Introduction
Hepatocellular carcinoma (HCC) is one of the leading
causes for cancer-associated mortality, with one of the
fastest-rising morbidity and mortality rates worldwide (1–3). Although
surgical resection, systemic chemotherapy and targeted cancer
therapy are widely used, the response to therapy and prognosis of
patients with HCC remain suboptimal due to the development of drug
resistance and severe adverse side effects (4–8).
Therefore, it is essential to explore and develop novel strategies
for the control and treatment of HCC.
Cancer cells are characterized by uncontrolled
proliferation and the deregulation of apoptotic signaling (9). Cell proliferation is primarily regulated
by cell cycle checkpoints. One of the major cell cycle checkpoints
is the G1/S checkpoint; G1/S progression is regulated by the
pro-proliferative cyclin D1/cyclin-dependent kinase (CDK)4 complex
(10,11). The expression of cyclin D1 and CDK4
are often upregulated in various types of cancer (12–14). Bcl-2
serves a critical role in inhibiting apoptosis and is overexpressed
in numerous types of cancer (15–18). The
ability to inhibit excessive proliferation and induce the apoptosis
of cancer cells is paramount in the development of anticancer
drugs.
MicroRNAs (miRNAs/miRs) are a class of endogenous
short noncoding RNAs that primarily suppress gene expression by
specifically binding to the 3′-untranslated region of target mRNAs
(19–21). A single miRNA can modulate the
expression of hundreds of different targets and may therefore be
implicated in a broad range of physiological and pathological
processes (22,23). It has been demonstrated that miRNAs
may function as oncogenes or tumor suppressors to modulate multiple
oncogenic cellular processes, including cell proliferation,
apoptosis, invasion and metastasis (24–26).
miR-16 is localized at chromosome 13q14.3, and is downregulated in
the majority of patients with chronic lymphocytic leukemia (CLL)
(27) and HCC (28). It has been reported that the
upregulation of miR-16 inhibits cell proliferation, induces cell
cycle arrest and increases the rate of apoptosis by downregulating
the expression of Bcl-2 in CLL, colorectal cancer and HCC (29,30). It
has also been demonstrated that miR-16 may inhibit tumor cell
proliferation by targeting cyclin D1 and CDK4 to induce cell cycle
arrest (31–33).
Traditional Chinese medicine (TCM) has been used in
China for thousands of years and may provide treatment with
multi-target and multi-level intervention against various types of
cancer, with relatively few side effects (34,35). Pien
Tze Huang (PZH), a well-known TCM formula that originated in the
Chinese Ming Dynasty >450 years ago, has been widely used in
China and Southeast Asia as a remedy for various diseases,
including cancer (36). It was
previously demonstrated that PZH may inhibit colon cancer growth
via multiple mechanisms (37–49) and PZH has exhibited promising
therapeutic effects in clinical trials regarding HCC (50,51).
However, the effect of PZH on HCC, including on miR-16 expression
level, has not been evaluated; therefore, the present study aimed
to explore the effect of PZH on the proliferation and apoptosis of
the HCC BEL-7402 cell line.
Materials and methods
Materials and reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin, streptomycin and trypsin-EDTA were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). A Hoechst
staining kit was purchased from the Beyotime Institute of
Biotechnology (Shanghai, China). A BD Pharmingen™ Cell
Cycle kit was obtained from BD Biosciences (San Jose, CA, USA). An
RNAiso Plus for Total RNA kit, an RNAiso for microRNA kit, a
PrimeScript™ RT reagent kit and a SYBR®
PrimeScript™ miRNA RT-PCR kit were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). SYBR® Select
Master Mix was purchased from Thermo Fisher Scientific, Inc.
Antibodies against CDK4 (cat no. 2906S), cyclin D1 (cat no. 2978S),
Bcl-2 (cat no. 15071S) and β-actin (cat no. 4967S), horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G,
(cat no. 7074P2) and HRP-conjugated horse anti-mouse IgG (cat no.
7076S) were obtained from Cell Signaling Technology, Inc. (Beverly,
MA, USA).
Preparation of PZH
PZH was obtained from and authenticated by Zhangzhou
Pien Tze Huang Pharmaceutical Co., Ltd. (Zhangzhou, China; Chinese
Food and Drug Administration approval no. Z35020242). PZH was
prepared by dissolving in PBS to a stock concentration of 20 mg/ml,
which was stored at −20°C. Dissolving the stock solution in
RPMI-1640 to varying concentrations produced the working
concentrations of PZH.
Cell culture
Human HCC BEL-7402 cells were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in RPMI-1640 medium containing 10% (v/v) FBS,
100 U/ml penicillin and 100 µg/ml streptomycin, and maintained in a
humidified incubator at 37°C with 5% CO2.
Evaluation of cell viability by MTT
assay
Cell viability was assessed by an MTT assay.
BEL-7402 cells were seeded in 96-well plates at a density of
8×103 cells/well in 100 µl medium. Cells were incubated
overnight and treated with various concentrations (0, 0.25, 0.5 and
0.75 mg/ml) of PZH for 24, 48 or 72 h. An MTT assay was
subsequently performed by the addition of 100 µl MTT reagent
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and 0.5 mg/ml PBS into each well, followed by incubation for
4 h at 37°C. The resulting purple-blue MTT formazan precipitate was
dissolved in 100 µl DMSO. The optical density (OD) at 570 nm was
measured with an ELISA reader (ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA). Cell viability was determined using the
following formula: Cell viability = (absorbance of the experimental
samples/absorbance of the control samples) ×100%.
Observation of cell confluence
BEL-7402 cells were seeded into 6-well plates at a
density of 2.5×105 cells/well and treated with 0, 0.25,
0.5 or 0.75 mg/ml PZH for 24 h. Cell confluence was observed using
a phase-contrast microscope (Leica Microsystems GmbH, Wetzlar,
Germany). Images were captured at ×200 magnification.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry
using a FACSCalibur system (Becton-Dickinson, San Jose, CA, USA).
Following treatment with 0, 0.25, 0.5 or 0.75 mg/ml PZH for 24 h,
BEL-7402 cells were collected at a final concentration of
1×106 cells/ml, then fixed in 70% ethanol at 4°C
overnight. The cells were subsequently washed twice with ice cold
PBS and incubated with propidium iodide (10 µg/ml) and a BD
Pharmingen™ Cell Cycle kit, which contained DNase (8 µg/ml; BD
Biosciences) for 30 min. The fluorescence signal was observed in
the FL2 channel and the proportion of DNA in each phase was
analyzed using Modfit LT software version 3.0 (Verity Software
House, Inc., Topsham, ME, USA).
Colony formation assay
BEL-7402 cells were seeded into 6-well plates at a
density of 2.5×105 cells/well and treated with 0, 0.25,
0.5 or 0.75 mg/ml PZH for 24 h. The cells were subsequently
reseeded into 6-well plates in RPMI-1640 without PZH at a density
of 1×103 cells/well. Following incubation for 8 days,
cell colonies were fixed with 4% paraformaldehyde for 10 min at
room temperature, stained with 0.1% crystal violet for 15 min at
room temperature and observed using phase-contrast microscopy. The
number of colonies per plate were counted.
Detection of apoptosis with Hoechst
staining
BEL-7402 cells were seeded into 12-well plates at a
density of 1×105 cells/well, and treated with 0, 0.25,
0.5 or 0.75 mg/ml PZH for 24 h. Cells were subsequently washed with
PBS, fixed with 4% polyoxymethylene for 10 min at room temperature
and washed twice more in PBS. Cells were then incubated in Hoechst
33258 for 10 min in the dark at 37°C and observed using a
phase-contrast fluorescence microscope (Leica Microsystems GmbH).
Images were captured at ×200 magnification.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
BEL-7402 cells were seeded into 6-well plates at a
density of 2.5×105 cells/well in 2 ml medium. The cells
were treated with 0, 0.25, 0.5 or 0.75 mg/ml PZH for 24 h.
Analysis of Bcl-2, CDK4 and cyclin D1
expression
Total RNA was isolated with RNAiso Plus reagent, and
1 µg of total RNA was reverse-transcribed with the PrimeScript™ RT
reagent kit according to the manufacturer's protocol. The produced
cDNA was used to determine the mRNA expression of Bcl-2, CDK4 and
cyclin D1 by qPCR with the SYBR® Select Master Mix using
the ABI 7500 Fast instrument (both from Thermo Fisher Scientific,
Inc.) under the following thermocycling conditions: 50°C for 2 min,
95°C for 2 min and 40 cycles at 95°C for 1 sec and 60°C for 30 sec.
GAPDH was used as an internal control. Primer sequences are listed
in Table I.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Gene | Primers (5′ to
3′) |
|---|
| Bcl-2 | F:
CAGCTGCACCTGACGCCCTT |
|
| R:
GCCTCCGTTATCCTGGATCC |
| Cyclin D1 | F:
TGGATGCTGGAGGTCTGCGAGGAA |
|
| R:
GGCTTCGATCTGCTCCTGGCAGGC |
| Cyclin-4 dependent
kinase | F:
CATGTAGACCAGGACCTAAGC |
|
| R: AACTGGCGCATC
AGATCCTAG |
| GAPDH | F:
CGACCACTTTGTCAAGCTCA |
|
| R:
AGGGGTCTACATGGCAACTG |
Analysis of miR-16 expression
Total miRNA was isolated with the RNAiso for
microRNA kit. Total miRNA (1 µg) was reverse-transcribed with the
SYBR® PrimeScript™ miRNA RT-PCR kit according to the
manufacturer's protocol. The resulting cDNA was used to determine
the expression of miR-16 by qPCR; U6 was used as an internal
control. The primers for U6 (cat no. D356-03) and miR-16 (cat no.
DHM0135) were obtained from Takara Biotechnology Co., Ltd. qPCR was
performed with the SYBR® Premix Ex Taq II using the ABI
7500 Fast instrument under the following thermocycling conditions:
95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for 30
sec.
Quantification of qPCR results
The mRNA or miRNA expression levels were determined
as ∆Cq = Cq (sample) - Cq (U6 or GAPDH) and relative quantities
between different samples were determined as ∆∆Cq = ∆Cq (sample 1)
- ∆Cq (sample 2); values were expressed as 2−∆∆Cq. All
qPCR reactions were conducted in triplicate.
Western blot analysis
BEL-7402 cells were seeded into 25 cm2
culture flasks at a density of 2.5×105 cells/ml and
treated with 0, 0.25, 0.5 or 0.75 mg/ml PZH for 24 h. Cells were
lysed with cell lysis buffer (CWBIO, Beijing, China) containing
protease and phosphatase inhibitor cocktails, and the resulting
total protein concentration was determined by a bicinchoninic acid
assay. Proteins (50 µg/lane) were resolved on 10% SDS-PAGE gels and
electroblotted onto polyvinyl difluoride membranes. The membranes
were blocked for 1 h at room temperature with blocking buffer
(Beyotime Institute of Biotechnology) and probed with primary
antibodies for Bcl-2, CDK4, cyclin D1 and β-actin (dilution,
1:1,000) overnight at 4°C, then incubated with an appropriate
HRP-conjugated secondary antibody for 1 h at room temperature
(dilution, 1:5,000). Protein bands were subsequently detected using
Thermo Scientific™ SuperSignal™ West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance with Bonferroni's multiple comparison test
using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Data were
presented as the mean of three individual experiments. P<0.05
was considered to indicate a statistically significant
difference.
Results
PZH inhibits the proliferation of
BEL-7402 cells
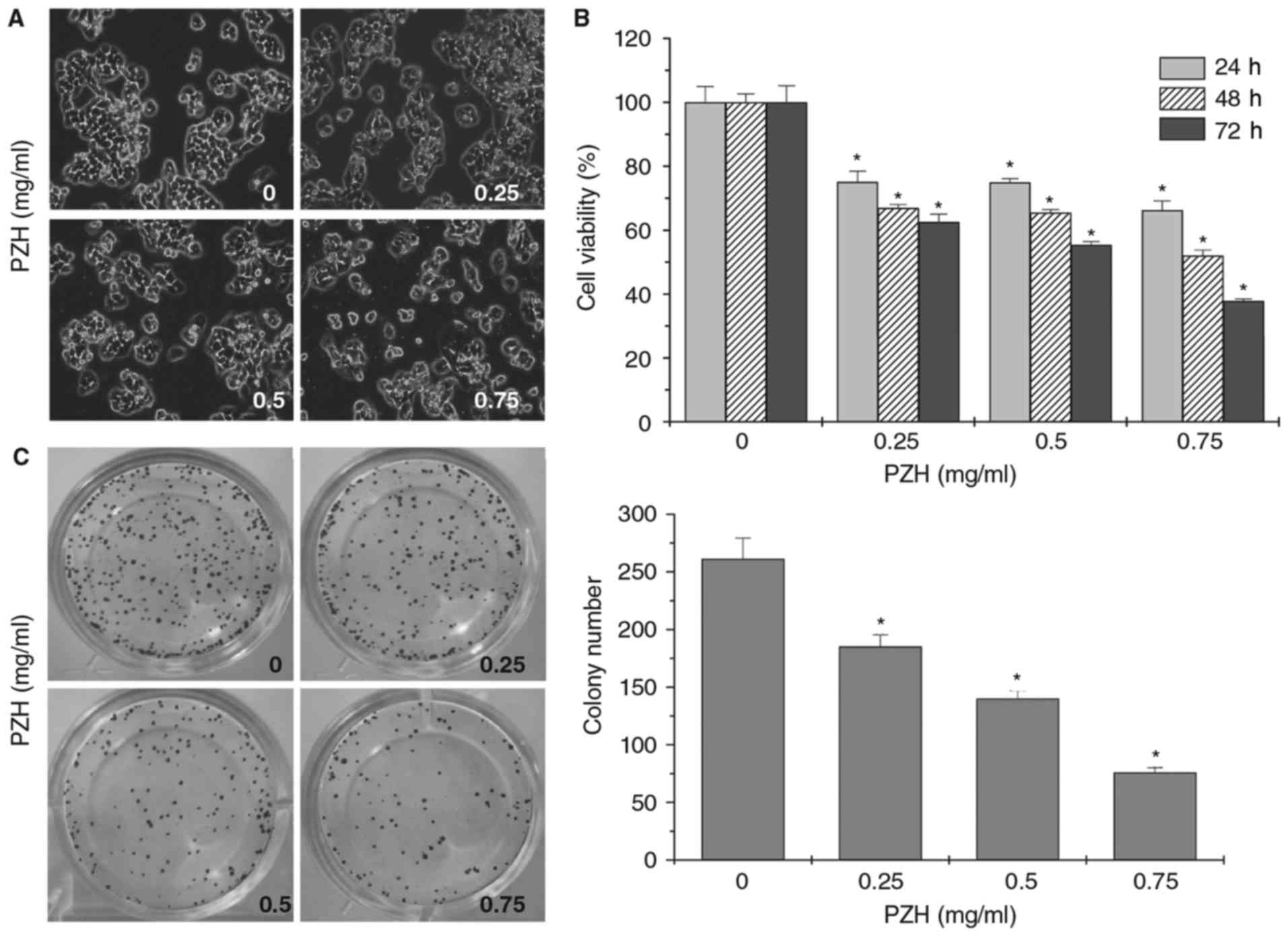
The effect of PZH on the proliferation of BEL-7402
cells was evaluated using phase-contrast microscopy. PZH treatment
decreased the confluence and cell density of BEL-7402 cells in a
dose-dependent manner (Fig. 1A).
Furthermore, the MTT assays demonstrated that PZH treatment
significantly decreased the cell viability of BEL-7402 cells in a
dose- and time-dependent manner (Fig.
1B; P<0.05). In addition, a colony formation assay indicated
that PZH treatment significantly reduced the clonogenicity rate of
BEL-7402 cells (Fig. 1C;
P<0.05).
PZH inhibits G1/S transition in
BEL-7402 cells
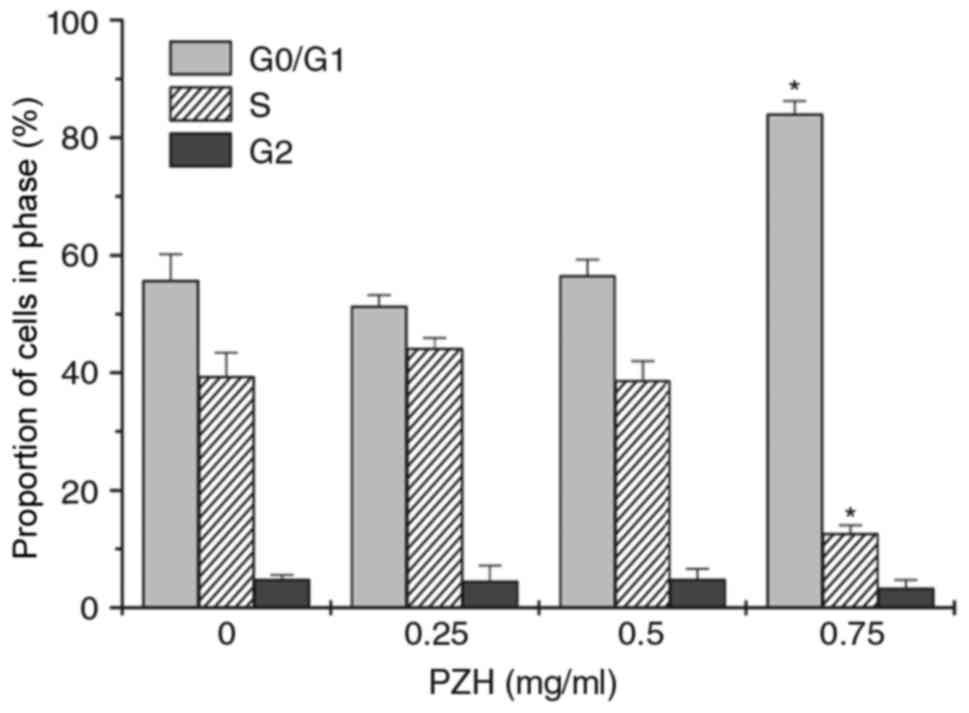
Cell cycle analysis was then performed using flow
cytometry. There was a significant increase in the number of
BEL-7402 cells in G0/G1 phase following treatment with 0.75 mg/ml
PZH, whereas the percentage of cells in S phase was decreased,
compared with untreated control cells (Fig. 2; P<0.05).
PZH induces the apoptosis of BEL-7402
cells
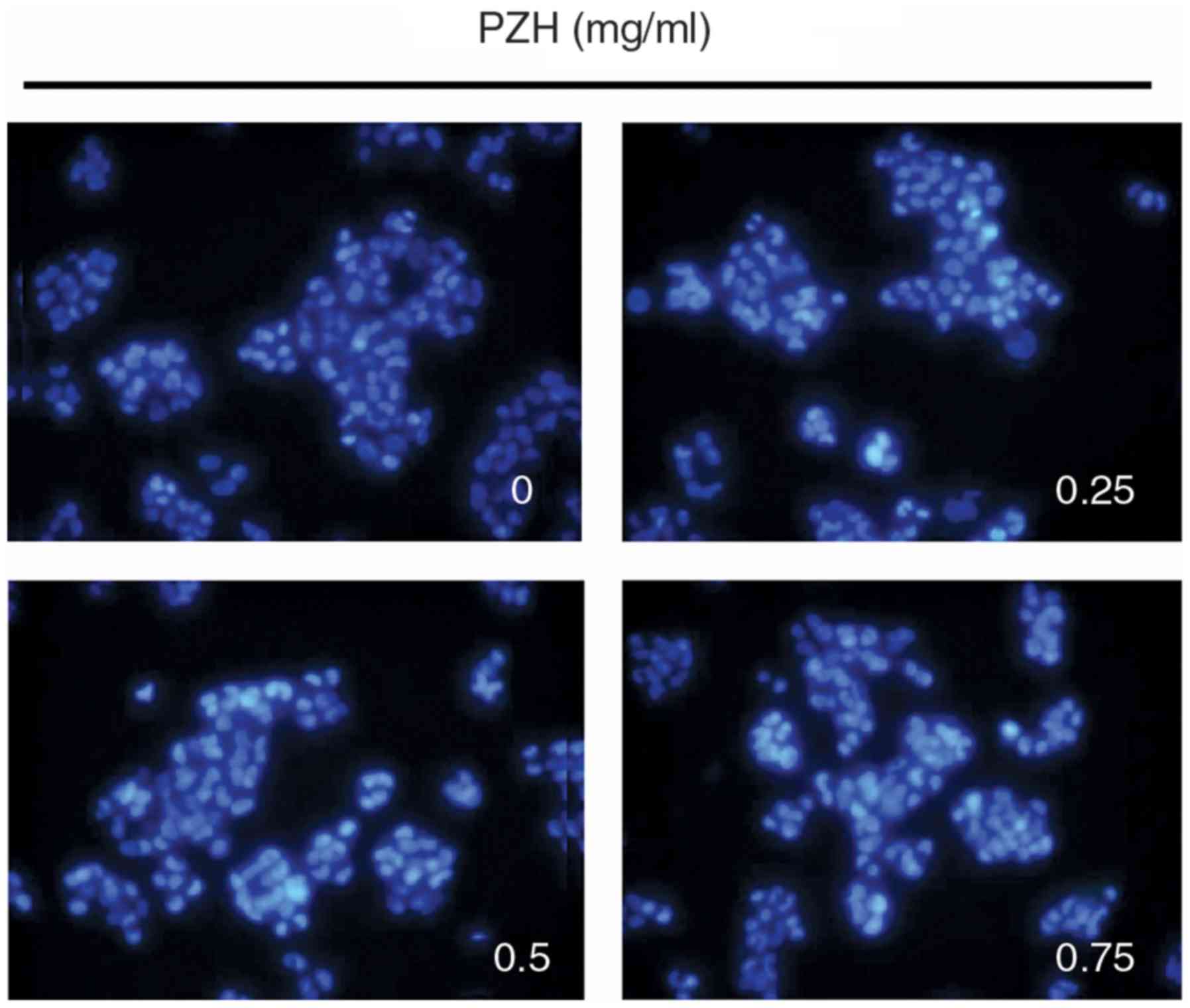
The rate of BEL-7402 cell apoptosis following PZH
treatment was determined using Hoechst staining. PZH-treated cells
exhibited the typical morphological features of apoptosis,
including chromatin condensation and nuclear fragmentation, at a
greater rate compared with the untreated control cells, which
exhibited more homogenous staining of the nuclei (Fig. 3).
PZH modulates the expression of Bcl-2,
cyclin D1, CDK4 and miR-16 in BEL-7402 cells
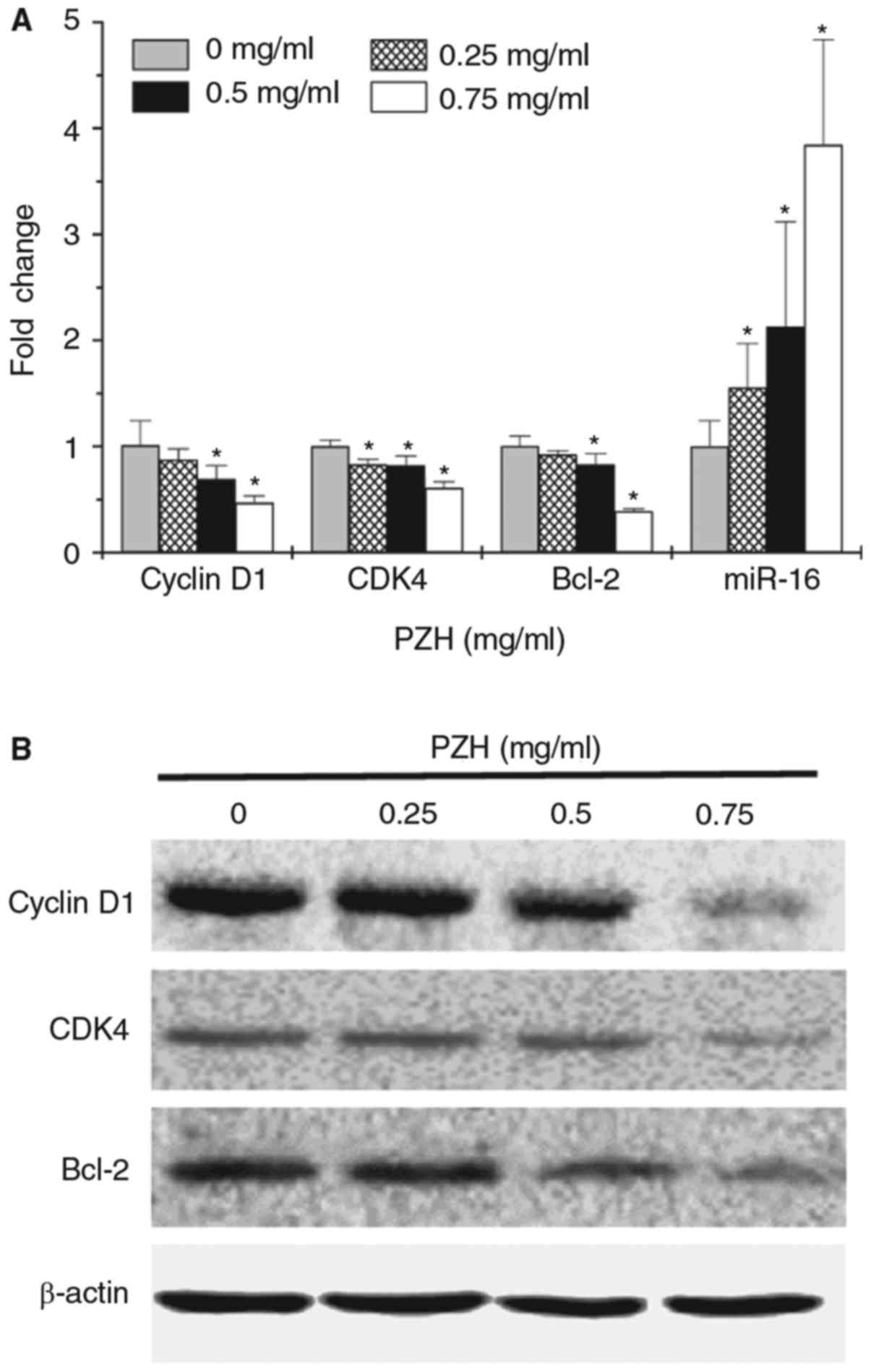
In order to determine the underlying mechanisms for
the pro-apoptotic and anti-proliferative activity of PZH, the
expression of key factors associated with cell cycle regulation and
apoptosis was examined using RT-qPCR and western blot analysis. As
demonstrated in Fig. 4A, miR-16
expression was significantly upregulated following treatment with
PZH in a dose-dependent manner (P<0.05). In addition, PZH
treatment decreased the mRNA (Fig.
4A; P<0.05 for doses ≥0.5 mg/ml) and protein expression
levels (Fig. 4B) of Bcl-2, cyclin D1
and CDK4.
Discussion
HCC is one of the leading causes of
cancer-associated mortality worldwide (1–3). Current
treatment strategies for HCC include surgery, chemotherapy and
targeted drug therapy (4–7). However, there are significant
limitations in the effectiveness of current treatment options due
to the development of drug resistance and non-specific
cytotoxicity. The TCM treatment of cancer has received widespread
attention due to its therapeutic efficacy and relatively few side
effects. Previous studies have demonstrated that the commonly used
TCM formula PZH can inhibit the growth and metastasis of colorectal
cancer cells (34–46). However, its effects on HCC and the
underlying mechanisms remain unclear.
miRNAs may serve crucial functions in liver
tumorigenesis by regulating various proliferation and
apoptosis-associated pathways. Previous studies have reported that
miR-16 upregulation inhibited cell proliferation and induced cell
cycle arrest (29,30). Thus, determining the effect of miRNAs,
particularly miR-16, in HCC and the effect of PZH treatment on
miR-16 expression may be of critical importance. The aim of the
present study, was to demonstrate the inhibitory effect of PZH
treatment on the proliferation and apoptosis of the BEL-7402 HCC
cell line and explore the underlying mechanisms. It was identified
that PZH treatment significantly decreased the viability and
survival of BEL-7402 cells in a dose- and time-dependent manner. In
addition, higher doses of PZH increased the percentage of cells in
the G0/G1 phase and prevented G1/S cell cycle progression.
Consistent with these data, PZH treatment significantly
downregulated the expression of the anti-apoptotic gene Bcl-2, and
the pro-proliferative factors cyclin D1 and CDK4. In addition, the
miR-16 expression level in BEL-7402 cells was significantly
upregulated following PZH treatment.
To conclude, the data of the present study
demonstrate that PZH suppresses HCC growth through inhibiting
cancer cell proliferation and inducing apoptosis. Modulating the
expression of miR-16 and its target genes may be a possible
mechanism for PZH's anti-tumor action.
Acknowledgements
The present study was sponsored by the National
Natural Science Foundation of China (grant no. 81673721), the Joint
Project of the Education and Health Bureaus of Fujian Province
(grant no. WKJ-FJ-21) and the Research Fund of the Education Bureau
of Fujian Province (grant no. JA14162).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
PZH
|
Pien Tze Huang
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: SEER cancer statistics review, 1975–2010Natl Cancer Inst.
Bethesda, MD, USA: 2013
|
|
2
|
Simard EP, Ward EM, Siegel R and Jemal A:
Cancers with increasing incidence trends in the United States: 1999
through 2008. CA Cancer J Clin. 62:118–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma JM, Zou ZH and Jemal A:
Cancer Statistics, 2014. CA Cancer J Clin. 64:9–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin S, Hoffmann K and Schemmer P:
Treatment of hepatocellular carcinoma: A systematic review. Liver
Cancer. 1:144–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu SC: Where are we in the chemoprevention
of hepatocellar carcinoma? Hepatology. 51:734–736. 2010.PubMed/NCBI
|
|
7
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterol.
140:1410–1426. 2011. View Article : Google Scholar
|
|
8
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: A review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:547–550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haraken S, Abu-EI-Ardat K, Diab-Assaf M,
Niedzwiecki A, EI-Sabban M and Rath M: Epigallocatechin-3-gallate
induces apoptosis and cell cycle arrest in HTLV-1-positive and
-negative leukemia cells. Med Oncol. 25:30–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Purohit A, Hejaz HA, Walden L,
MacCarthy-Morrogh L, Packham G, Potter BVL and Reed MJ: The effect
of 2-methoxyoestrone-3-O-sulphamate on the growth of breast cancer
cells and induced mammary tumors. Int J Cancer. 85:584–589. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kessel D and Luo Y: Cells in
cryptophycin-induced cell-cyclearrest are susceptible to apoptosis.
Cancer Lett. 151:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cory S and Adams JM: The Bcl-2 family:
Regulators of the cellular life-of-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng J, Ding J, Tan C, Baggenstoss B,
Zhang Z, Lapolla SM and Lin J: Oligomerization of membrane-bound
Bcl-2 is involved in its pore formation induced by tBid. Apoptosis.
14:1145–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng J, Tan C, Roberts GJ, Nikolaeva O,
Zhang Z, Lapolla SM, Primorac S, Andrews DW and Lin J: tBID elicits
a conformational alteration in membrane-bound Bcl-2 such that it
inhibits Bax pore formation. J Biol Chem. 281:35802–35811. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valencia-Sachez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bagga S and Pasquinelli AE: Identification
and analysis of microRNAs. Genet Eng (NY). 27:1–20. 2006.
View Article : Google Scholar
|
|
21
|
Bar N and Dikstein R: miR-22 forms a
regulatory loop in PTEN/AKT pathway and modulates signaling
kinetics. PLoS One. 5:e108592010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartel DP: MicroRNA: Target recognition
and regulatory function. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leskelä S, Leandro-García LJ, Mendiola M,
Barriuso J, Inglada-Pérez L, Muñoz I, Martínez-Delgado B, Redondo
A, de Santiago J, Robledo M, et al: The mir-200 family controls
beta-tubulin III expression and is associated with paclitaxel-based
treatment response and progression-free survival in ovarian cancer
patients. Endocr Relat Cancer. 18:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhlmann S, Zhang J, Schwäger A,
Mannsperger H, Riazalhosseini Y, Burmester S, Ward A, Korf U,
Wiemann S and Sahin Ö: miR-200bc/429 cluster targets PLCgamma1 and
differentially regulates proliferation and EGF-driven invasion than
miR-200a/141 in breast cancer. Oncogene. 29:4297–4306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Tang J, Lei H, Cai P, Zhu H, Li B,
Xu X, Xia Y and Tang W: Decreased miR-200a/141 suppress cell
migration and proliferation by targeting PTEN in Hirschsprung's
disease. Cell Physiol Biochem. 34:543–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 3q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:pp. 15524–15529. 2002, View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YH, Lin KH, Chen HC, Chang ML, Hsu
CW, Lai MW, Chen TC, Lee WC, Tseng YH and Yeh CT: Identification of
postoperative prognostic microRNA predictors in hepatocellular
carcinoma. PLoS One. 7:e371882012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cimmino A, Calin GA, Fabbri M, Lorio MV,
Ferracin M, Shimizu M, Wojcik SE, Ageilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:pp. 13944–13949. 2005, View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsang WP and Kwok TT: Epigallocatechin
gallate up-regulation of miR-16 and induction of apoptosis in human
cancer cells. J Nutr Biochem. 21:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Q, Zhang Y, Zhao M, Li Q, Chen R,
Long X, Fang W and Liu Z: miR-16 induction after CDK4 knockdown is
mediated by c-Myc suppression and inhibits cell growth as well as
sensitizes nasopharyngeal carcinoma cells to chemotherapy. Tumour
Biol. 37:2425–2433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pekarsky Y and Croce CM: Role of miR-15/16
in CLL. Cell Death Differ. 22:6–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma
YL, Ji ZW, Li XX, Han K, Gao J, et al: miR-15a and miR-16-1
downregulate CCND1 and induce apoptosis and cell cycle arrest in
osteosarcoma. Oncol Rep. 28:1764–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combination in
the fight against cancer and dementia? EMBO Rep. 10:194–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chinese pharmacopoeia commission,
pharmacopoeia of the Peoples Repubic of Chian. Beijing: Chinese
medical science and technology press; pp. 2632010
|
|
37
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhuang Q, Hong F, Shen A, Zheng L, Zeng J,
Lin W, Chen Y, Sferra TJ, Hong Z and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in colorectal cancer mouse. Int J
Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
39
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen A, Hong F, Liu L, Lin J, Wei L, Cai
Q, Hong Z and Peng J: Pien Tze Huang inhibits the proliferation of
human colon carcinoma cells by arresting G1/S cell cycle
progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
41
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Shen A, Zhang Y, Chen Y, Lin J,
Lin W, Sferra T and Peng J: Pien Tze Huang inhibits hypoxia-induced
epithelial-mesenchymal transition in human colon carcinoma cells
through suppression of the HIF-1 pathway. Exp Ther Med.
7:1237–1242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shen A, Chen H, Chen Y, Lin J, Lin W, Liu
L, Sferra TJ and Peng J: Pien Tze Huang overcomes multidrug
resistance and epithelial-mesenchymal transition in human
colorectal carcinoma cells via suppression of TGF-β pathway. Evid
Based Complement Alternat Med. 2014:6794362014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen H, Feng J, Zhang Y, Shen A, Chen Y,
Lin J, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced angiogenesis via HIF-1a/VEGF-A pathway in
colorectal cancer. Evid-Based Complement Alternat Med.
2015:4542792015.PubMed/NCBI
|
|
46
|
Shen A, Lin W, Chen Y, Liu L, Chen H,
Zhuang Q, Lin J, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 46:685–690.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qi F, Wei L, Shen A, Chen Y, Lin J, Chu J,
Cai Q, Pan J and Peng J: Pien Tze Huang inhibits the proliferation,
and induces the apoptosis and differentiation of colorectal cancer
stem cells via suppression of the Notch1 pathway. Oncol Rep.
35:511–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin J, Feng J, Jin Y, Yan Z, Lai Z and
Peng J: Pien Tze Huang suppresses VEGF-C-mediated lymphangiogenesis
in colorectal cancer. Oncol Rep. 36:3568–3576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu YY and Yu EX: Clinical analysis of the
analysis of the effect of Pien Tze Huang in treatment of 42
patients with moderate or advanced liver cancer. Shanghai J Tradit
Chin Med. 12:4–5. 1994.
|
|
51
|
Xu YY: The effect of Pien Tze Huang in
treatment of liver cancer is good. Med World. 11:622001.
|