Introduction
Lung carcinomas, the second most frequent type of
cancer worldwide, are the leading cause of cancer-associated
mortality (1). Lung adenocarcinoma is
a major pathological type of non-small cell lung cancer (NSCLC),
which usually occurs following chronic inflammation (2,3).
Chemokines are a superfamily of small chemotactic
cytokines, which exert various biological functions by activating
7-transmembrane-domain G protein-coupled receptors on their target
cells (4). Chemokines and their
receptors are expressed by numerous types of neoplastic cells
(5,6).
In tumor tissues, chemokines and chemokine receptors promote tumor
growth, angiogenesis and escape of antitumor immune surveillance
via autocrine and paracrine mechanisms (6,7).
C-C motif chemokine ligand 20 (CCL20), alternatively
termed liver and activation-regulated chemokine, is the only
chemokine known to interact with C-C motif chemokine receptor 6
(CCR6), a property shared with the antimicrobial β-defensins
(8). The ligand-receptor pair
CCL20/CCR6 is responsible for the chemoattraction of immature
dendritic cells, effector/memory T cells and B cells, and also
serves a role in the skin and mucosal surfaces under homeostatic
and inflammatory conditions, as well as in pathological processes,
such as those associated with cancer and rheumatoid arthritis
(8). Furthermore, CCL20/CCR6 is
involved in the pathogenesis of interstitial lung fibrosis and
chronic obstructive pulmonary disease, which are considered
smoking-associated chronic inflammatory conditions (9,10). A
previous study demonstrated that CCL20/CCR6 are involved in the
metastasis of a variety of tumors, including pancreatic, hepatic,
prostate and colorectal carcinomas (11). This suggests that CCL20 and CCR6 may
also be involved in the occurrence and metastasis of lung
adenocarcinoma.
In nonmalignant cells, extracellular
signal-regulated kinase [ERK; also known as p44/42
mitogen-activated protein kinases (MAPKs)] signaling cascades are
involved in regulating cell proliferation, survival and
differentiation (12).
Chemokine/chemokine receptor-mediated activation of ERK also serves
a crucial role in cancer cell proliferation and invasiveness
(13–15). Brand et al (16) reported that CCL20 activated Akt,
ERK1/2 and stress-activated protein kinase/c-Jun N-terminal kinase
MAPKs, and increased interleukin-8 protein expression, resulting in
a 2.6-fold increase of cell migration as well as a significant
increase of cell proliferation in colorectal cancer. The occurrence
and development of lung cancer are also considered to be associated
with the ERK signaling pathway (17).
However, the mechanisms of CCL20 and CCR6 in lung
adenocarcinoma remain unclear. The present study was performed to
evaluate the roles of the CCL20/CCR6 and the ERK signal pathway in
lung adenocarcinoma growth.
Patients and methods
Study population
A total of 162 patients [62.75±9.76 years (mean age
± standard deviation); 87 male and 75 female] with lung
adenocarcinoma, whose diagnosis (pathological stage I or II,
pre-operative assessments) was confirmed by histological
examination between February 2009 and July 2011 at the Hebei
General Hospital (Shijiazhuang, China), were enrolled in the
present study. All the patients underwent pulmonary resection by
video-assisted thoracoscopic surgery (VATS). The patients were
divided into a recurrence group (n=50) and a non-recurrence group
(n=112) according to their recurrence and metastasis status within
2 years following surgery. Staging prior to and following surgery
was based on histopathological analysis according to the
International Union Against Cancer tumor-node-metastasis staging
system (18). The study was approved
by the Medical Ethics Committee on Human Research of Hebei General
Hospital (no. 2005-123). Informed written consent was obtained from
each patient. Patients were followed up for 2 years
post-operatively.
Surgical procedures and sample
collection
General anesthesia with double-lumen endotracheal
intubation and one-lung ventilation was used for all patients
during surgery. For the VATS procedure, three working ports were
established for the insertion of thoracoscopic instruments; these
were located at the 7th or 8th intercostal space at the
mid-axillary line, the 4th or 5th intercostal space at the anterior
axillary line, and the 8th or 9th intercostal space at the scapular
line. Specimen bags were used for removing the samples. No muscles
(latissimus dorsi or serratus anterior muscles) or ribs were cut. A
proportion of the lung tissues were fixed in 10% formalin, for
48–72 h at 4°C for immunohistochemistry, while others were stored
at −80°C for subsequent use in reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analyses.
Immunohistochemistry and histological
evaluation
The 5-µm-thick tissue sections were deparaffinized
in xylene for 20 min twice and hydrated in a graded series of
alcohol baths (100% for 5 min twice, 95% for 5 min twice, 90% for 5
min once and 80% for 5 min once). The sections were then washed
with PBS, immersed in 3% hydrogen peroxide for 15 min to block
endogenous peroxidase activity at 37°C prior to being washed again
with PBS and microwaved (medium power, 92–98°C) for 20 min for
antigen retrieval. Subsequently, the sections were incubated with
goat anti-human CCL20 monoclonal antibody (dilution, 1:100; cat.
no. AF360; R&D Systems, Inc., Minneapolis, MN, USA) and mouse
anti-human CCR6 monoclonal antibody (dilution, 1:200; cat. no.
MAB195; R&D Systems, Inc.) overnight at 4°C. The sections were
subsequently incubated with secondary anti-goat horseradish
peroxidase-conjugated antibody (dilution, 1:500; cat. no. HAF019;
R&D Systems, Inc.) or secondary anti-mouse horseradish
peroxidase-conjugated antibody (dilution, 1:500; cat. no.
abs20002A; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA),
respectively, for 30 min at room temperature. The sections were
washed with PBS then incubated for 3–5 min with
3,3′-diaminobenzidine (dilution, 1:1,000; cat. no. K3468 Dako;
Agilent Technologies, Inc.), followed by counterstaining with
hematoxylin for 30 sec at room temperature and were dehydrated and
mounted. Negative controls were performed in all cases by omitting
the primary antibody.
The slides were independently evaluated and scored
under a CX41 light microscope (magnification, ×200; Olympus
Corporation, Tokyo, Japan) by two pathologists without knowledge of
clinical data who randomly captured and assessed 20 fields of view.
The staining intensity was assigned a scored between 0 and 3, as
follows: 0, negative; 1, weak; 2, moderate; or 3, strong. The
percentages of positively stained tumor cells were counted and
grouped into four levels (0–25, score 0; 26–50, score 1; 51–75,
score 2; 76–100%, score 3). Samples with >50% positive cells
were defined as having high expression. The total immunostaining
score was calculated as the sum of each intensity score multiplied
by the corresponding percentage, for example CCR6 index = (total
staining intensity score of CCR6 × total positive tumor cells %
score for CCR6)/100.
Total RNA extraction and RT-qPCR of
CCL20 and CCR6
In total, 50 mg of each tissue sample was subjected
to total RNA isolation using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. To ensure that total RNA fulfilled the
requirements for the qPCR, the purity and quantity of RNA for each
sample was analyzed by nanodrop (a minimum A260/A280 ratio of
>1.8 was applied for all samples) and RNA integrity was assessed
by formaldehyde modified gel electrophoresis. cDNA was synthesized
using PrimeScript™ RT reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China). DNase (Takara
Biotechnology Co., Ltd.) was used prior to the reverse
transcription reaction. The following primer sequences were used:
CCL20 forward, 5′-ATGTGCTGTACCAAGAGTTT-3′ and reverse,
5′-CAAGTCTGTTTTGGATTTGC-3′; CCR6 forward,
5′-CCATTCTGGGCAGTGAGTCA-3′ and reverse, 5′-AGCAGCATCCCGCAGTTAA-3′;
GAPDH forward, 5′-ATCCCATCACCATCTTCCAG-3′ and reverse,
5′-GAGTCCTTCCACGATACCAA-3′. qPCR was performed using a thermocycler
for 35 cycles according to the following program: 5 min at 95°C, 15
sec at 95°C, 30 sec at 58°C and 30 sec at 72°C. The PCR mixture was
prepared using Eastep® qPCR Master Mix (Shanghai Promega
Biological Products Co., Ltd., Shanghai, China) according to the
manufacturer's protocol. Expression levels of each mRNA were
determined using the 2−ΔΔCq method (19) using GAPDH (Takara Biotechnology Co.,
Ltd.) as an endogenous control. The experiment was repeated three
times.
Western blot analysis of CCL20 and
CCR6
Protein samples were prepared using
radioimmunoprecipitation assay buffer. The total protein
concentration was measured by using the BCA method and 60 µg of
each sample was subjected to 10% SDS-PAGE and then transferred to
polyvinylidene fluoride membranes. Subsequent to blocking at room
temperature for 1 h with 5% fat-free milk powder with 1% Triton
X-100 in TBS (20 mmol/l Tris-Cl, 150 mmol/l NaCl, pH 7.4), the
films were incubated at 4°C overnight with antibodies against CCL20
(dilution, 1:200; cat. no. AF360; R&D Systems, Inc.), CCR6
(dilution, 1:200; cat. no. MAB195; R&D Systems, Inc.) and
β-actin (dilution, 1:2,000; cat. no. sc-47778; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Corresponding secondary
anti-mouse horseradish peroxidase-conjugated antibody (dilution,
1:5,000; cat. no. abs20002A; DakoCytomation, Glostrup, Denmark) was
then applied for 1 h at room temperature. The bands were detected
on an X-ray film following the application of Pierce ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). Relative
optical density of all bands was analyzed by GelPro Analyzer
(version 4.0; Media Cybernetics, Inc., Rockville, MD, USA).
A549 cell culture
The human alveolar epithelial A549 cell line was
purchased from Cell Resource Center, Shanghai Institute of Life
Sciences, Chinese Academy of Sciences (Shanghai, China) and grown
in RPMI-1640 medium, supplemented with 1% penicillin/streptomycin
and 10% fetal bovine serum. RPMI-1640 medium and 10% fetal bovine
serum were purchased from Thermo Fisher, Scientific, Inc. Cultures
were maintained on tissue culture-treated petri dishes (BD Falcon;
BD Biosciences, Franklin Lakes, NJ, USA) in a 5% CO2
incubator at 37°C.
Western blot analysis of
phosphorylated (p)-ERK and ERK
Prior to western blot analysis, A549 cells were
treated with CCL20 (500 ng/ml; R&D Systems, Inc.) for 0, 5, 15,
30 and 60 min. A549 cells with various treatments were harvested
and lysed on ice in RIPA buffer (1% Triton X-100, 150 mM NaCl, 10
mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0. 2 mM Na3VO4,
0.2 mM phenylmethylsulfonyl fluoride and 0.5 % NP-40; cat. no.
89900; Thermo Fisher Scientific, Inc.) containing a protease
inhibitor. The total protein concentration was measured using the
BCA method. Proteins (60 µg per lane) were subjected to SDS-PAGE
using a 10% gel, and blotted onto nitrocellulose membranes (Hybond
ECL; GE Healthcare, Chicago, IL, USA). Membranes were blocked by
incubation in TBS containing 5% nonfat dry milk and 0.1% Tween-20
for 2 h at room temperature and then incubated overnight at 4°C
with rabbit anti-human p-ERK antibodies (dilution, 1:1,000; cat.
no. sc-101760; Santa Cruz Biotechnology, Inc.), ERK antibodies
(dilution, 1:1,000; cat. no. sc-292838; Santa Cruz Biotechnology,
Inc.) and β-actin (dilution, 1:1,000; cat. no. sc-130656; Santa
Cruz Biotechnology, Inc.). Blots were then washed with TBS/Tween-20
three times and incubated at room temperature for 1 h with
horseradish peroxidase-conjugated goat anti-rabbit antibodies (cat.
no. ab97051; Abcam, Cambridge, UK). The bands were detected on an
X-ray film following application of Pierce ECL Western Blotting
Substrate Thermo Fisher Scientific, Inc.). Relative optical density
of all bands was analyzed using GelPro Analyzer 4.0.
Colony formation assay
The A549 cells were treated with various
concentrations of CCL20 (10, 50 and 250 ng/ml) and PD98059 (20
µl/ml) (Cell Signaling Technology Biological Reagents Co., Ltd.,
Shanghai, China) at 37°C for 14 days. After macroscopically visible
clones appeared, the culture was terminated; the cells were rinsed
with PBS twice, fixed with methanol at 37°C for 15 min and
subjected to Giemsa staining (Giemsa stain, modified; cat. no.
GS1L-1L; Sigma-Aldrich; Merck KGaA, Darmstadt. Germany), performed
according to manufacturer's protocol. The number of colonies was
counted in 10 different fields under a CX41 light microscope
(magnification, ×100; Olympus Corporation).
Statistical analysis
Analyses were performed using SPSS 17.0 for Windows
(SPSS, Inc., Chicago, IL, USA). Data are expressed as percentages
or as the mean ± standard deviation. The mean differences of
continuous variables between groups were analyzed using an unpaired
Student's t-test. The differences of categorical variables between
groups were analyzed using the χ2 test. The mean
differences of continuous variables among groups were analyzed
using one-way analysis of variance, and the Newman-Keuls post hoc
test was used in the event of a significant F-ratio. All
significant tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of CCL20 and CCR6 in the
non-recurrence and recurrence groups of patients with lung
adenocarcinoma
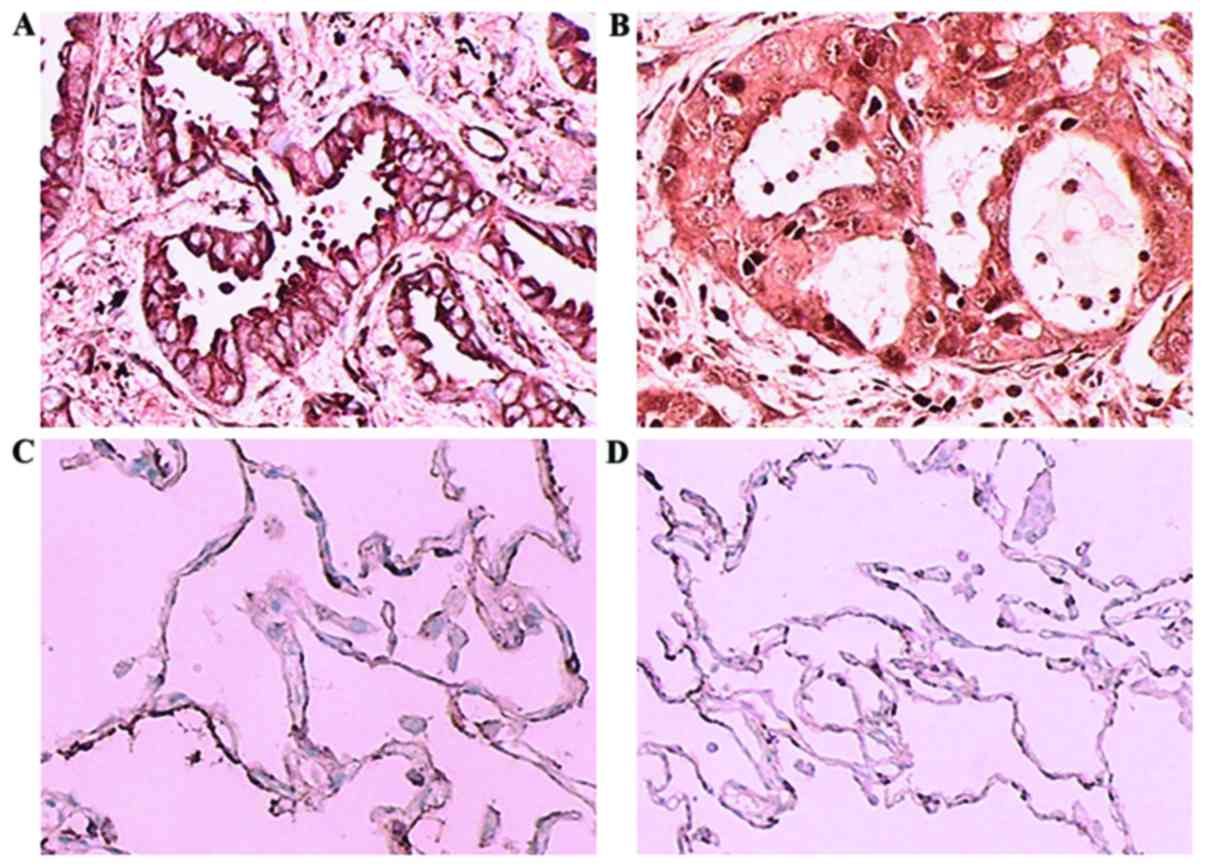
As shown in Fig. 1,
CCL20 was expressed in the cell membrane and cytoplasm, whereas
CCR6 was predominantly expressed in the cytoplasm of lung
adenocarcinoma tissues. In cancer-adjacent tissues, there was
scarcely any expression of CCL20 or CCR6. The percentages of
high-expression samples (with >50% of cells stained) in the
recurrence group were 76 and 66%, respectively, which were
significantly higher compared with those in non-recurrence group (8
and 6%; P<0.001). The sum of the staining indexes (total
immunostaining score) of CCL20 and CCR6 in the recurrence group
were 149.3 and 134.4, respectively, significantly higher than those
in non-recurrence group, which were 57.2 and 58.0, respectively
(P<0.001; Tables I–III).
 | Table I.CCL20 and CCR6 scores representing the
percentage of positively stained tumor cells in the non-recurrence
(n=112) and recurrence (n=50) groups. |
Table I.
CCL20 and CCR6 scores representing the
percentage of positively stained tumor cells in the non-recurrence
(n=112) and recurrence (n=50) groups.
|
| Non-recurrence group,
no. of patients (collective score) | Recurrence group, no.
of patients (collective score) |
|---|
|
|
|
|
|---|
| Percentage of
positive tumor cells (score) | CCL20 | CCR6 | CCL20 | CCR6 |
|---|
| 0–25% (0) | 81 (0) | 80 (0) | 9 (0) | 13 (0) |
| 26–50% (1) | 22 (22) | 25 (25) | 3 (3) | 4 (4) |
| 51–75% (2) | 5 (10) | 4 (8) | 8 (16) | 7 (14) |
| 76–100% (3) | 4 (12) | 3 (9) | 30 (90) | 26 (78) |
| Total | 112 (44) | 112 (42) | 50 (109) | 50 (96) |
 | Table III.Expression rate of CCL20 and CCR6 in
non-recurrence and recurrence groups. |
Table III.
Expression rate of CCL20 and CCR6 in
non-recurrence and recurrence groups.
| Group | High expression,
n | Low expression,
n | Total, n | High expression
ratio, % |
|---|
| Non-recurrence
group |
|
|
|
|
|
CCL20 | 9 | 103 | 112 | 8 |
|
CCR6 | 7 | 105 | 112 | 6 |
| Recurrence
group |
|
|
|
|
|
CCL20 | 38 | 12 | 50 | 76 |
|
CCR6 | 33 | 17 | 50 | 66 |
CCL20 and CCR6 mRNA and protein
expression levels are higher in patients with recurrent compared
with non-recurrent lung adenocarcinoma
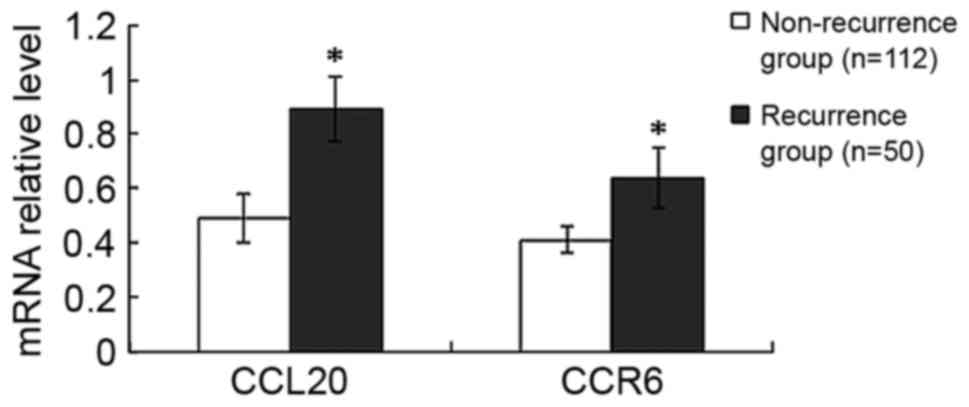
Compared with the non-recurrence group, the mRNA
expression levels of CCL20 and CCR6 were increased by 82%
(P<0.001) and 56% (P<0.001), respectively, in the recurrence
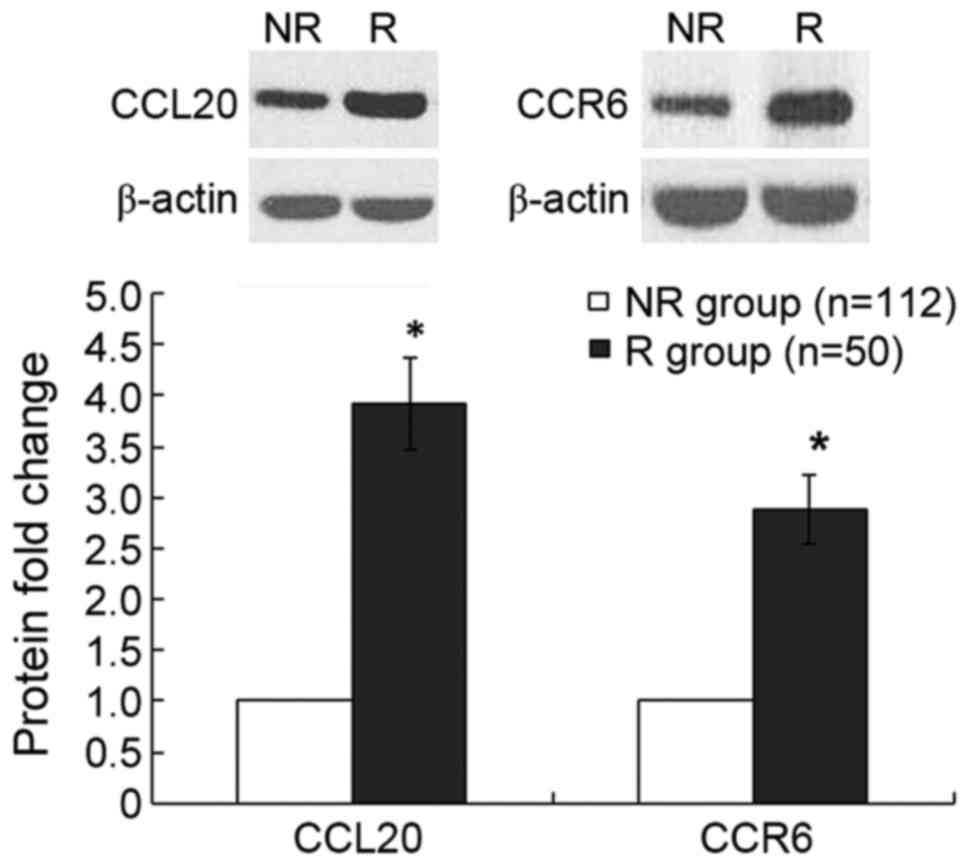
group (Fig. 2). Furthermore, compared
with the non-recurrence group, the relative protein expression
levels of CCL20 and CCR6, measured by western blotting, were
increased by 282 (P<0.001) and 188% (P<0.001), respectively,
in the recurrence group (Fig. 3).
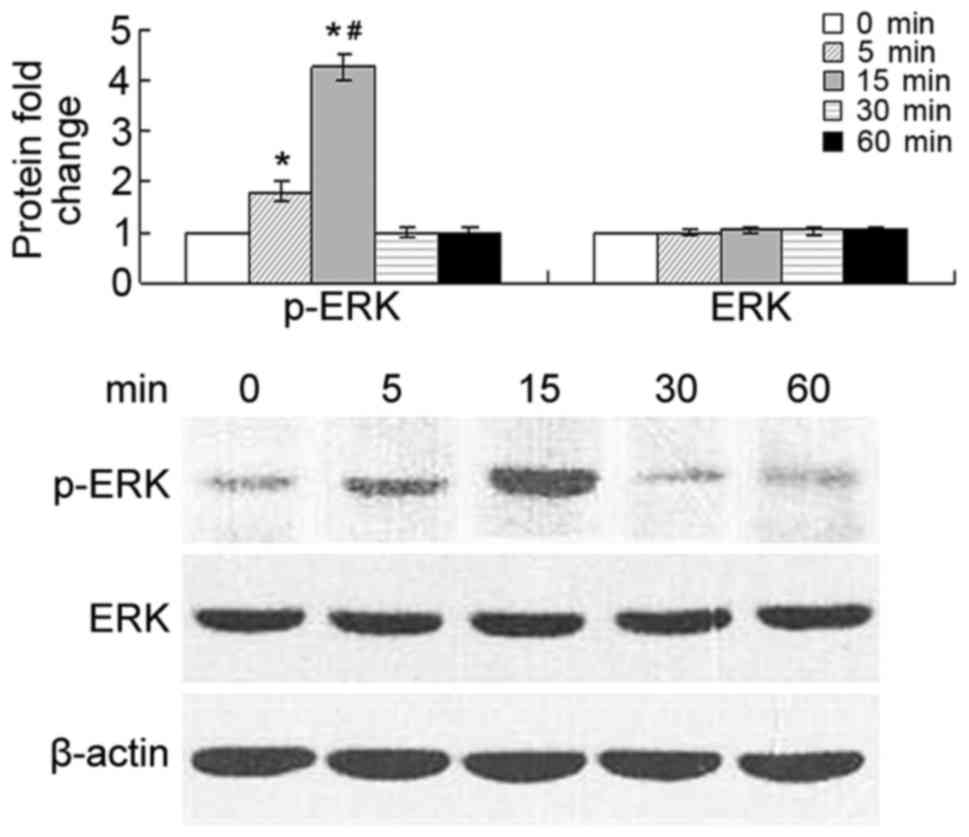
CCL20 induces ERK phosphorylation
Phosphorylation of ERK in tumor cells was detected
at 5 and 15 min after stimulation of the cells with 500 ng/ml
CCL20, while the level of overall ERK remained unaffected. The
level of phosphorylation was significantly increased at 5 and 15
min compared with that at 0 min (P<0.01), and markedly increased
at 15 min compared with that at 5 min (P<0.01). The p-ERK
protein level decreased at 30 and 60 min (Fig. 4).
CCL20 promotes A549 cell colony
formation, which is attenuated by treatment with an ERK inhibitor
(PD98059)
The colony formation was significantly increased
following stimulation with 50 or 250 ng/ml CCL20 compared with 0
and 10 ng/ml, in a dose-dependent manner (P<0.01; Table IV).
 | Table IV.Colony formation by A549 cells in
response to stimulation with increasing concentrations of CCL20
with or without the ERK inhibitor PD98059. |
Table IV.
Colony formation by A549 cells in
response to stimulation with increasing concentrations of CCL20
with or without the ERK inhibitor PD98059.
| CCL20
concentration, ng/ml | Without
PD98059 | With PD98059 | t | P-value |
|---|
| 0 | 160±15.2 | 149±13.4 | 1.5354 | 0.147 |
| 10 | 280±16.8 | 212±15.1 | 8.5146 | <0.001 |
| 50 | 325±17.5 | 240±13.9 | 10.7576 | <0.001 |
| 250 | 440±19.8 | 255±14.7 | 21.2187 | <0.001 |
In the absence of CCL20 stimulation, there was no
change in colony formation when cells were treated with specific
ERK inhibitor compared with untreated cells (P=0.147), indicating
no effect of PD98059 on the basal capacity of colony formation of
A549 cells. However, treatment with PD98059 significantly
attenuated the CCL20-induced increase in colony formation ability
(0 vs. 20 µl/ml PD98059, P<0.001 for all concentrations of
CCL20; Table IV).
Discussion
It is well-known that numerous types of cells,
including cancer cells and leukocytes, express chemokines and their
receptors (20–22). Tumor-derived chemokines perform an
important role in the characteristic recruitment of leukocytes,
including tumor-infiltrating lymphocytes, macrophages and dendritic
cells, to the tumor environment (8).
Chemokines and their receptors expressed by tumor cells enhance
tumor growth, recurrence and metastatic potential (6,7). This
suggests that CCL20/CCR6 may serve an important role in the
development of lung cancer.
The present study showed that CCL20 was expressed in
the cell membrane and cytoplasm of lung adenocarcinoma tissue,
while CCR6 was only expressed in the cytoplasm. CCL20 and CCR6
expression levels in the recurrence group were significantly higher
compared with those in the non-recurrence group. The mRNA and
protein expression levels of CCL20 and CCR6 were also significantly
increased in the recurrence group of lung adenocarcinoma tissue
compared with the non-recurrence group. The abnormal expression of
these molecules may lead to the occurrence and development of lung
adenocarcinoma. Consistent with this finding, Kleeff et al
(23) reported that, compared with
normal human pancreatic tissues, CCL20 transcription in pancreatic
ductal adenocarcinoma tissue is highly upregulated, as measured by
northern blotting. Furthermore, another study that utilized RT-qPCR
analysis and immunohistochemical staining demonstrated that CCL20
mRNA and protein were significantly overexpressed in breast
adenocarcinoma compared with peritumoral areas (24). Shimizu et al (25) found that CCL20 and CCR6 were strongly
expressed in chronically inflamed liver and hepatocellular
carcinoma. These previous findings suggested that CCL20 and CCR6
interactions in the tumor environment may have a pro-carcinogenic
function.
In the present study, the A549 cell line was
selected to identify the potential mechanisms of CCL20/CCR6
interactions and the ERK signaling pathway in lung adenocarcinoma
pathogenesis in vitro. Phosphorylation of ERK in tumor cells
was detected following stimulation with CCL20, but there was no
effect on the level of overall ERK. The colony formation ability of
the cells was stimulated by CCL20 in a dose-dependent manner, and
this effect was markedly attenuated by treatment with the ERK
inhibitor PD98059, without any change in the basal (unstimulated)
colony formation capacity of the cells treated with PD98059.
Therefore, the CCL20-induced increase in cancerous cell colony
formation was dependent, at least in part, on ERK phosphorylation
and signaling. A study by Kimsey et al (26) also showed that co-localization of
CCL20 and CCR6 promotes pancreatic cancer cell invasion into type
IV collagen. This finding continues to highlight the importance of
CCL20/CCR6 in the progression of pancreatic cancer. In colorectal
cancer, tumor cells express CCL20 and CCR6 in a non-polarized
manner, providing a basis for efficient autocrine and paracrine
loops, and CCR6 is upregulated in colorectal cancer compared with
normal colon mucosa (27). In another
study, CCL20/CCR6 was shown to promote the growth of colorectal
cancer cells through ERK phosphorylation (16). The present study demonstrated that
CCL20/CCR6 serves a crucial role in NSCLC development, at least
partially through the ERK signaling pathway.
However, the present study had several limitations,
including the relatively small sample size and the requirement for
validation studies in independent samples. Additional studies are
required to clarify how the production of CCL20 was potentiated by
cancerous cells.
In conclusion, the present study indicated that CCR6
and CCL20 may serve a role in lung adenocarcinoma, leading to
proliferation and migration via autocrine or paracrine mechanisms.
This effect is partially dependent on the ERK signaling pathway.
The disruption of CCL20/CCR6 interactions may be a promising
strategy in the treatment of cancer.
Acknowledgements
The authors would like to thank their colleagues in
the Department of Thoracic Surgery (Hebei General Hospital) for
collecting the samples and Dr Bing-jie Li (Hebei General Hospital)
for providing technical assistance.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spiro SG and Silvestri GA: One hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Radzikowska E, Głaz P and Roszkowski K:
Lung cancer in women: Age, smoking, histology, performance status,
stage, initial treatment and survival. Population-based study of 20
561 cases. Ann Oncol. 13:1087–1093. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy PM, Baggiolini M, Charo IF, Hébert
CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ and Power CA:
International union of pharmacology. XXII. Nomenclature for
chemokine receptors. Pharmacol Rev. 52:145–176. 2000.PubMed/NCBI
|
|
5
|
Mantovani A, Allavena P, Sozzani S, Vecchi
A, Locati M and Sica A: Chemokines in the recruitment and shaping
of the leukocyte infiltrate of tumors. Semin Cancer Biol.
14:155–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantovani A: Chemokines in neoplastic
progression. Semin Cancer Biol. 14:147–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beider K, Abraham M, Begin M, Wald H,
Weiss ID, Wald O, Pikarsky E, Abramovitch R, Zeira E, Galun E, et
al: Interaction between CXCR4 and CCL20 pathways regulates tumor
growth. PLoS One. 4:e51252009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schutyser E, Struyf S and Van Damme J: The
CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor
Rev. 14:409–426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bracke KR, Demedts IK, Joos GF and
Brusselle GG: CC-chemokine receptors in chronic obstructive
pulmonary disease. Inflamm Allergy Drug Targets. 6:75–79. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demedts IK, Bracke KR, Van Pottelberge G,
Testelmans D, Verleden GM, Vermassen FE, Joos GF and Brusselle GG:
Accumulation of dendritic cells and increased CCL20 levels in the
airways of patients with chronic obstructive pulmonary disease. Am
J Respir Crit Care Med. 175:998–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghadjar P, Rubie C, Aebersold DM and
Keilholz U: The chemokine CCL20 and its receptor CCR6 in human
malignancy with focus on colorectal cancer. Int J Cancer.
125:741–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YC, Hsiao YC, Chen YJ, Wei YY, Lai
TH and Tang CH: Stromal cell-derived factor-1 enhances motility and
integrin up-regulation through CXCR4, ERK and NF-kappaB-dependent
pathway in human lung cancer cells. Biochem Pharmacol.
74:1702–1712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganju RK, Brubaker SA, Meyer J, Dutt P,
Yang Y, Qin S, Newman W and Groopman JE: The alpha-chemokine,
stromal cell-derived factor-1alpha, binds to the transmembrane
G-protein-coupled CXCR-4 receptor and activates multiple signal
transduction pathways. J Biol Chem. 273:23169–23175. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
16
|
Brand S, Olszak T, Beigel F, Diebold J,
Otte JM, Eichhorst ST, Göke B and Dambacher J: Cell differentiation
dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt
signaling resulting in proliferation and migration of colorectal
cancer cells. J Cell Biochem. 97:709–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee W, Jiang Z, Liu J, Haverty PM, Guan Y,
Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, et al: The mutation
spectrum revealed by paired genome sequences from a lung cancer
patient. Nature. 465:473–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mountain CF: Revisions in the
International System for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Dleta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JM, Deng X, Gong W and Su S:
Chemokines and their role in tumor growth and metastasis. J Immunol
Methods. 220:1–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kleeff J, Kusama T, Rossi DL, Ishiwata T,
Maruyama H, Friess H, Büchler MW, Zlotnik A and Korc M: Detection
and localization of MIP-3alpha/LARC/Exodus, a macrophage
proinflammatory chemokine, and its CCR6 receptor in human
pancreatic cancer. Int J Cancer. 81:650–657. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bell D, Chomarat P, Broyles D, Netto G,
Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA and
Banchereau J: In breast carcinoma tissue, immature dendritic cells
reside within the tumor, whereas mature dendritic cells are located
in peritumoral areas. J Exp Med. 190:1417–1426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu Y, Murata H, Kashii Y, Hirano K,
Kunitani H, Higuchi K and Watanabe A: CC-chemokine receptor 6 and
its ligand macrophage inflammatory protein 3alpha might be involved
in the amplification of local necroinflammatory response in the
liver. Hepatology. 34:311–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimsey TF, Campbell AS, Albo D, Wilson M
and Wang TN: Co-localization of macrophage inflammatory
protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes
pancreatic cancer cell invasion. Cancer J. 10:374–380. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghadjar P, Coupland SE, Na IK, Noutsias M,
Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C and
Keilholz U: Chemokine receptor CCR6 expression level and liver
metastasis in colorectal cancer. J Clin Oncol. 24:1910–1916. 2006.
View Article : Google Scholar : PubMed/NCBI
|