Introduction
Glioblastoma multiforme (GBM) remains incurable
despite advanced treatments including surgery, chemotherapy, and
irradiation (1). Temozolomide (TMZ),
the most widely used chemotherapeutic agent, prolonged the median
survival of GBM patients by only up to 14.6 month (2). TMZ resistance is one of the greatest
obstacles to successful GBM treatment; the tumors tend to recur
within a few months and result in death (3). Several factors contribute to the
regulation of the acquired and intrinsic pathway of resistance to
chemotherapy (4). A P-type ATPase
copper transporter ATP7B plays a role in the elicitation of
multi-drug resistance (5); its
expression was elevated in human malignancies including ovarian-,
gastric-, and breast cancer (6–8).
We investigated the expression of ATP7B in GBM and
its role in the resistance of glioblastoma to TMZ. We show for the
first time that the high expression of ATP7B is correlated with the
poor overall survival of GMB patients and that ATP7B
over-expressing cell lines are resistant to TMZ.
Materials and methods
Patients and tissue samples
Surgical specimens from 79 consecutive GMB patients
who underwent total tumor removal surgery from 2007 to 2015 at the
Department of Neurosurgery, Kagoshima University Hospital, were
available for immunohistochemical study. All were treated with TMZ
(75 mg/m2/day) concurrently with conventional radiation
therapy conventional radiation 40 Gy plus CyberKnife 35 Gy/5
fractions. This was followed by maintenance TMZ treatment (150
mg/m2 for 5 consecutive days of every 28 days). We
retrospectively reviewed the clinical records of all patients. Of
these, 12 underwent the second operation followed by same adjuvant
therapy due to the recurrence; tissue samples from the 1st and 2nd
surgical procedure were studied.
Our retrospective analysis was approved by the
ethics committee of Kagoshima University Hospital (reference
27–05). The authors certify that the study involving human subjects
was conducted in accordance with the 2013 Declaration of Helsinki.
The data we collected are routinely obtained and are essential for
the adequate management of glioblastoma. They were analyzed
anonymously in a linkable fashion to protect patient privacy.
Informed patient consent was waived due to the retrospective nature
of the analysis; the information was obtained from anonymized
medical charts and records.
Immunohistochemical staining and
evaluation
All surgical specimens were fixed in 10%
formaldehyde, embedded in paraffin, and cut into 3-µm slices. After
microwave antigen retrieval in citrate buffer (pH 6.0) the samples
were incubated with anti-ATP7B rabbit antibody (Novus Biologicals,
Littleton, CO. USA) (1:100 dilution) and mouse
anti-O6-methylguanine-DNA methyltransferase monoclonal antibody
(MGMT, Chemicon international, USA) (1:200 dilution) as the primary
antibody. The cells in three microscopic fields (magnification
×400) were counted independently by two researchers (M. F and S.Y).
The criterion for a positive reaction was staining of a vesicle or
vesicles in cytoplasm for ATP7B and clear nuclear staining for
MGMT. The ratio of positive cells (%) was obtained by dividing the
number of immunopositive cells by the total number of cells per
field. The mean of six ratios of positive cells (2 readers, 3
fields each) was used for analysis. When more than 10% of the tumor
cells were positive, the tumor was recorded as high-ATP7B GBM;
tumors with less than 10% ATP7B-positive cells were categorized as
low-ATP7B GBM. Anti-isocitrate dehydrogenase 1 (IDH1) R132H
(Dianova GmbH, Hamburg, Germany) (1:50 dilution) was used as
primary antibody following same immunohistochemical staining
protocol mentioned above.
Cell culture and transfection
All cells were cultured in DMEM (Thermo Fisher
Scientific, Grand Island, NE, USA) containing 10% fetal calf serum
(Thermo Scientific, Logan, UT, USA) and 100 units/ml
antibiotic-antimycotic solution (Thermo Fisher Scientific) in 5%
CO2 at 37°C. Wild type (WT) ATP7B-expressing cell lines
(U251/WD-1 and U251/WD-2) were established by WT ATP7B transfection
of the human glioblastoma cell line U251 using lipofectamin-2000
(ThermoFisher Scientific) as described previously (5). The control, U251/EV, was established
from U251 cells transfected with vector (pRc/CMV) only.
Protein extraction and
immunoblotting
Protein from cell lysates was prepared as described
(9). The protein concentration was
determined with the Bio-Rad protein assay kit according to the
manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA, USA).
Total protein (100 µg) was subjected to 7.5% SDS-PAGE under
reducing conditions. Immunoblotting was as described elsewhere
(10). The polyclonal anti-ATP7B
antibody (Novus Biologicals, Littleton, CO, USA) or anti-GAPDH
monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA)
was used as the primary antibody. Horseradish peroxidase-conjugated
anti-rabbit IgG and anti-mouse IgG (GE Healthcare, Buckinghamshire,
UK) were the secondary antibody respectively. Immunoreactive bands
were visualized with enhanced chemiluminescence using the ECL prime
Western blotting system (GE Healthcare).
Cell sutvival assay
Equal numbers of cells (2×103) were
inoculated into each well of multi-well plates and treated for 7
days with TMZ (Sigma-Aldrich, St., Louis, MO, USA). Then their
sensitivity to each of the administered TMZ dose was assessed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
(Sigma-Aldrich) colorimetric assay (9). The drug concentration that reduced the
number of cells to 50% of cells grown in control medium was
recorded as the IC50 value.
Statistical analysis
Data were analyzed using the Statistical Package for
the Social Sciences (SPSS, v22; IBM, Armonk, NY, USA). For
intergroup comparisons we used the Student t-test for continuous-
and the Chi square test for categorical variables. Overall survival
was plotted by applying Kaplan-Meier methods. Multivariable Cox
proportional hazards regression analysis was used to obtain hazard
ratios and 95% confidence intervals (CIs). Quantitative data were
expressed as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
ATP7B expression in human
glioblastoma
In all 79 GBM tissue samples obtained at the initial
surgery, ATP7B expression were immunohistochemically assessed. The
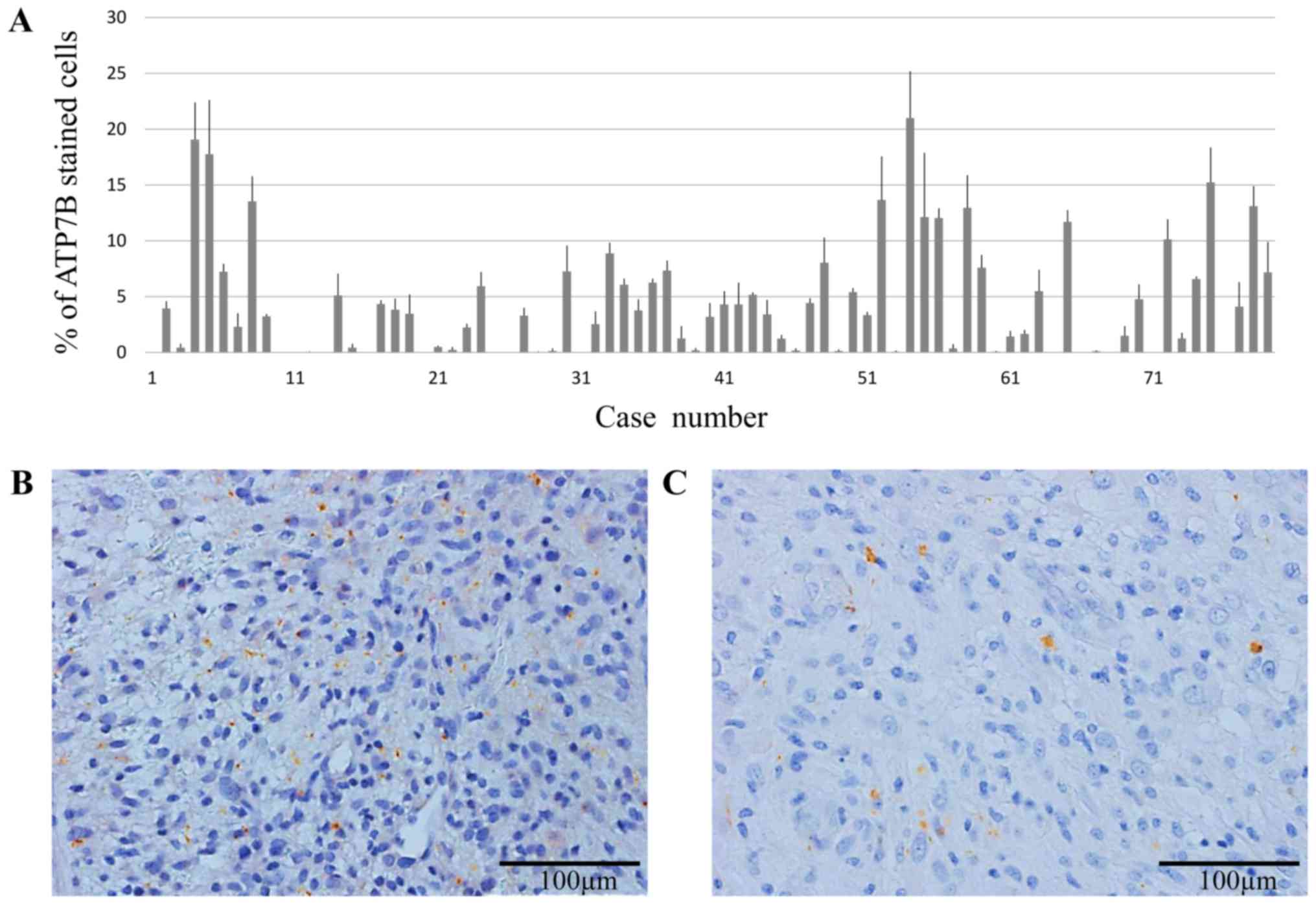
degree of cytoplasmic staining in tumor cells varied (Fig. 1A); the average rate of ATP7B protein
expression was 4.4±0.98%. Two representative cases of GBM tumors
exhibiting high (>10%) and low ATP7B expression (<10%) are
shown in Fig. 1B and C,
respectively.
Prognostic relevance of ATP7B
expression
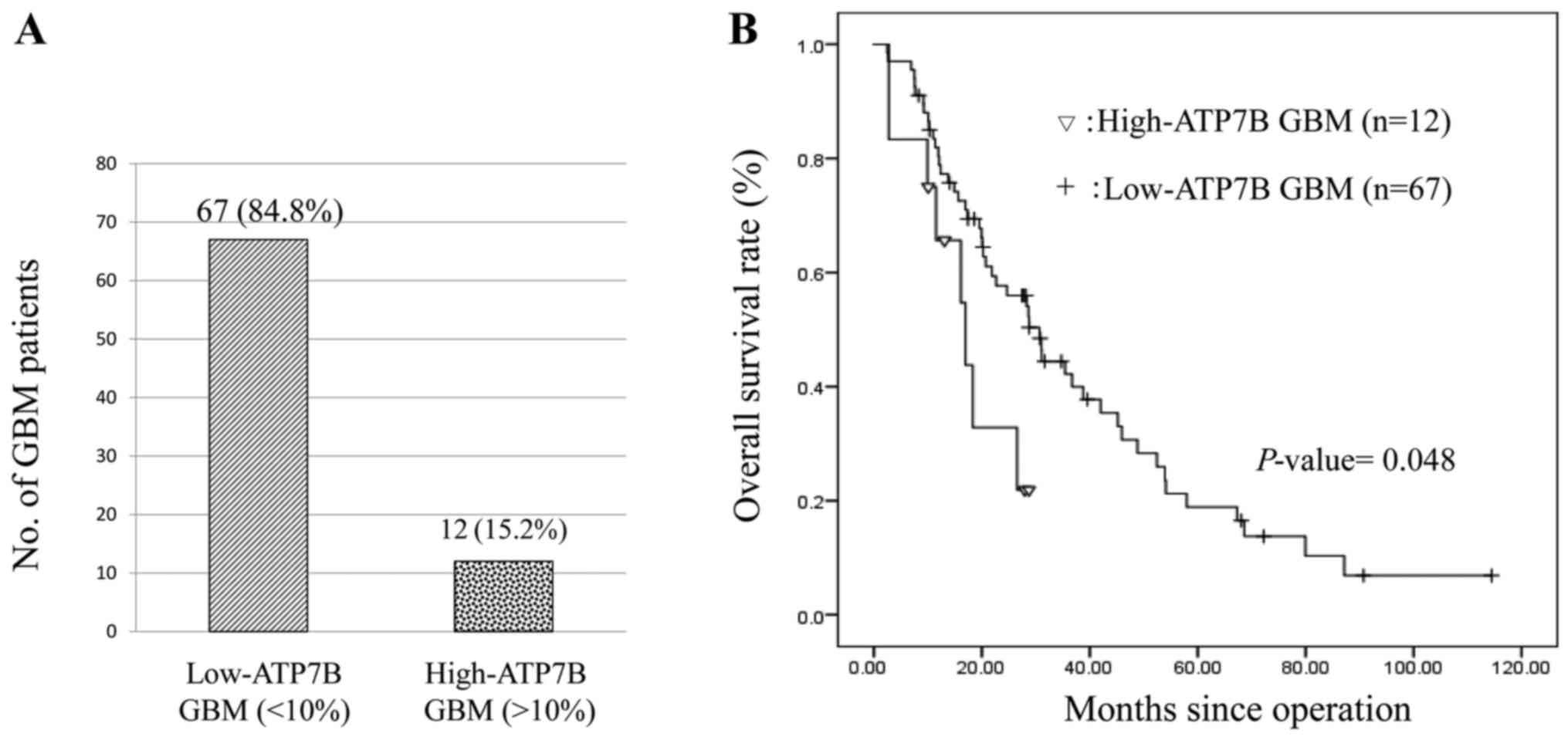
Of our 79 patients, 12 (15.2%) harbored high-ATP7B
GBM; 14.4±3.0% of the tumor cells were ATP7B positive. The other 67
patients had low-ATP7B GBM; 2.6±0.64% of the cells were ATP7B
positive (Fig. 2A). Investigation of
the relationship between patient characteristics and ATP7B
expression revealed no significant association with the patient
gender and age, and the GBM tumor location. None of our patients
younger than 50 years bore high-ATP7B GBM (Table I). The Kaplan-Meier estimate of
overall survival in Fig. 2B was
significantly shorter in patients with high-ATP7B GBM (hazard ratio
0.452, 95% CI 0.206–0.994, P=0.048). The median overall survival of
patients with high- and low ATP7B GBM was 14.6 and 24.7 months,
respectively. In addition, the MGMT expression in low-ATP7B GBM
(5.7±1.2%) and high-ATP7B GBM (5.3±1.3%) has no significant
difference. IDH1 mutant is negative in all 79 GBM cases.
 | Table I.ATP7B protein detection in
Glioblastoma specimens: Correlation with clinical data. |
Table I.
ATP7B protein detection in
Glioblastoma specimens: Correlation with clinical data.
|
|
| Immunohistochemical
expression of ATP7B protien |
|
|---|
|
|
|
|
|
|---|
| Correlatives
data | Specimens No.
(%) | Low n (%) | High n (%) | P-value |
|---|
| Sex |
|
|
|
|
| Male | 45 (57.0) | 38 (84.4) | 7 (15.6) | 0.9 |
|
Female | 34 (43.0) | 29 (85.3) | 5 (14.7) |
|
| Age (years) |
|
|
|
|
|
<50 | 11 (13.9) | 11 (100) | 0 (0) | 0.1 |
|
>50 | 68 (79.7) | 56 (82.4) | 12 (17.6) |
|
| Tumor Location |
|
|
|
|
|
Frontal | 27 (34.2) | 26 (96.3) | 1 (3.7) | 0.2 |
|
Parital | 19 (24.1) | 16 (84.2) | 3 (15.8) |
|
|
Occipital | 3 (3.8) | 3 (100) | 0 (0) |
|
|
Temporal | 23 (29.1) | 17 (73.9) | 6 (25.1) |
|
|
Other | 7 (8.9) | 5 (71.4) | 2 (28.6) |
|
ATP7B expression in recurrent GBMs
after treatment with TMZ
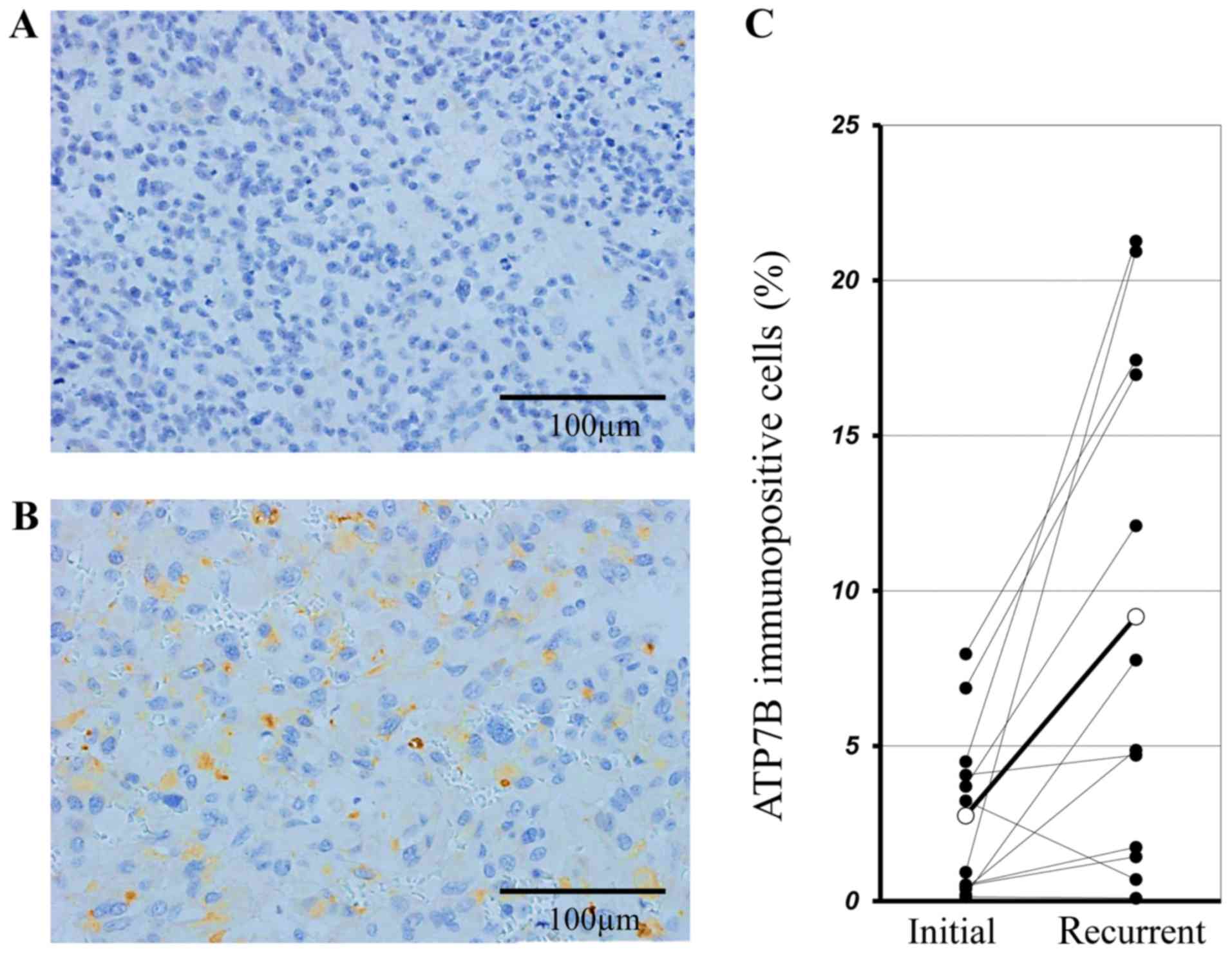
Of our 79 patients, 12 underwent the second
operation due to the recurrence. Fig. 3A
and B show the immunohistochemical staining for ATP7B of
tissues removed at the 1st and 2nd surgical procedure from the
identical patient. In this representative case, the mean ATP7B
positivity ratio was 0.93±0.15% and 20.93±7.79% in samples from the
1st and 2nd operation, respectively. In all 12 recurrent cases the
expression of ATP7B in the 2nd surgical samples was significantly
higher than the 1st ones (9.17±2.56% vs. 2.75±0.55%, P=0.008 by the
paired t-test) (Fig. 3C).
Expression of ATP7B expressing GBM
cells were resistant to TMZ
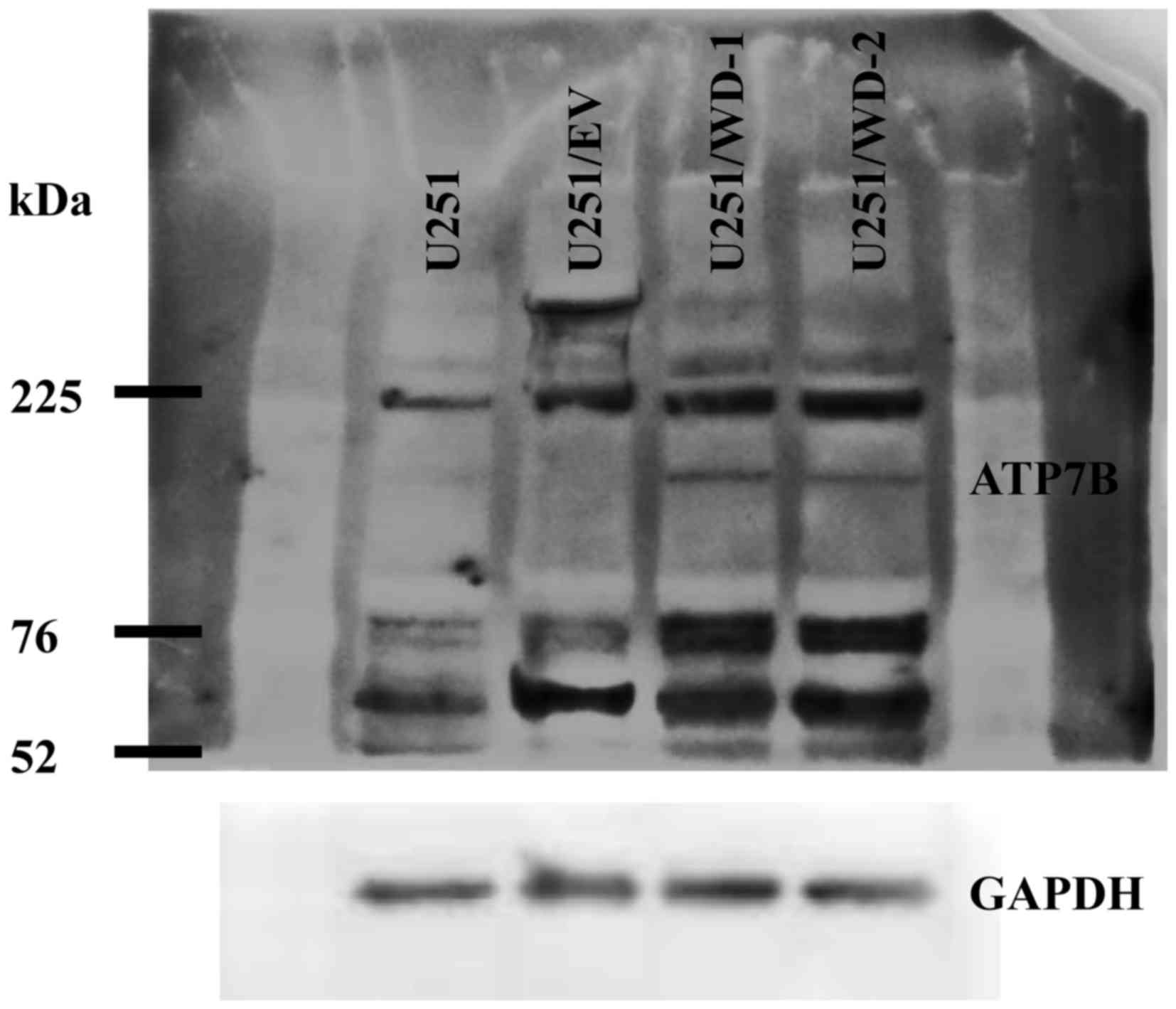
We detected ATP7B 165 kDa bands in the both
transfected cell lines but not in U251 and U251/EV cells by
immunoblotting (Fig. 4). Lower bands
(105 kDa) are likely to be specifically digested ATP7B protein.
The sensitivity of the transfected cells to TMZ was
evaluated by MTT assay. Interestingly, U251/WD-1 cells and
U251/WD-2 cells were 3.8 and 1.7 times more resistant to TMZ than
U251/EV cells (Table II), indicating
that the resistance to TMZ was correlated with ATP7B expression in
these cells.
 | Table II.Chemosensitivity of ATP7B
overexpressing cells. |
Table II.
Chemosensitivity of ATP7B
overexpressing cells.
| Cells | U251/EV | U251/WD-1 | U251/WD-2 |
|---|
| Agent | IC50 | IC50 | RRa | IC50 | RRa |
| TMZ (µM) | 61±4.8 | 240±59 | 3.84 | 110±21 | 1.71 |
Discussion
GBM resistance mechanisms to alkylating agents, e.g.
O6-methylguanine-DNA methyltransferase (MGMT), isocitrate
dehydrogenase (IDH1/2), Y-box binding protein-1 (YB-1), and
maternal embryonic leucine-zipper kinase (MELK) have been
identified (11–14). Recently we reported that ATP7B confers
drug resistance against several structurally unrelated
chemotherapeutic agents through drug sequestration in acidic
vesicles (5). We expected ATP7B as a
candidate protein for the mediation of chemoresistance to TMZ.
P-type ATPase copper transporters ATP7A and ATP7B play key roles in
regulating copper homeostasis. Their function is directly
influenced by their subcellular localization. ATP7A is ubiquitously
expressed and ATP7A mutations elicit Menkes disease (15); ATP7B is primarily expressed in the
liver and mutant ATP7B is involved in Wilson disease (16,17). ATP7A
and ATP7B are expressed in normal brain tissue, especially in the
polarized epithelial tissue of the choroid plexus; they are also
observed in the visual cortex, anterior cingulate cortex, caudate
putamen, and cerebellum (18).
The high expression of ATP7B protein has been shown
in human ovarian-, gastric-, and breast cancer (6–8). We
earlier reported that ATP7A and ATP7B were involved in multi-drug
resistance (5,19) Highly expressed ATP7B is a poor
prognostic marker in patients with ovarian and oral squamous cell
cancers treated with cisplatin-based chemotherapy (6,20). In the
present study we first document the presence of different levels of
ATP7B expression in GBM tissues. We also report that the prognosis
of patients with ATP7B high-expressing tumors was poorer than of
patient with low ones. We included only patients who underwent
total tumor removal to exclude the influence of different degree of
surgical removal. We regard the standardizing sample back ground is
very important to estimate the correct influence of ATP7B on
patient survival. The patients with residual tumor after surgery
were excluded because these patients must bring heterogeneity in
treatment affecting on the survival. We also found that MGMT and
IDH1 had no significant influence in these particular groups.
Postoperative adjuvant therapy, including radio- and chemotherapy,
was administered under the same protocol to all 79 patients. As the
patient gender and age, and the tumor location exhibited no
significant relationship with ATP7B expression, its elevation in
patients with GBM can serve as a marker for a poor prognosis.
We also found that GBM that recurred after treatment
with TMZ and radiotherapy exhibited higher ATP7B expression than
the initially operated tumor. This observation consistent with the
ideas that adjuvant therapy induced the higher expression of ATP7B
and ATP7B overexpressing rendered GBM cells resistant to TMZ.
Others reported that ATP-dependent drug efflux
mechanisms involving P-glycoprotein (P-gp, ABCB1), breast
cancer-resistance protein1 (BCRP1, ABCG2), and
multidrug-resistance-associated proteins (MRPs, ABCC1) confer drug
resistance to gliomas (21). P-gp and
BCRP1 are mainly expressed in endothelial cells of the tumor
vasculature instead of the glioma cells themselves (22,23) and
both are also present at comparable levels in the normal brain
(24). Thus, chemotherapeutic agents
that inhibit P-gp and BCRP1 functions may be more toxic in normal
tissues than neoplastic ones, thereby precluding an acceptable
clinical response. As ATP7B was abundant in GBM cells, cellular
micro-environmental modifications targeting ATP7B may help to abate
their drug resistance. We reported that the drug resistance of an
ATP7B over-expressing epidermoid carcinoma cell line could be
controlled by increasing the endo-lysosomal pH level (5). This finding may lead to the development
of a drug delivery procedure that targets acidic vesicles and helps
to overcome the ATP7B-mediated drug resistance of GBM cells.
In the present study we indicated ATP7B expressing
U251 cells are more resistant to TMZ. However there are
possibilities such effects cannot be observed in some other cells,
since the cancer cells have different genetic background. The
further studies are needed to make clear the effect of ATP7B
expression in other GBM cell lines and identify important back
ground factors. Our investigation of the expression of ATP7B in GBM
strongly suggests that ATP7B plays a role in TMZ resistance.
Studies are underway to identify the mechanism(s) implicated in the
acquisition of drug resistance and to develop specific maneuvers to
overcome the ATP7B-mediated drug resistance of glioblastoma.
Acknowledgements
The authors thank Ms. Tomoko Takajyo for performing
the immunostaining. The present study was supported by JSPS KAKENHI
grant nos. JP15K10338, JP26870456, JP16K07121.
References
|
1
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verhoeff JJ, van Tellingen O, Claes A,
Stalpers LJ, van Linde ME, Richel DJ, Leenders WP and van Furth WR:
Concerns about anti-angiogenic treatment in patients with
glioblastoma multiforme. BMC Cancer. 9:4442009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohka F, Natsume A and Wakabayashi T:
Current trends in targeted therapies for glioblastoma multiforme.
Neurol Res Int. 2012:8784252012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moinuddin FM, Shinsato Y, Komatsu M,
Mitsuo R, Minami K, Yamamoto M, Kawahara K, Hirano H, Arita K and
Furukawa T: ATP7B expression confers multidrug resistance through
drug sequestration. Oncotarget. 7:22779–22790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama K, Kanzaki A, Terada K, Mutoh M,
Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K,
et al: Prognostic value of the Cu-transporting ATPase in ovarian
carcinoma patients receiving cisplatin-based chemotherapy. Clin
Cancer Res. 10:2804–2811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanzaki A, Toi M, Neamati N, Miyashita H,
Oubu M, Nakayama K, Bando H, Ogawa K, Mutoh M, Mori S, et al:
Copper-transporting P-type adenosine triphosphatase (ATP7B) is
expressed in human breast carcinoma. Jpn J Cancer Res. 93:70–77.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohbu M, Ogawa K, Konno S, Kanzaki A,
Terada K, Sugiyama T and Takebayashi Y: Copper-transporting P-type
adenosine triphosphatase (ATP7B) is expressed in human gastric
carcinoma. Cancer Lett. 189:33–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinsato Y, Furukawa T, Yunoue S, Yonezawa
H, Minami K, Nishizawa Y, Ikeda R, Kawahara K, Yamamoto M, Hirano
H, et al: Reduction of MLH1 and PMS2 confers temozolomide
resistance and is associated with recurrence of glioblastoma.
Oncotarget. 4:2261–2270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Che XF, Zheng CL, Owatari S, Mutoh M,
Gotanda T, Jeung HC, Furukawa T, Ikeda R, Yamamoto M, Haraguchi M,
et al: Overexpression of survivin in primary ATL cells and sodium
arsenite induces apoptosis by down-regulating survivin expression
in ATL cell lines. Blood. 107:4880–4887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joshi K, Banasavadi-Siddegowda Y, Mo X,
Kim SH, Mao P, Kig C, Nardini D, Sobol RW, Chow LM, Kornblum HI, et
al: MELK-dependent FOXM1 phosphorylation is essential for
proliferation of glioma stem cells. Stem Cells. 31:1051–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang P, Zhang W, Wang Y, Peng X, Chen B,
Qiu X, Li G, Li S, Wu C, Yao K, et al: IDH mutation and MGMT
promoter methylation in glioblastoma: Results of a prospective
registry. Oncotarget. 6:40896–40906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Fotovati A, Lee C, Wang M, Cote G,
Guns E, Toyota B, Faury D, Jabado N and Dunn SE: Inhibition of
Y-box binding protein-1 slows the growth of glioblastoma multiforme
and sensitizes to temozolomide independent O6-methylguanine-DNA
methyltransferase. Mol Cancer Ther. 8:3276–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vulpe C, Levinson B, Whitney S, Packman S
and Gitschier J: Isolation of a candidate gene for Menkes disease
and evidence that it encodes a copper-transporting ATPase. Nat
Genet. 3:7–13. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bull PC, Thomas GR, Rommens JM, Forbes JR
and Cox DW: The Wilson disease gene is a putative copper
transporting P-type ATPase similar to the Menkes gene. Nat Genet.
5:327–337. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanzi RE, Petrukhin K, Chernov I,
Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L,
Brzustowicz LM, et al: The Wilson disease gene is a copper
transporting ATPase with homology to the Menkes disease gene. Nat
Genet. 5:344–350. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies KM, Hare DJ, Cottam V, Chen N,
Hilgers L, Halliday G, Mercer JF and Double KL: Localization of
copper and copper transporters in the human brain. Metallomics.
5:43–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owatari S, Akune S, Komatsu M, Ikeda R,
Firth SD, Che XF, Yamamoto M, Tsujikawa K, Kitazono M, Ishizawa T,
et al: Copper-transporting P-type ATPase, ATP7A, confers multidrug
resistance and its expression is related to resistance to SN-38 in
clinical colon cancer. Cancer Res. 67:4860–4868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyashita H, Nitta Y, Mori S, Kanzaki A,
Nakayama K, Terada K, Sugiyama T, Kawamura H, Sato A, Morikawa H,
et al: Expression of copper-transporting P-type adenosine
triphosphatase (ATP7B) as a chemoresistance marker in human oral
squamous cell carcinoma treated with cisplatin. Oral Oncol.
39:157–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veringa SJ, Biesmans D, van Vuurden DG,
Jansen MH, Wedekind LE, Horsman I, Wesseling P, Vandertop WP, Noske
DP, Kaspers GJ and Hulleman E: In vitro drug response and efflux
transporters associated with drug resistance in pediatric high
grade glioma and diffuse intrinsic pontine glioma. PLoS One.
8:e615122013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henson JW, Cordon-Cardo C and Posner JB:
P-glycoprotein expression in brain tumors. J Neurooncol. 14:37–43.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawada T, Kato Y, Sakayori N, Takekawa Y
and Kobayashi M: Expression of the multidrug-resistance
P-glycoprotein (Pgp, MDR-1) by endothelial cells of the
neovasculature in central nervous system tumors. Brain Tumor
Pathol. 16:23–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daood M, Tsai C, Ahdab-Barmada M and
Watchko JF: ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2)
expression in the developing human CNS. Neuropediatrics.
39:211–218. 2008. View Article : Google Scholar : PubMed/NCBI
|