Introduction
Nasopharyngeal carcinoma is a radiotherapy-sensitive
tumor, but with the extension of radiotherapy, the percentage of
hypoxic cells gradually increased (up to 10 to 50%), and the
increased percentage of hypoxic cells can lead to the failure of
solid tumor chemotherapy, the recurrence and metastasis of tumors
(1). Tumor cell hypoxia on the one
hand can reduce the production of oxygen free radicals, resulting
in reduction in radiotherapy-induced DNA breakage, on the other
hand can increase the release of hypoxia-inducible factor-1
(HIF-1), increase the expression of vascular endothelial growth
factor (VEGF), and inhibit tumor cell apoptosis (2). Therefore, the use of hypoxic cytotoxic
drugs combined with radiotherapy and chemotherapy can improve the
efficacy of cancer treatment. Toll-like receptor (TLR), as an
important component of innate immunity, is related to the
pathogenesis of nasopharyngeal carcinoma, breast cancer, pancreatic
cancer, basal cell carcinoma and other malignant tumors (3,4).
TLR-9/myeloid differentiation factor 88 (MyD88) signaling pathway
can activate inflammatory and immune responses (5), mediate cell proliferation and apoptosis
regulated by nuclear transcription factor (NF-κB) (6), stimulate the secretion of matrix
metalloproteinases and integrins, and induce tumor cell invasion
and metastasis (7). Based on this, we
investigated the correlation between the expression of Toll-like
receptor-9 (TLR-9) and cell proliferation and apoptosis in hypoxic
nasopharyngeal carcinoma cells.
Materials and methods
Experimental materials
Human nasopharyngeal carcinoma cell line HNE-1 (EBV
positive) and CNE-1 (EBV negative) were purchased from Sangon
(Shanghai, China). Occurrence of nasopharyngeal carcinoma is
closely related to EB virus infection, so both EBV-positive and
-negative cell lines were used. Cells were cultured with RPMI-1640
cell culture medium (Beyotime Biotechnology, Jiangsu, China)
containing 10% fetal bovine serum, 100 U/ml penicillin and 40 U/ml
gentamicin in an incubator (37°C, 5% CO2). Cell recovery
and subculture were performed using the same method. Cells were
collected at logarithmic growth phase for following
experiments.
Experimental methods
Cells were divided into normal control group,
hypoxia group and hyperoxia group. Hypoxic conditions were 5%
CO2 and 0.01% partial pressure of oxygen, hyperoxia
conditions were 5% CO2 and 10% partial pressure of
oxygen. After culture for 6, 12 and 24 h, cells in hypoxia group
and hyperoxia group were cultured in normoxic condition for another
4 h. RT-PCR and western blot analysis were used to detect the
expression of TLR-9 mRNA and protein at 6, 12 and 24 h after the
beginning of cell culture.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to detect the cell proliferation rate and flow
cytometry was used to detect the cell apoptosis rate.
Detection method
RT-PCR
Total RNA was extracted by 1 ml of TRIzol reagent
(Beyotime Biotechnology, Jiangsu, China). The purity and
concentration of RNA samples were checked by 1.5% agarose gel
electrophoresis and UV spectrophotometer (HyClone, Logan, UT, USA).
cDNA was synthesized using 2 µg total RNA and reverse transcription
kit (Sigma, St. Louis, MO, USA). Primers were designed by Sangon
(Shanghai, China) accroding to the gene sequences downloaded from
GenBank. The following primers were used: TLR-9 forward,
5′-GGTTTCATCCAGGATCGAGCAGG-3′ and reverse,
5′-ACAAAGATGGTCACGGTCTGCC-3′; endogenous control GAPDH forward,
5′-CGCGAGAAGATGACCCAGAT-3′ and reverse, 5′-GCACTGTGTTGGCGTACAGG-3′.
The PCR reaction system was: 2 µl of cDNA + 3 µl of each primer +
0.5 µl of Taq polymerase (Sigma) + 1 µl of dNTPs + 2 µl of 10X
buffer, water was added to make a final volume of 20 µl. Reaction
conditions were 95°C for 5 min, followed by 30 cycles of 95°C for
30 sec, 58°C for 30 sec and 72°C for 60 sec, and 72°C for 10 min.
PCR product (6 ml) was subjected to 2% agarose gel electrophoresis,
and results were checked and pictures were taken using a gel
imaging system. The grey scale values were analyzed.
Western blot analysis
RIPA lysate (Beyotime Biotechnology) was added to
extract total protein, and BCA quantitative kit (Beijing Zhongshan
Golden Bridge Biology Co., Ltd., Beijing, China) was used to
quantify protein, and β-actin was used as endogenous control for
normalization. Thirty micrograms of protein from each sample was
subjected to 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) electrophoresis, followed by
transmembrane to PVDF membrane. Membrane was then incubated with
mouse anti-human TLR-9 monoclonal primary antibody (dilution,
1:500; cat. no. 12-9099-82; Thermo Fisher Scientific Inc., MA, USA)
or mouse anti-human β-actin primary antibody (dilution, 1:2,000;
Sigma-Aldrich, Inc., MA USA) overnight at 4°C. After washing,
membranes were incubated with goat anti-mouse polyclonal secondary
antibody (1:500; Sigma-Aldrich, Inc.) at room temperature for 4 h.
After washing with PBS, color development was performed with ECL.
Results were scanned and recorded. Lab Works 4.5 gel imaging
software (Invitrogen, Carlsbad, CA, USA) was used for
semi-quantitative analysis.
MTT
Cells were resuspended to make a concentration of
2×106/ml and transferred to a 96-well plate with 100 µl
for each well. After incubation for 6, 12 and 24 h, 10 µl of 5
mg/ml MTT (Bio-Rad, Hercules, CA, USA) was added to each well,
followed by incubation for another 4 h. Culture medium was
discarded and 150 µl of dimethyl sulfoxide (DMSO; Bio-Rad) was
added to each well and shaken for 10 min. Optical density (OD) at
A490 nm was measured by a microplate reader (Bio-Rad). OD values
were measured 3 times and the average value was calculated. Cell
proliferation rate = sample/control OD value ×100%.
Flow cytometry
Cells were centrifuged at 1,500 × g for 10 min to
remove the supernatant. After washing with PBS, cells were
collected. Cells were mixed with 100 µl of binding buffer and 10 µl
of FITC-labeled Annexin V (20 µg/ml) (both from Beyotime
Biotechnology), followed by incubation at room temperature for 30
min. After that, 5 µl of propidium iodide (PI, 50 µg/ml; Beyotime
Biotechnology) was added and incubated at room temperature for 5
min. After that, 400 µl of binding buffer was added and detection
was performed within 1 h. Cell solution without Annexin V-FITC and
PI was used as negative control. FACSCalibur flow cytometry (BD
Biosciences, Lake Franklin, NJ, USA) was used here.
Statistical analysis. Statistical analysis was
performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Measurement data were expressed as mean ± standard deviation,
comparisons among multiple groups were performed using single
factor ANOVA analysis, comparisons between two groups were
performed using LSD-t method, and comparisons between different
time-points were performed using analysis of variance of repeated
measure data. P<0.05 was considered to be statistically
significant.
Results
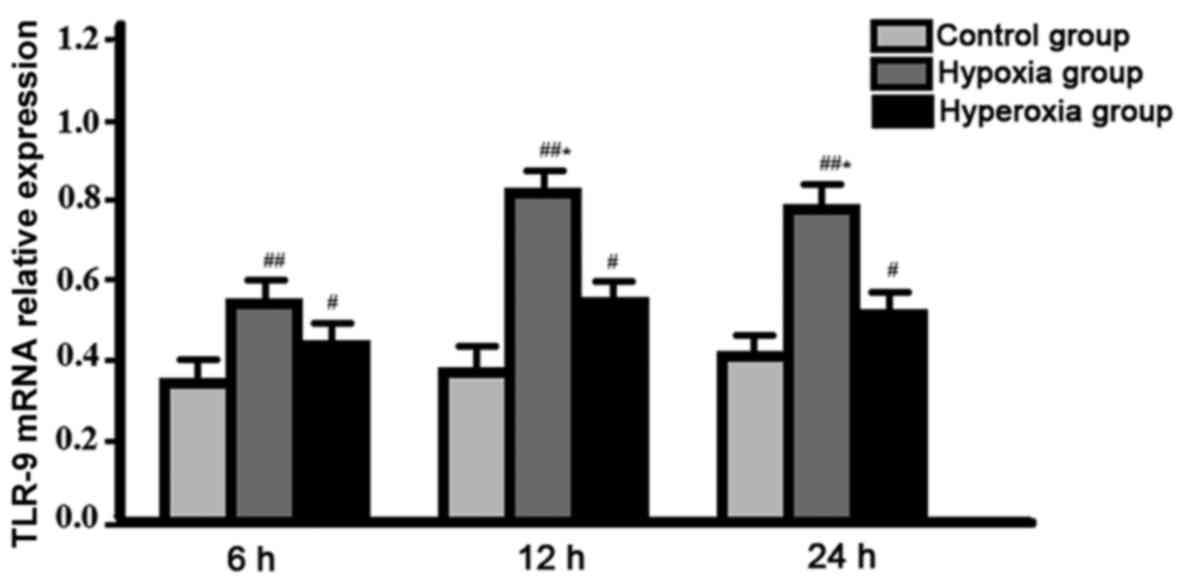
Comparison of expression level of TLR-9 mRNA between
groups. Expression level of TLR-9 mRNA in hypoxia group reached the
peak at 12 h after the beginning of cell culture, and was
significantly higher than that of hyperoxia group at all
time-points, control group was the lowest, difference between
groups were all statistically significant (P<0.05). No
significant changes in expression level of TLR-9 mRNA were found in
control group and hyperoxia group between different time-points
(P>0.05) (Fig. 1).
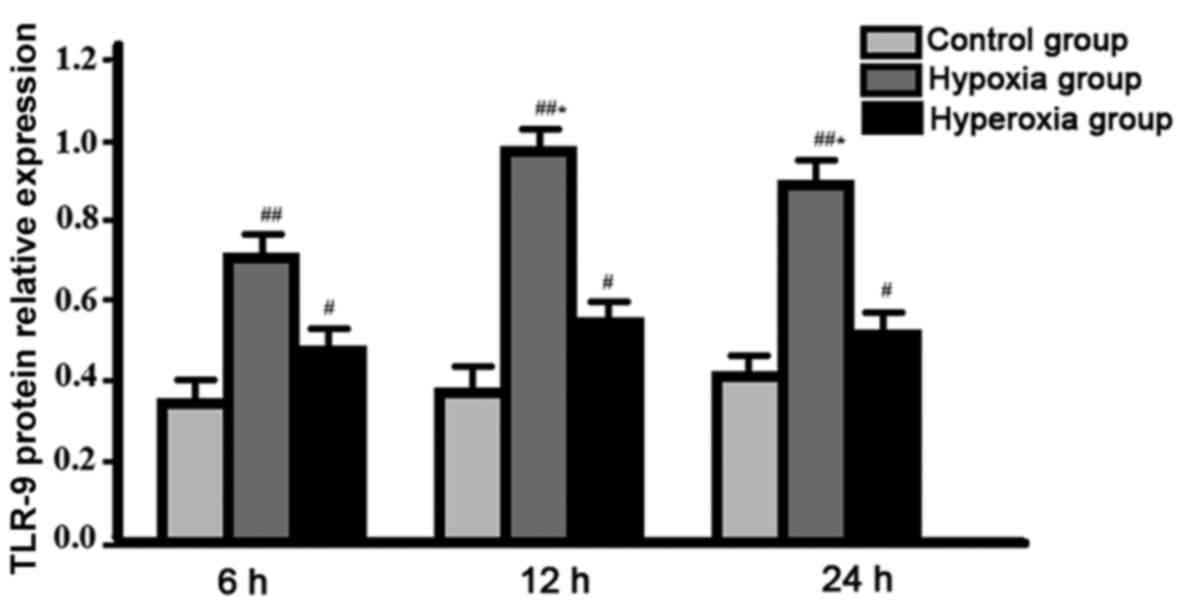
Comparison of expression level of TLR-9 protein
between groups. Expression level of TLR-9 protein in hypoxia group
reached the peak at 12 h after the beginning of cell culture, and
was significantly higher than that of hyperoxia group at all
time-points, control group was the lowest, difference between
groups were all statistically significant (P<0.05). No
significant changes in expression level of TLR-9 protein were found
in control group and hyperoxia group between different time-points
(P>0.05) (Fig. 2).
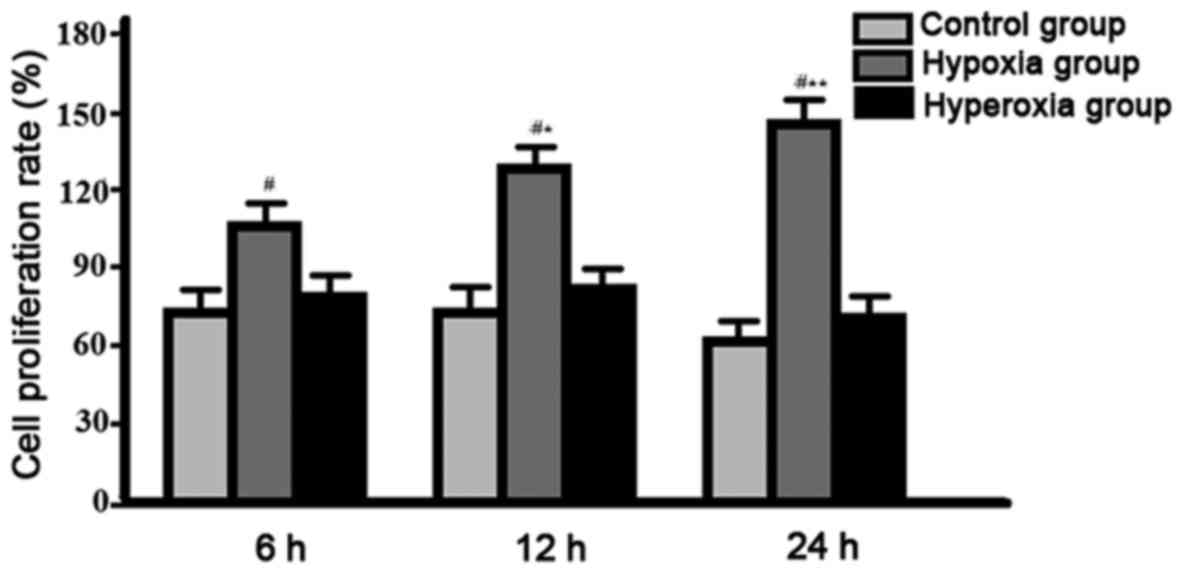
Comparison of cell proliferation rate among groups.
Compared with other two groups, cell proliferation rate was
gradually decreased in hypoxia group, significant differences were
found between hypoxia group, and control group and hyperoxia group
(P<0.05), no significant differences were found between control
group and hyperoxia group (P>0.05) (Fig. 3).
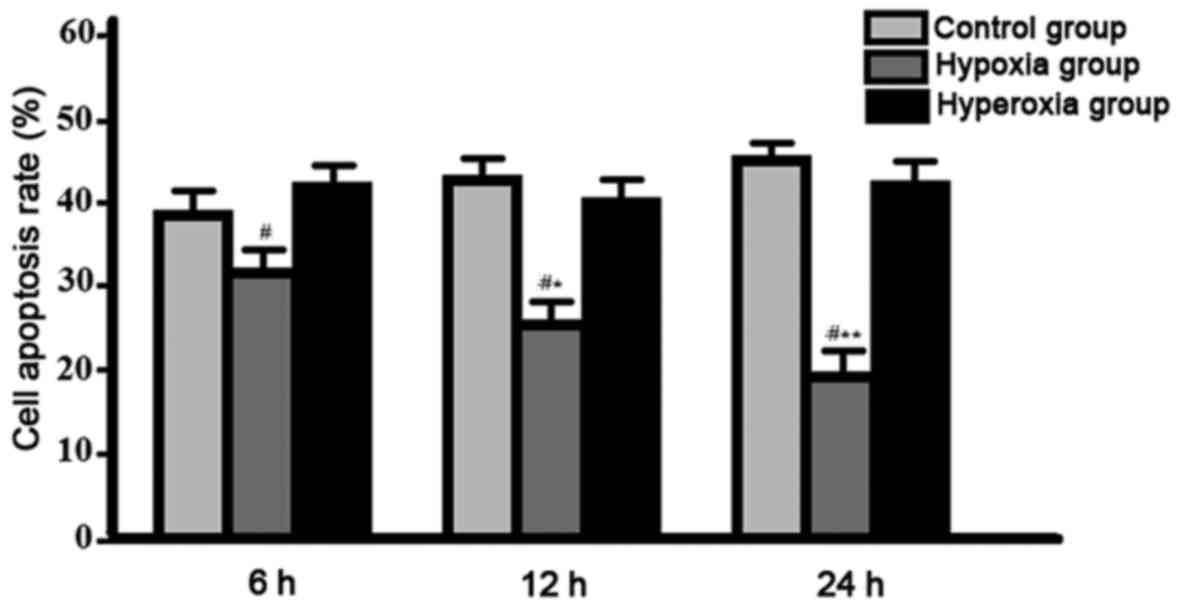
Comparison of cell apoptotic rate among groups.
Compared with other two groups, cell apoptotic rate was gradually
decreased in hypoxia group, significant differences were found
between hypoxia group, and control group and hyperoxia group
(P<0.05), no significant differences were found between control
group and hyperoxia group (P>0.05) (Fig. 4).
Discussion
Studies have shown that solid tumors can overexpress
HIF-1α in hypoxic microenvironment and regulate the expression of a
series of genes that are compatible with hypoxia to maintain
metabolic stability and promote tumor growth and metastasis. HIF-1α
can exert anti-apoptotic and pro-apoptotic effects simultaneously,
HIF-1α can increase the anaerobic metabolism and glucose
extraction, and downregulate the expression of apoptotic genes to
play an anti-apoptotic role (8); at
the same time, HIF-1α can increase the level of p53 protein by
inhibiting its degradation to play a pro-apoptotic role (9). Therefore, how to regulate HIF-1α to
increase tumor cell apoptosis is an important task for studies on
treatment of tumors.
This study showed that expression levels of TLR-9
mRNA and protein in hypoxia group reached the peak at 12 h after
the beginning of cell culture, and were significantly higher than
those of hyperoxia group at all time-points, expression levels of
TLR-9 mRNA and protein of control group were the lowest,
differences between groups were all statistically significant
(P<0.05). No significant changes in expression levels of TLR-9
mRNA and protein were found in control group and hyperoxia group
between different time-points; compared with other two groups, cell
proliferation rate was gradually decreased and apoptotic rate was
gradually decreased in hypoxia group, significant differences were
found between hypoxia group, and control group and hyperoxia group,
but no significant differences were found between control group and
hyperoxia group, indicating that hypoxic nasopharyngeal carcinoma
cells can highly express TLR-9 to regulate cell proliferation and
apoptosis, which may be an important mechanism of tumorigenesis and
a potential target for intervention therapy.
Clinical study of nasopharyngeal carcinoma showed
that (10,11), expression level of TLR-9 was
significantly higher in tumor cells than in adjacent normal tissue
and the tissue from healthy volunteers, and was closely related to
tumor clinical stage, pathological grade and the efficacy of
radiotherapy and chemotherapy. TLR-9-1486T/CCC genotype can reduce
the sensitivity of nasopharyngeal carcinoma patients to
radiotherapy, promote tumor proliferation, migration and
recurrence, and upregulate the expression of VEGF and other
cytokines (12,13). TLR-9/MyD88 signaling pathway plays an
important role in hypoxia and inflammatory responses, the
expression of HIF-1α and VEGF (14),
differentiation and activation of immune cells (15), and release of inflammatory factors
such as IL-6 and TNF-α (16). In
addition, TLR-9/MyD88 signaling pathway can mediate the
transcription of NF-κB, whereas upstream promoter region of NF-κB
contains HIF-1α binding site, which can affect the transcription
and expression of HIF-1α, so NF-κB can participate in
HIF-1α-regulated tumor cell proliferation and apoptosis and other
biological activities (8,17).
The innovation of this study is that the hypoxic
environment can induce high expression level of TLR-9 in
nasopharyngeal carcinoma cells, which may affect the cell
proliferation and apoptosis, and this may be an important mechanism
of tumorigenesis and potential target of intervention therapy.
Further studies may focus on the effects of TLR-9 gene invention on
tumor development, so as to provide reference for clinical
treatment.
References
|
1
|
Yip C, Cook GJ, Wee J, Fong KW, Tan T and
Goh V: Clinical significance of hypoxia in nasopharyngeal carcinoma
with a focus on existing and novel hypoxia molecular imaging. Chin
Clin Oncol. 5:242016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu T and Xiao D: Oleuropein enhances
radiation sensitivity of nasopharyngeal carcinoma by downregulating
PDRG1 through HIF1α-repressed microRNA-519d. J Exp Clin Cancer Res.
36:32017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ou C, Sun Z, Zhang H, Xiong W, Ma J, Zhou
M, Lu J, Zeng Z, Bo X, Chen P, et al: SPLUNC1 reduces the
inflammatory response of nasopharyngeal carcinoma cells infected
with the EB virus by inhibiting the TLR9/NF-κB pathway. Oncol Rep.
33:2779–2788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karki K, Pande D, Negi R, Khanna S, Khanna
RS and Khanna HD: Correlation of serum toll like receptor 9 and
trace elements with lipid peroxidation in the patients of breast
diseases. J Trace Elem Med Biol. 30:11–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng Y, Qin Z, Ye Q, Chen P, Wang Z, Yan
Q, Luo Z, Liu X, Zhou Y, Xiong W, et al: Lactoferrin suppresses the
Epstein-Barr virus-induced inflammatory response by interfering
with pattern recognition of TLR2 and TLR9. Lab Invest.
94:1188–1199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammadi A, Billard C, Faussat AM and Kolb
JP: Stimulation of iNOS expression and apoptosis resistance in
B-cell chronic lymphocytic leukemia (B-CLL) cells through
engagement of Toll-like receptor 7 (TLR-7) and NF-kappaB
activation. Nitric Oxide. 19:138–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan M, Zhang Z, Li S, Yan M, Liu S, Yang
W, Wang L and Zhang C: Activation of Toll-like receptor-9 promotes
cellular migration via up-regulating MMP-2 expression in oral
squamous cell carcinoma. PLoS One. 9:e927482014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu SL, Li YJ, Liao K, Shi L, Zhang N, Liu
S, Hu YY, Li SL and Wang Y: 2-Methoxyestradiol inhibits the
proliferation and migration and reduces the radioresistance of
nasopharyngeal carcinoma CNE-2 stem cells via NF-κB/HIF-1 signaling
pathway inactivation and EMT reversal. Oncol Rep. 37:793–802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung WW, Chu YC, Chen PR, Liao MH and Lee
JW: Positive regulation of HIF-1A expression by EBV oncoprotein
LMP1 in nasopharyngeal carcinoma cells. Cancer Lett. 382:21–31.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Xie G, Li L, Jiang Z, Yue Z and Pan
Z: The effect of TLR4/MyD88/NF-κB signaling pathway on
proliferation and apoptosis in human nasopharyngeal carcinoma 5–8F
cells induced by LPS. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 29:1012–1015. 2015.(In Chinese). PubMed/NCBI
|
|
11
|
Dai Q, Li XP, Chai L, Long HA and Yang ZH:
Polymorphisms of Toll-like receptor 9 are associated with
nasopharyngeal carcinoma susceptibility. Tumour Biol. 35:3247–3253.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bottoni U, Paolino G, Didona D, Corsetti
P, Clerico R, Cantisani C, Richetta AG, Arcidiacono V, Scali E,
Pranteda G, et al: Improvement of survival in patients with
melanoma and non-melanoma skin cancers compared to patients without
double cutaneous malignancies. Eur Rev Med Pharmacol Sci.
19:1640–1644. 2015.PubMed/NCBI
|
|
13
|
Hold GL, Rabkin CS, Gammon MD, Berry SH,
Smith MG, Lissowska J, Risch HA, Chow WH, Mowat NA, Vaughan TL, et
al: CD14-159C/T and TLR9-1237T/C polymorphisms are not associated
with gastric cancer risk in Caucasian populations. Eur J Cancer
Prev. 18:117–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grimmig T, Moench R, Kreckel J, Haack S,
Rueckert F, Rehder R, Tripathi S, Ribas C, Chandraker A, Germer CT,
et al: Toll-like receptor 2, 4, and 9 signaling promotes
autoregulative tumor cell growth and VEGF/PDGF expression in human
pancreatic cancer. Int J Mol Sci. 17:E20602016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan M, Thorn K, Liu S, Li S, Yang W and
Zhang C and Zhang C: The secretion of IL-6 by CpG-ODN-treated
cancer cells promotes T-cell immune responses partly through the
TLR-9/AP-1 pathway in oral squamous cell carcinoma. Int J Oncol.
44:2103–2110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang ZH, Dai Q, Gu YJ, Guo QX and Gong L:
Cytokine and chemokine modification by Toll-like receptor
polymorphisms is associated with nasopharyngeal carcinoma. Cancer
Sci. 103:653–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han S, Xu W, Wang Z, Qi X, Wang Y, Ni Y,
Shen H, Hu Q and Han W: Crosstalk between the HIF-1 and Toll-like
receptor/nuclear factor-κB pathways in the oral squamous cell
carcinoma microenvironment. Oncotarget. 7:37773–37789. 2016.
View Article : Google Scholar : PubMed/NCBI
|