress the expression of
p16INK4a and p19ARF (which are proteins from
the same genetic locus as cyclin-dependent kinase inhibitor 2A) by
impairing the transcription of this locus and therefore leading to
a series of dysfunctions in the cell cycle and cell
proliferation.
In the present study, the expression of miR-218 in
APL tissues and its functions in cell growth were investigated
in vitro. The results indicated that miR-218 is
downregulated in APL tissues and HL-60 cells, and it may suppress
cell proliferation and induce apoptosis by inhibiting BMI-1
expression.
Materials and methods
Clinical specimens and cell
culture
A total of 40 marrow tissues from patients with APL
(25 female; 15 male) and 20 normal marrow tissues were collected
and stored in liquid nitrogen in Yuhuangding Hospital during
(Yantai, China) between April 2011 to May 2014. The age range of
the patients was between 24 and 65 years and the median age was 49
years. None of the patients included in the present study accepted
chemotherapy prior to biopsy. All clinical values for white blood
cell count (WBC), hemoglobin (HGB) and platelet count (PLT) were
obtained from the medical records of patients. The present study
was approved by the Ethics Committee of Yantai Yuhuangding Hospital
and written informed consent was obtained from all patients. The
human APL cell line HL-60 was purchased from The Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Gibco®
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and maintained at 37°C in a
cell culture incubator with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from marrow tissues and HL-60
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 20 µg cDNA was used for the SYBR® Green Real-Time PCR
Master Mixes (Invitrogen; Thermo Fisher Scientific, Inc.) system.
The relative expression of miR-218 was detected using a Bulge-Loop™
miRNA RT-qPCR Starter kit (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) according to the manufacturer's protocol. The BMI-1 mRNA
expression level was measured using a Quant One Step qRT-PCR kit
(Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's protocol. The Bulge-Loop™ miR-218 primer kit (cat.
no. miRQ0000275-1-2) was purchased from RiboBio Co., Ltd
(Guangzhou, China). BMI-1 and β-actin primers were synthesized by
Beijing Augct DNA-Syn Biotechnology Co., Ltd. (Beijing, China) and
the sequences were as follows: BMI-1 forward,
5′-GTGCTTTGTGGAGGGTACTTCAT-3′ and reverse,
5′-TTGGACATCACAAATAGGACAATACTT-3′; β-actin forward,
5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse, 5′-GCTGTCACCTTCACCGTTCC-3′.
The 2−ΔΔCq method (14)
was used to calculate differences in mRNA expression.
miRNA transfection
HL-60 cell transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 5×105 cells were cultured in 6-well culture
plates containing 1.5 ml RPMI-1640 medium per well without FBS.
Amounts of 100 pmol of miR-218 mimic (cat. no., miR10000275-1-5) or
scrambled miRNA mimic (cat. no., miR01201-1-5) (Guangzhou RiboBio
Co., Ltd.) were mixed with 5 µl Lipofectamine® 2000 in
500 µl Opti-MEM® I Reduced Serum Medium (Gibco; Thermo
Fisher Scientific, Inc.) and incubated for 15 min at room
temperature. The complex was added to the cells and the full
distribution over the plate surface was ensured. After 8 h of
incubation, whole media were replaced and the cells were incubated
for 24, 48 and 72 h.
Cell viability assay
The viability of cells was assessed using the Cell
Counting Kit-8 (CCK-8, Sangon Biotech Co., Ltd., Shanghai, China).
The CCK-8 assay was performed at 24, 48 and 72 h after
transfection. A 10 µl volume of CCK-8 solution was added to
5×105 HL-60 cells suspended in 100 µl RPMI-1640 medium
and incubated for 4 h at 37°C in the dark. Absorbance was
determined using a spectrophotometer at 450 nm.
Bromodeoxyuridine (BrdU) incubation
assay
HL-60 cells (7×104) were cultured on
coverslips at 48 h post-transfection and their proliferative
ability was detected using a BrdU Cell Proliferation Assay kit
(Cell Signaling Technology, Inc., Danvers, MA, USA) according to
the manufacturer's protocol. Absorbance was determined using a
spectrophotometer at 450 nm.
Flow cytometric analysis
HL-60 cells were collected and diluted to
5×105 48 h after transfection. Cell cycle phase and the
proportion of apoptotic cells were evaluated using Annexin
V-fluorescein isothiocyanate and propidium iodine staining (Roche
Diagnostics, Basel, Switzerland) according to the manufacturer's
protocol and analyzed using BD FACSCalibur flow cytometry system
(BD Biosciences, Franklin Lakes, NJ, USA) with the software FCS
Express v3.0 (De Novo Software, Glendale, CA, USA).
Caspase 3/7 activity assay
HL-60 cells (2×104) were collected and
cultured on 96-well plates 48 h after transfection. The activity of
caspase 3/7 in transfected cells was measured using the
Apo-ONE® Homogeneous Caspase-3/7 assay kit (Promega
Corporation, Madison, WI, USA). The data were calculated using a
microplate reader at a wavelength of 499 nm.
Protein isolation and western blot
analysis
Cells were washed twice with PBS and proteins were
isolated using 1% Triton X-100 reagent (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) on ice. The lysate was centrifuged at 13,000 ×
g and 4°C for 15 min. The supernatant was obtained and the protein
content was detected using a bicinchoninic acid assay kit (EMD
Millipore, Billerica, MA, USA). Proteins were separated by vertical
electrophoresis and transferred onto polyvinylidene fluoride
membranes (EMD Millipore). Anti-BMI-1 (cat. no. sc-10745; dilution,
1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
-β-actin (cat. no. sc-4778; dilution, 1:5,000; Santa Cruz
Biotechnology, Inc.) antibodies were used to detect the expression
of each protein. Secondary horseradish peroxidase (HRP)-conjugated
goat anti-rabbit or anti-mouse antibodies (cat. no. ABIN361237,
dilution, 1:5,000; Abgent, Inc., San Diego, CA, USA) were used and
bands were developed using Immobilon Western Chemiluminescent HRP
Substrate (WBKLS0500, EMD Millipore, Billerica, MA, USA).
Statistical analysis
Experiments were repeated in triplicate, and data
are presented as the mean ± standard deviation. SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA) was used to calculate
all statistical results. Pearson's correlation analysis was used to
examine the correlation between miR-218 and BMI-1 mRNA expression
levels. Differences in two groups were determined by the two-tailed
Student's t-test. A one-way analysis of variance was used to
analyze the difference for the data from the CCK-8 assays.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-218 is overexpressed in APL marrow
tissues
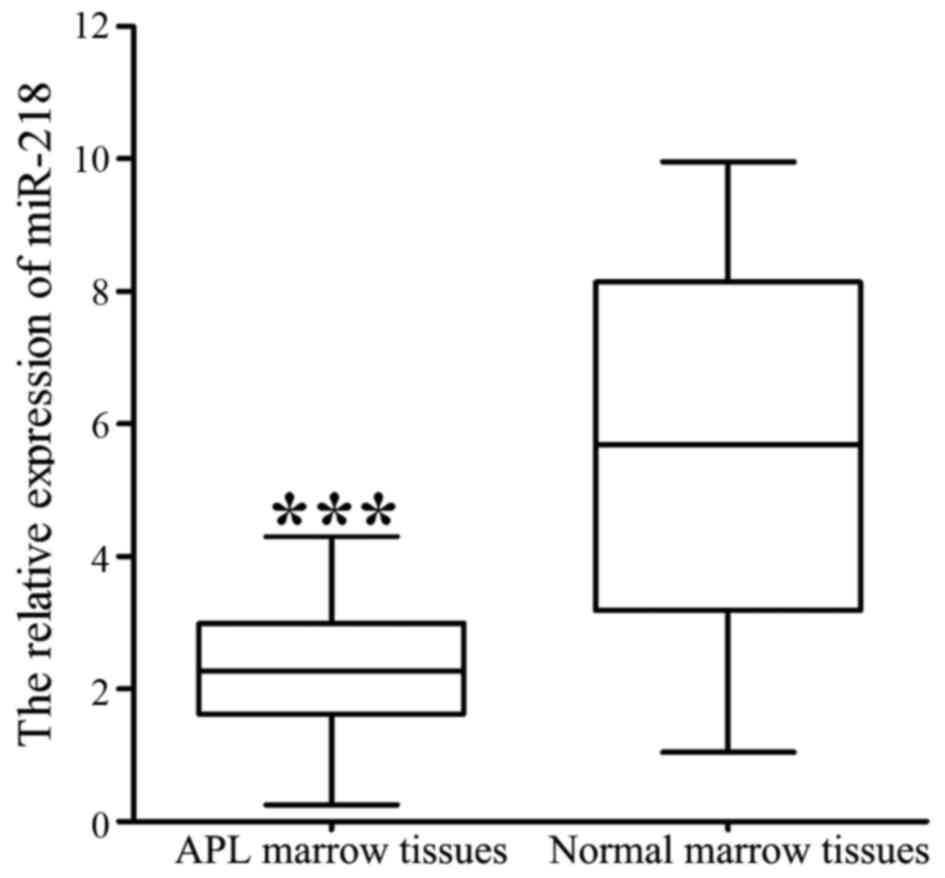
RT-qPCR analysis demonstrated that the expression of
miR-218 was significantly decreased in 40 APL marrow tissues
compared with 20 normal marrow tissues (2.327±0.063 vs.
5.734±0.121; P<0.001; Fig. 1). The
association of miR-218 expression with clinical features is
summarized in Table I. Lower levels
of miR-218 were associated with a higher WBC count (P=0.004) and
bone marrow (BM) promyelocyte percentage (P=0.027), and lower
hemoglobin level (P=0.007) and blood platelet count (P=0.002).
 | Table I.miR-218 expression in APL marrow
tissues from patients with APL (n=40), compared according to
different clinicopathological characteristics. |
Table I.
miR-218 expression in APL marrow
tissues from patients with APL (n=40), compared according to
different clinicopathological characteristics.
| Clinicopathological
characteristic | Classification | Relative miR-218
level | t | P-value |
|---|
| Sex | Male | 2.379±0.201 | 0.791 | 0.533 |
|
| Female | 2.181±0.192 |
|
|
| Age, years | <60 | 2.403±0.191 | 0.905 | 0.341 |
|
| ≥60 | 2.191±0.207 |
|
|
| WBC |
<10×109 | 3.283±0.111 | 3.017 | 0.004a |
|
|
≥10×109 | 1.105±0.097 |
|
|
| HGB, g/l | <80 | 1.091±0.241 | 2.826 | 0.007a |
|
| ≥80 | 2.973±0.182 |
|
|
| PLT |
<50×109 | 1.313±0.132 | 3.341 | 0.002a |
|
|
≥50×109 | 2.589±0.107 |
|
|
| Promyelocytes in BM,
% | <50 | 2.813±0.121 | 2.382 | 0.027a |
|
| ≥50 | 1.321±0.237 |
|
|
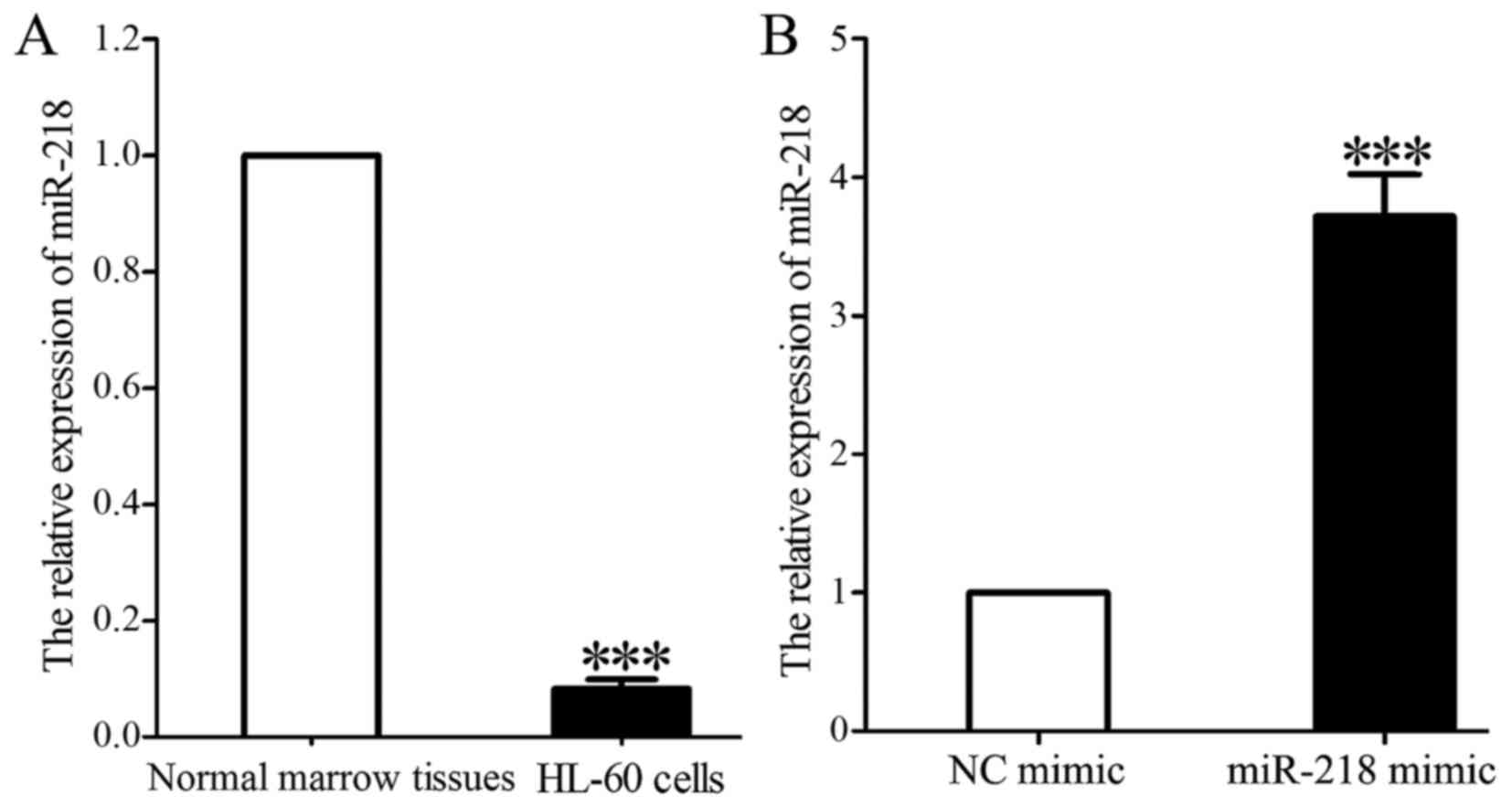
miR-218 mimic upregulates miR-218
expression in HL-60 cells
miR-218 expression in HL-60 cells was significantly
decreased compared with that in normal marrow tissues (0.118±0.021
vs. 1.000; P<0.001; Fig. 2A).
miR-218 expression was significantly increased in HL-60 cells 48 h
following transfection with miR-218 mimic compared with that in
cells transfected with control mimic (3.731±0.346 vs. 1.000;
P<0.001; Fig. 2B).
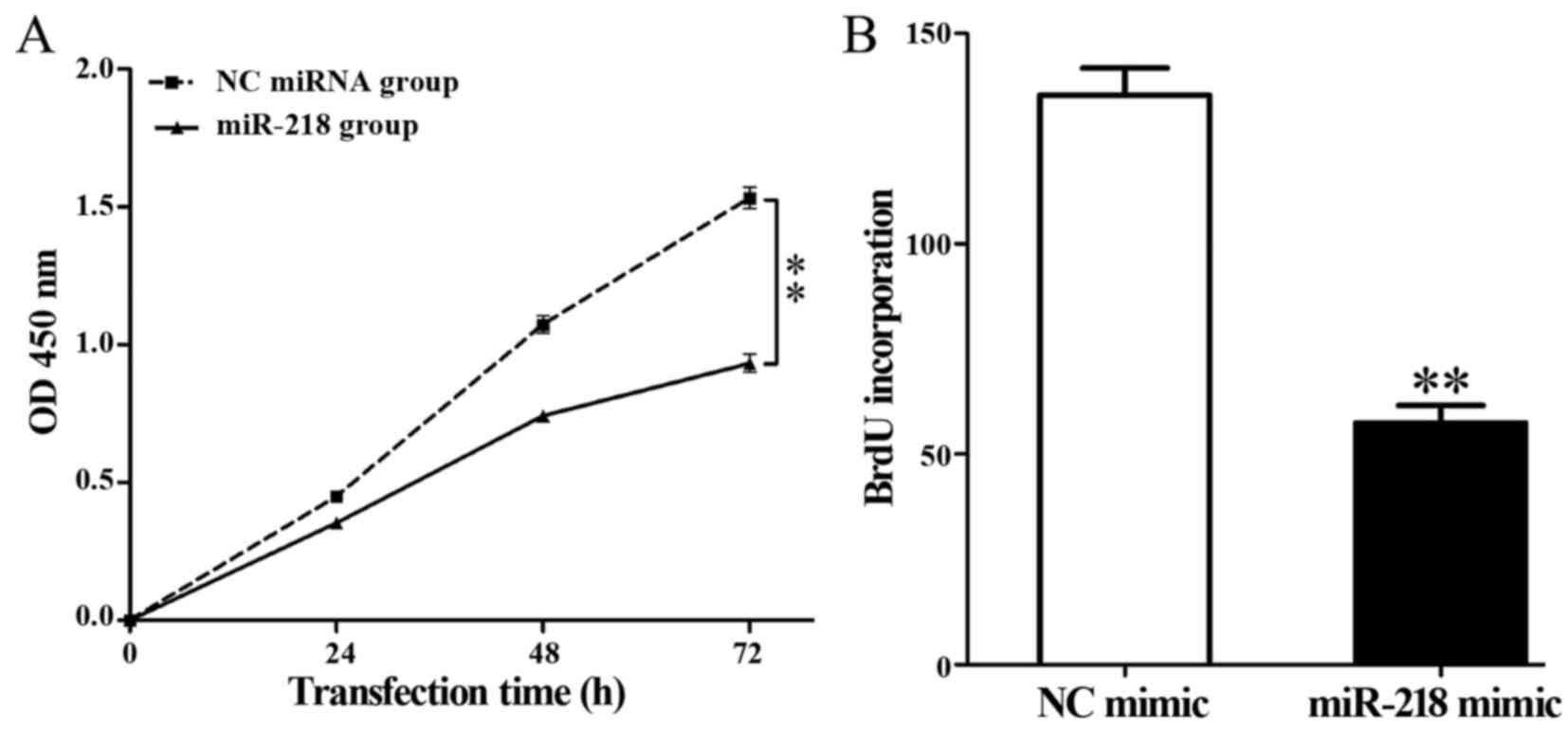
Overexpression of miR-218 inhibits
viability and proliferation of HL-60 cells
Overexpression of miR-218 significantly decreased
the viability of HL-60 cells compared with the control group
(P<0.01; Fig. 3A). In addition, a
BrdU incubation assay demonstrated that miR-218 overexpression
significantly repressed DNA synthesis in HL-60 cells compared with
the control group (57.39±4.20 vs. 135.37±6.43; P=0.002; Fig. 3B).
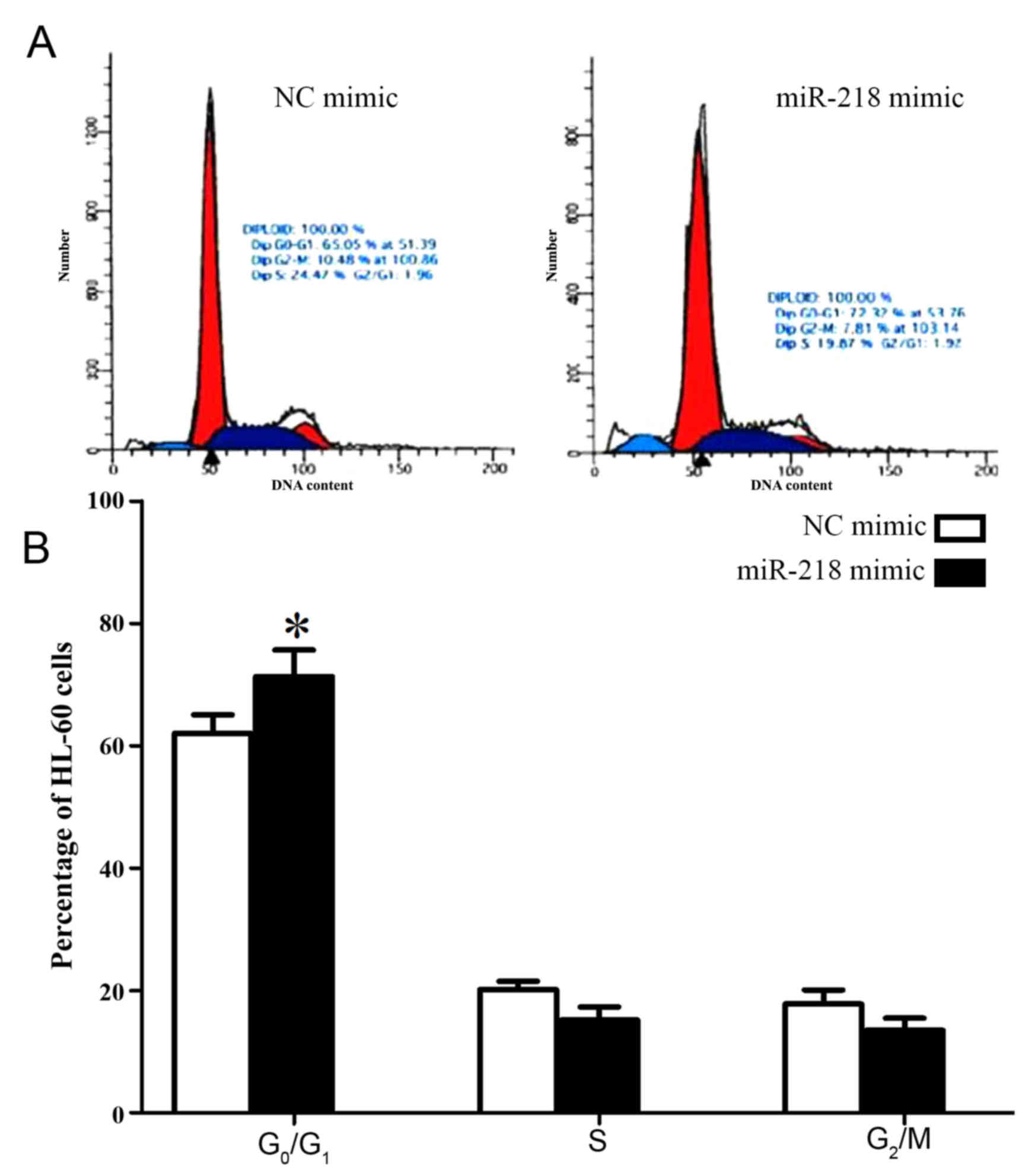
Overexpression of miR-218 arrests
cells in G0/G1 phase
In order to investigate the molecular mechanisms
underlying the effect of miR-218 on cell proliferation, the cell
cycle of HL-60 cells was analyzed following transfection with
miR-218 mimic. Significantly increased numbers of HL-60 cells
transfected with miR-218 mimic were arrested in
G0/G1 phase compared with cells transfected
with control mimic (71.32±4.31 vs. 62.05±3.02, P=0.034; Fig. 4; Table
II).
 | Table II.Proportion of HL-60 cells in each
phase of the cell cycle following transfection with control and
miR-218 mimic. |
Table II.
Proportion of HL-60 cells in each
phase of the cell cycle following transfection with control and
miR-218 mimic.
|
| Cell cycle |
|---|
|
|
|
|---|
| Group |
G0/G1 phase (% of
cells) | S phase (% of
cells) | G2/M
phase (% of cells) |
|---|
| miR-218 |
71.32±4.31a | 15.18±2.15 | 13.50±1.98 |
| NC | 62.05±3.02 | 19.17±1.33 | 18.78±2.31 |
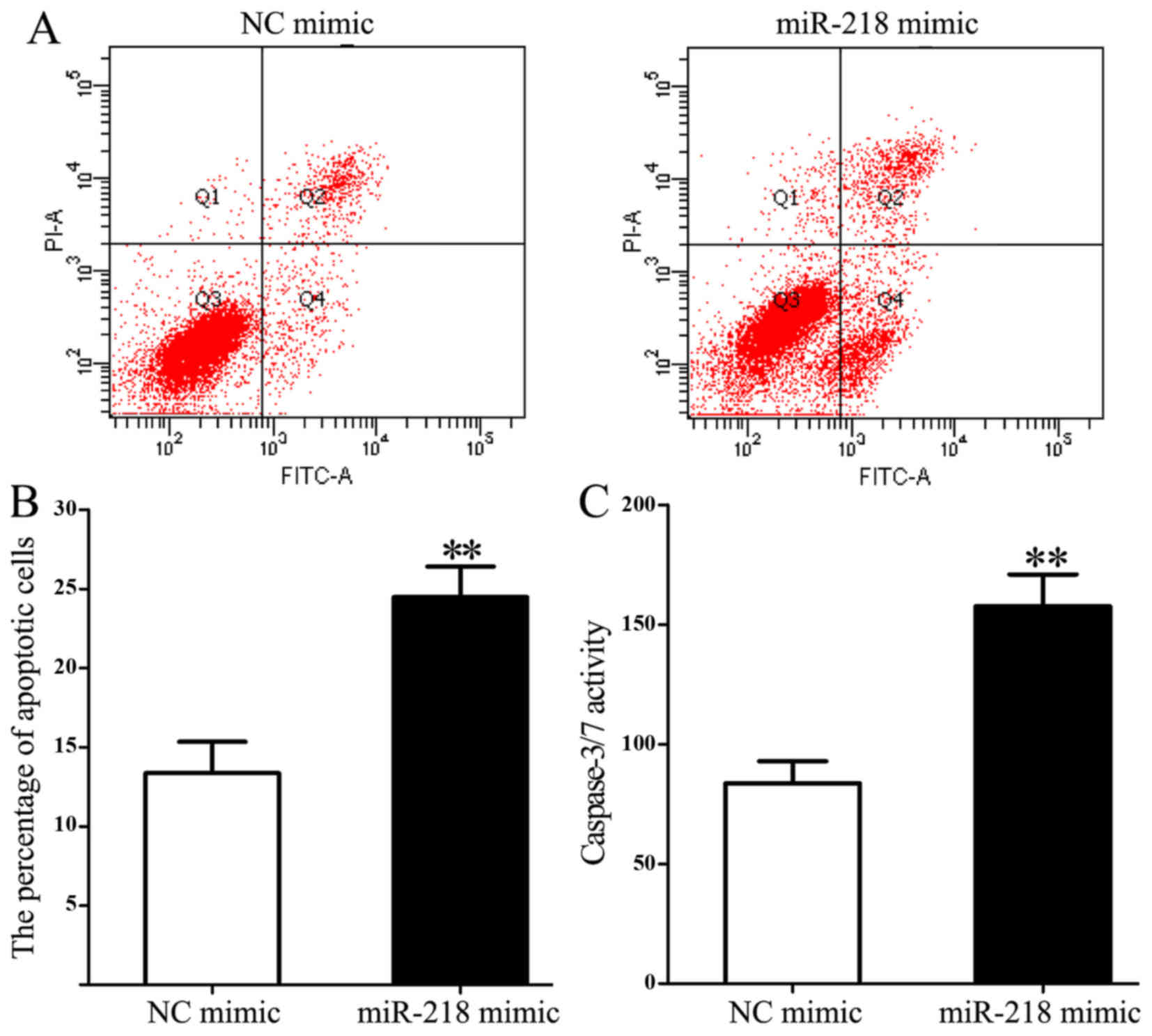
Overexpression of miR-218 increases
apoptosis and caspase 3/7 activity in HL-60 cells
Flow cytometry was used to measure the influence of
miR-218 on cell apoptosis. miR-218 overexpression significantly
increased the proportion of apoptotic cells compared with the
control group (24.482±1.943 vs. 13.352±1.981; P=0.003; Fig. 5A and B). In addition, miR-218
overexpression significantly increased caspase 3/7 activity
compared with the control group (157.65±13.28 vs. 83.71±9.24;
P=0.005; Fig. 5C).
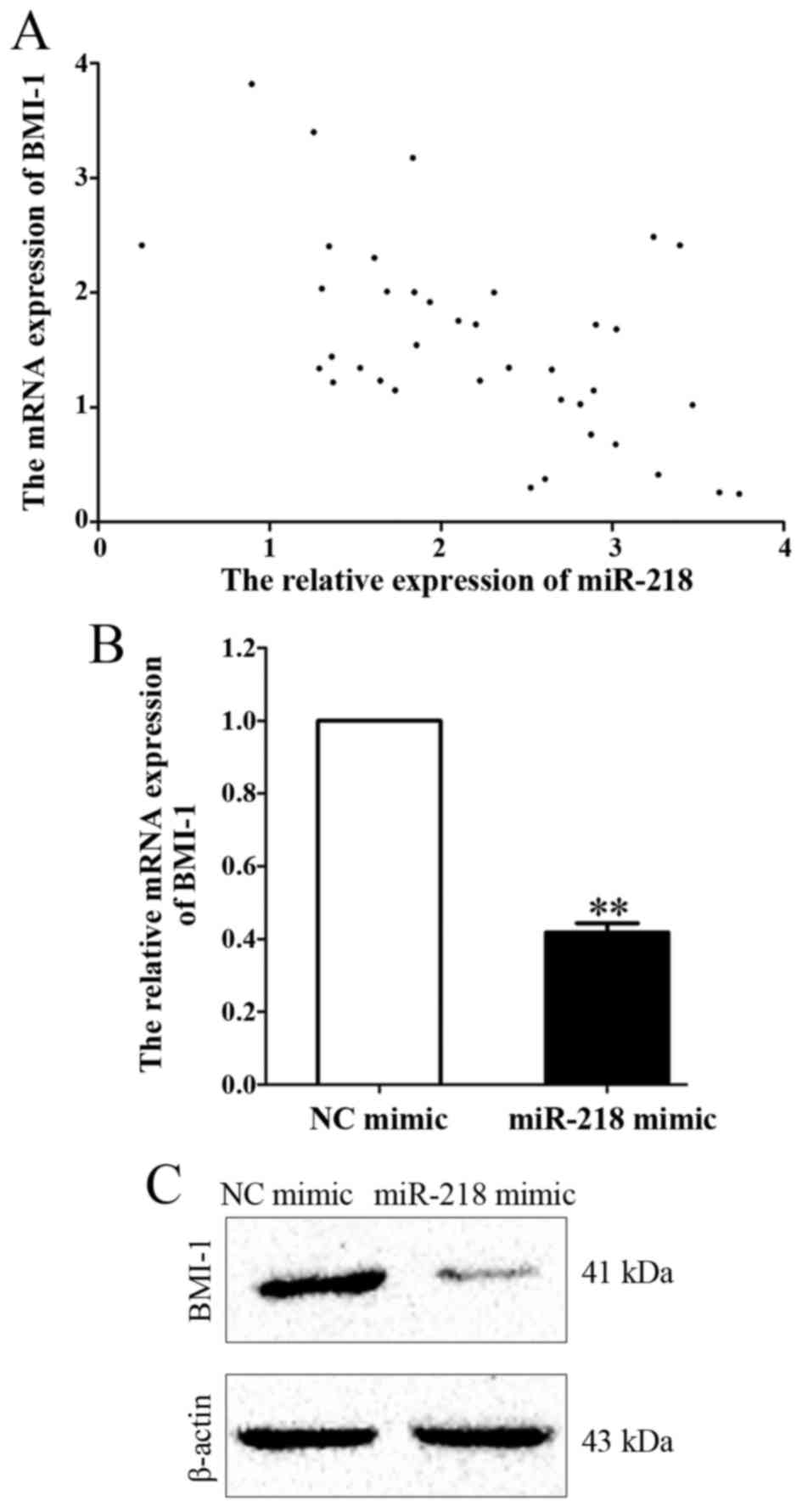
BMI-1 is a downstream target of
miR-218 in APL
In order to investigate the molecular mechanisms
underlying the effects of miR-218 on cell viability and apoptosis,
the mRNA expression of BMI-1, a possible downstream target of
miR-218 predicted by bioinformatics analysis (8), was measured in APL marrow tissues. A
Pearson's correlation analysis was used to examine the correlation
between miR-218 and BMI-1 mRNA expression levels. A negative
correlation was revealed between miR-218 and BMI-1 expression
(r=−0.546; P<0.001; Fig.
6A). In addition, mRNA and protein levels of BMI-1 were
downregulated following miR-218 overexpression compared with the
control group (P=0.007, Fig. 6B and
C).
Discussion
APL is a subtype of AML, and, following the
identification of the promyelocytic leukemia (PML)-retinoic acid
receptor (RAR)α fusion gene and the clinical applications of ATRA
and ATO, APL became the first type of human cancer that was able to
be treated with its tumor-specific antigen (15). However, the use of ATRA was reported
to cause hyperleucocytosis and certain lethal complications,
including retinoic acid syndrome (16). Therefore, further studies are required
to identify novel and safe therapeutic molecules for the treatment
of APL.
Previous studies have demonstrated that microRNAs
serve tumor suppressor and tumor promoter roles in different types
of cancer cell, and their abnormal expression is associated with
tumor growth, metastasis and recurrence (17). Sharifi et al (18) reported that miR-92a suppressed
proliferation and induced apoptosis of HL-60 cells by
downregulating tumor protein 63 expression. In addition, miR-21 has
been demonstrated as an oncogene in APL; Li et al (19) demonstrated that downregulation of
miR-21 using antisense oligonucleotides enhanced HL-60 cell
apoptosis induced by cytarabine (19). In the present study, the expression of
miR-218 in APL marrow tissues was significantly decreased compared
with normal marrow tissues and was identified to be associated with
certain adverse prognostic factors, including high WBC count and
marrow promyelocyte index, and low HGB and PLT counts. Therefore
differential expression of miR-218 in APL marrow tissues may serve
an important role in APL progression.
In a number of other types of cancer, miR-218 has
been suggested as a prognostic evaluation biomarker; in glioma, low
expression of miR-218 was associated with a higher World Health
Organization grade and lower Karnofsky scores (20). Furthermore, overexpression of miR-218
in human osteosarcoma Saos-2 cells repressed cell migration and
invasion by targeting T-lymphoma invasion and metastasis-inducing
protein 1, matrix metalloproteinase (MMP) 2 and MMP9 (21). In the present study, miR-218
overexpression significantly increased apoptosis and caspase 3/7
activity. Furthermore, CCK-8 and BrdU incubation assays
demonstrated that miR-218 overexpression inhibits cell viability
and proliferation compared with scrambled microRNA transfection.
Cell cycle analysis demonstrated that the majority of cells were
arrested in the G0/G1 phase; a result that
was in agreement with the result from the BrdU assay that miR-218
overexpression impairs DNA synthesis.
BMI-1 was the first functionally identified Polycomb
group gene family member, and serves roles in cell cycle
regulation, cell immortalization and cell senescence (22). BMI-1 is involved in the development
and progression of carcinomas and is a potent target for cancer
therapy (23). In addition, BMI-1
represses the cyclin-dependent kinase inhibitor 2A locus, which
encodes p16INK4A and p19ARF (24). BMI-1 may promote cell cycle
progression and enhance cell proliferation by downregulating
p16INK4A and p19ARF expression. The results
of the present study demonstrated a negative association between
miR-218 and BMI-1 mRNA expression. Overexpression of miR-218 in
HL-60 cells significantly repressed BMI-1 mRNA and protein
expression. Therefore the growth arrest effects of miR-218 may be
achieved by suppressing BMI-1 expression.
The results of the present study indicate that
expression of miR-218 is downregulated in patients with APL. To the
best of our knowledge, the present study is the first to
demonstrate that miR-218 is associated with clinical features of
APL and that miR-218 functions as a tumor suppressor by targeting
BMI-1. This suggests that miR-218 is a potential marker for risk
stratification in the treatment of APL.
References
|
1
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imani-Saber Z and Ghafouri-Fard S:
Promyelocytic leukemia gene functions and roles in tumorigenesis.
Asian Pac J Cancer Prev. 15:8021–8028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganzel C and Douer D: Extramedullary
disease in APL: A real phenomenon to contend with or not? Best
Pract Res Clin Haematol. 27:63–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandhu S and Mulligan SP: Ofatumumab and
its role as immunotherapy in chronic lymphocytic leukemia.
Haematologica. 100:411–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen SJ: A potential target of Tanshinone
IIA for acute promyelocytic leukemia revealed by inverse docking
and drug repurposing. Asian Pac J Cancer Prev. 15:4301–4305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y,
Pan Z, Li T, Hu M, Cui H, et al: MicroRNAs contribute to
promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol
Biochem. 32:1818–1829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharifi M, Salehi R, Gheisari Y and Kazemi
M: Inhibition of microRNA miR-92a induces apoptosis and inhibits
cell proliferation in human acute promyelocytic leukemia through
modulation of p63 expression. Mol Biol Rep. 41:2799–2808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu K, Li C, Zheng X, Yang W, Yao Y and Liu
Q: Prognostic significance of miR-218 in human hepatocellular
carcinoma and its role in cell growth. Oncol Rep. 32:1571–1577.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang F, Ren X and Zhang X: Role of
microRNA-150 in solid tumors. Oncol Lett. 10:11–16. 2015.PubMed/NCBI
|
|
10
|
Zhao J, Lu Q, Zhu J, Fu J and Chen YX:
Prognostic value of miR-96 in patients with acute myeloid leukemia.
Diagn Pathol. 9:762014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Luo XQ, Feng DD, Zhang XJ, Wu J,
Zheng YS, Chen X, Xu L and Chen YQ: Upregulation of microRNA-125b
contributes to leukemogenesis and increases drug resistance in
pediatric acute promyelocytic leukemia. Mol Cancer. 10:1082011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong Y, Zou J, Su S, Huang H, Deng Y, Wang
B and Li W: MicroRNA-218 and microRNA-520a inhibit cell
proliferation by downregulating E2F2 in hepatocellular carcinoma.
Mol Med Rep. 12:1016–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YL, Jiang SX, Yang YM, Xu H, Liu JL
and Wang XS: USP22 acts as an oncogene by the activation of
BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem
Biophys. 62:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miftakhova R, Sandberg T, Hedblom A,
Nevzorova T, Persson JL and Bredberg A: DNA methylation in
ATRA-treated leukemia cell lines lacking a PML-RAR chromosome
translocation. Anticancer Res. 32:4715–4722. 2012.PubMed/NCBI
|
|
16
|
Liu WJ, Jiang NJ, Guo QL and Xu Q: ATRA
and As2O3 regulate differentiation of human
hematopoietic stem cells into granulocyte progenitor via alteration
of HoxB8 expression. Eur Rev Med Pharmacol Sci. 19:1055–1062.
2015.PubMed/NCBI
|
|
17
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharifi M, Salehi R, Gheisari Y and Kazemi
M: Inhibition of microRNA miR-92a induces apoptosis and inhibits
cell proliferation in human acute promyelocytic leukemia through
modulation of p63 expression. Mol Biol Rep. 41:2799–2808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Zhu X, Gu J, Hu H, Dong D, Yao J,
Lin C and Fei J: Anti-miR-21 oligonucleotide enhances
chemosensitivity of leukemic HL60 cells to arabinosylcytosine by
inducing apoptosis. Hematology. 15:215–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng MW, Wang LL and Hu GY: Expression of
microRNA-218 and its clinicopathological and prognostic
significance in human glioma cases. Asian Pac J Cancer Prev.
16:1839–1843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Tian T, Sun W, Liu C and Fang X:
Bmi-1 overexpression as an efficient prognostic marker in patients
with nonsmall cell lung cancer. Medicine (Baltimore). 96:e73462017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin X, Ojo D, Wei F, Wong N, Gu Y and Tang
D: A novel aspect of tumorigenesis-BMI1 functions in regulating DNA
damage response. Biomolecules. 5:3396–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng S, Luo M, Sun H, Yu X, Shen M, Zhang
Q, Zhou R, Ju X, Tao W, Liu D, et al: Identification and
characterization of Bmi-1-responding element within the human p16
promoter. J Biol Chem. 285:33219–33229. 2010. View Article : Google Scholar : PubMed/NCBI
|