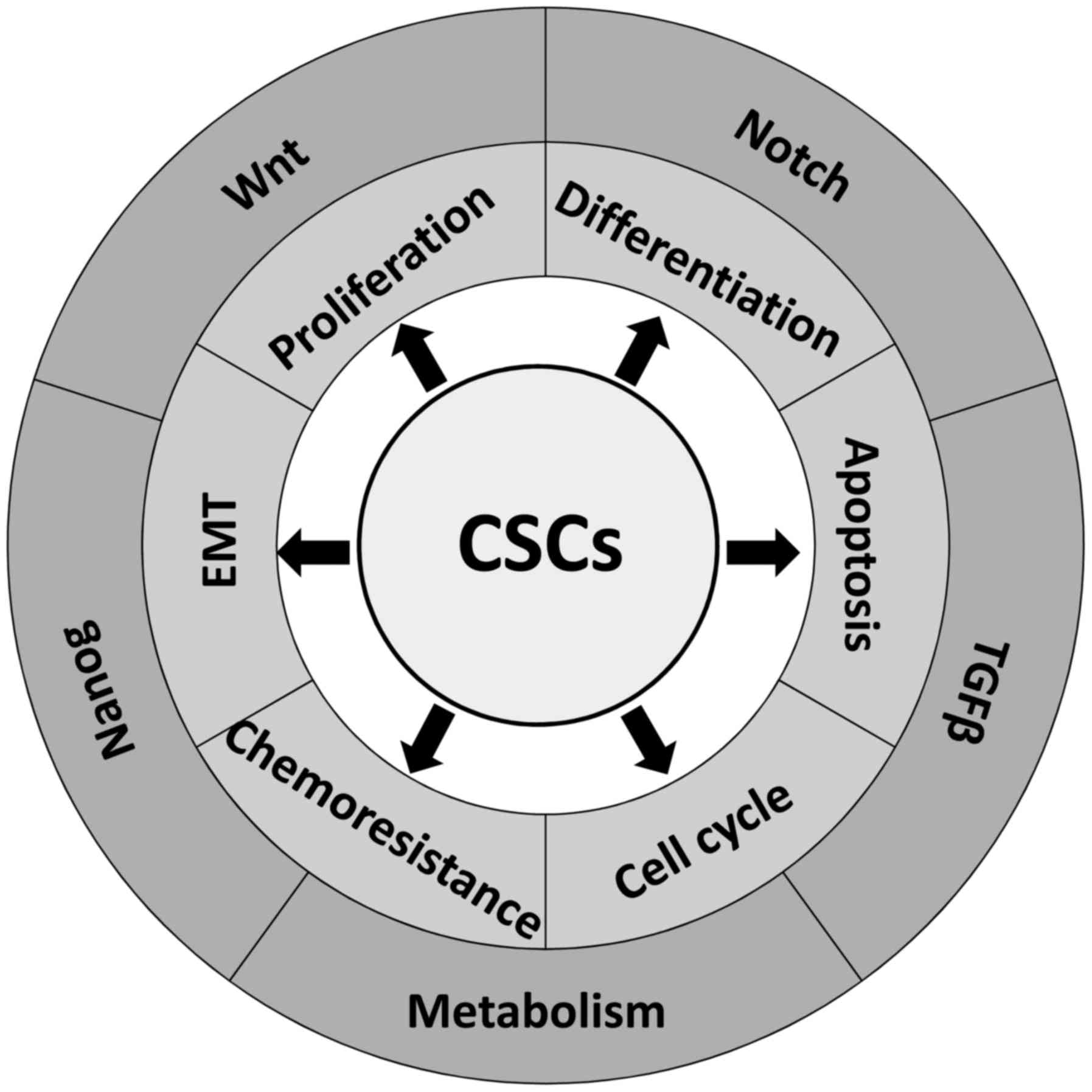
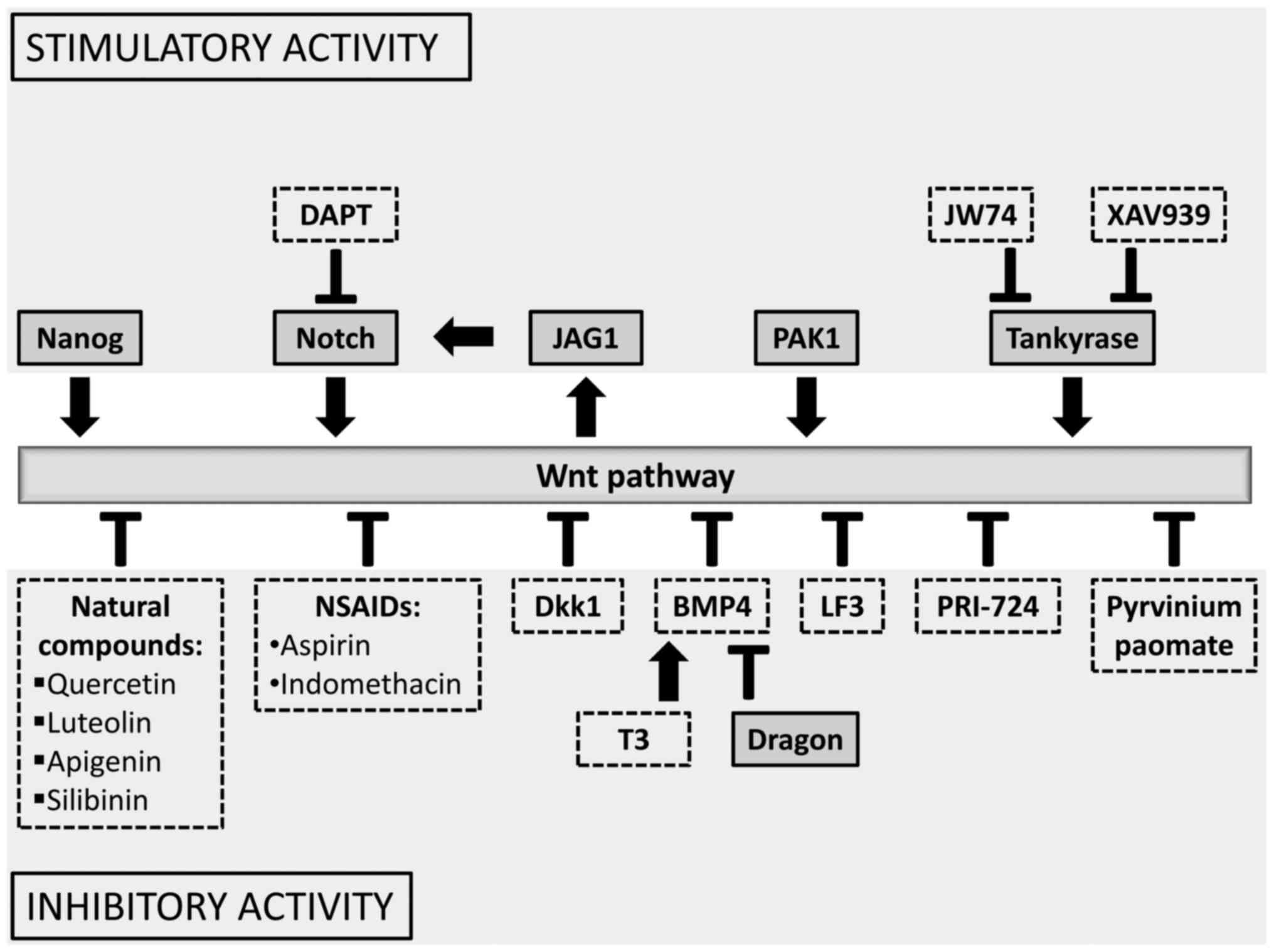
|
1
|
Amado NG, Predes D, Moreno MM, Carvalho
IO, Mendes FA and Abreu JG: Flavonoids and Wnt/β-catenin signaling:
Potential role in colorectal cancer therapies. Int J Mol Sci.
15:12094–12106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manhas J, Bhattacharya A, Agrawal SK,
Gupta B, Das P, Deo SV, Pal S and Sen S: Characterization of cancer
stem cells from different grades of human colorectal cancer. Tumour
Biol. 37:14069–14081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Franco S, Todaro M, Dieli F and Stassi
G: Colorectal cancer defeating? Mol Aspects Med. 39:61–81. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Cervantes A, Nordlinger B
and Arnold D; ESMO Guidelines Working Group, : Metastatic
colorectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 25 Suppl 3:iii1–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taieb J, Tabernero J, Mini E, Subtil F,
Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN,
Blons H, et al: Oxaliplatin, fluorouracil, and leucovorin with or
without cetuximab in patients with resected stage III colon cancer
(PETACC-8): An open-label, randomised phase 3 trial. Lancet Oncol.
15:862–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan K, Wale A, Brown G and Chau I:
Colorectal cancer with liver metastases: Neoadjuvant chemotherapy,
surgical resection first or palliation alone? World J
Gastroenterol. 20:12391–12406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paldino E, Tesori V, Casalbore P,
Gasbarrini A and Puglisi MA: Tumor initiating cells and
chemoresistance: Which is the best strategy to target colon cancer
stem cells? Biomed Res Int 2014. 8598712014.
|
|
11
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puglisi MA, Barba M, Corbi M, Errico MF,
Giorda E, Saulnier N, Boninsegna A, Piscaglia AC, Carsetti R,
Cittadini A, et al: Identification of Endothelin-1 and NR4A2 as
CD133-regulated genes in colon cancer cells. J Pathol. 225:305–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shmelkov SV, Butler JM, Hooper AT, Hormigo
A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et
al: CD133 expression is not restricted to stem cells, and both
CD133+ and CD133- metastatic colon cancer cells initiate tumors. J
Clin Invest. 118:2111–2120. 2008.PubMed/NCBI
|
|
15
|
Todaro M, Perez Alea M, Scopelliti A,
Medema JP and Stassi G: IL-4-mediated drug resistance in colon
cancer stem cells. Cell Cycle. 7:309–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang EH, Hynes MJ, Zhang T, Ginestier C,
Dontu G, Appelman H, Fields JZ, Wicha MS and Boman BM: Aldehyde
dehydrogenase 1 is a marker for normal and malignant human colonic
stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin SP, Lee YT, Yang SH, Miller SA, Chiou
SH, Hung MC and Hung SC: Colon cancer stem cells resist
antiangiogenesis therapy-induced apoptosis. Cancer Lett.
328:226–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z and Huang J: Intestinal stem
cells-types and markers. Cell Biol Int. 37:406–414. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P,
van Buren G II, Samuel S, Kim MP, Lim SJ and Ellis LM:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang EH and Wicha MS: Colon cancer stem
cells: Implications for prevention and therapy. Trends Mol Med.
14:503–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu ZQ, Zhang C, Wang H, Lao XY, Chai R,
Gao XH, Cao GW and Fu CG: Downregulation of ATP-binding cassette
subfamily C member 4 increases sensitivity to neoadjuvant
radiotherapy for locally advanced rectal carcinoma. Dis Colon
Rectum. 56:600–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozovska Z, Gabrisova V and Kucerova L:
Colon cancer: Cancer stem cells markers, drug resistance and
treatment. Biomed Pharmacother. 68:911–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:pp. 13427–13432. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta
B, Soong R and Salto-Tellez M: CD133 expression predicts for
non-response to chemotherapy in colorectal cancer. Mod Pathol.
23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du Y, Shi L, Wang T, Liu Z and Wang Z:
Nanog siRNA plus cisplatin may enhance the sensitivity of
chemotherapy in esophageal cancer. J Cancer Res Clin Oncol.
138:1759–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X,
Jin H, Cahuzac N, Mehrpour M, Lu Y and Chen Q: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berretta M, Alessandrini L, De Divitiis C,
Nasti G, Lleshi A, Di Francia R, Facchini G, Cavaliere C, Buonerba
C and Canzonieri V: Serum and tissue markers in colorectal cancer:
State of art. Crit Rev Oncol Hematol. 111:103–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Honoki K, Fujii H, Kubo A, Kido A, Mori T,
Tanaka Y and Tsujiuchi T: Possible involvement of stem-like
populations with elevated ALDH1 in sarcomas for chemotherapeutic
drug resistance. Oncol Rep. 24:501–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MP, Fleming JB, Wang H, Abbruzzese JL,
Choi W, Kopetz S, McConkey DJ, Evans DB and Gallick GE: ALDH
activity selectively defines an enhanced tumor-initiating cell
population relative to CD133 expression in human pancreatic
adenocarcinoma. PLoS One. 6:e206362011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koppaka V, Thompson DC, Chen Y, Ellermann
M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD and
Vasiliou V: Aldehyde dehydrogenase inhibitors: A comprehensive
review of the pharmacology, mechanism of action, substrate
specificity, and clinical application. Pharmacol Rev. 64:520–539.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu
Z, Stass SA and Jiang F: Aldehyde dehydrogenase 1 A1-positive cell
population is enriched in tumor-initiating cells and associated
with progression of bladder cancer. Cancer Epidemiol Biomarkers
Prev. 19:327–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Croker AK and Allan AL: Inhibition of
aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and
radiation resistance of stem-like ALDHhiCD44+ human breast cancer
cells. Breast Cancer Res Treat. 133:75–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nowak D, Stewart D and Koeffler HP:
Differentiation therapy of leukemia: 3 decades of development.
Blood. 113:3655–3665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung SS, Oliva B, Dwabe S and Vadgama JV:
Combination treatment with flavonoid morin and telomerase inhibitor
MST-312 reduces cancer stem cell traits by targeting STAT3 and
telomerase. Int J Oncol. 49:487–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martino-Echarri E, Henderson BR and
Brocardo MG: Targeting the DNA replication checkpoint by
pharmacologic inhibition of Chk1 kinase: A strategy to sensitize
APC mutant colon cancer cells to 5-fluorouracil chemotherapy.
Oncotarget. 5:9889–9900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pulito C, Donzelli S, Muti P, Puzzo L,
Strano S and Blandino G: microRNAs and cancer metabolism
reprogramming: The paradigm of metformin. Ann Transl Med.
2:582014.PubMed/NCBI
|
|
38
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zubeldia IG, Bleau AM, Redrado M, Serrano
D, Agliano A, Gil-Puig C, Vidal-Vanaclocha F, Lecanda J and Calvo
A: Epithelial to mesenchymal transition and cancer stem cell
phenotypes leading to liver metastasis are abrogated by the novel
TGFβ1-targeting peptides P17 and P144. Exp Cell Res. 319:12–22.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Sousa E, Melo F and Vermeulen L: Wnt
signaling in cancer stem cell biology. Cancers (Basel). 8:pii:
E602016. View Article : Google Scholar
|
|
43
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wierzbicki PM and Rybarczyk A: The Hippo
pathway in colorectal cancer. Folia Histochem Cytobiol. 53:105–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian H, Biehs B, Warming S, Leong KG,
Rangell L, Klein OD and de Sauvage FJ: A reserve stem cell
population in small intestine renders Lgr5-positive cells
dispensable. Nature. 478:255–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan KS, Chia LA, Li X, Ootani A, Su J, Lee
JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al: The intestinal
stem cell markers Bmi1 and Lgr5 identify two functionally distinct
populations. Proc Natl Acad Sci USA. 109:pp. 466–471. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim JS, Crooks H, Foxworth A and Waldman
T: Proof-of-principle: Oncogenic beta-catenin is a valid molecular
target for the development of pharmacological inhibitors. Mol
Cancer Ther. 1:1355–1359. 2002.PubMed/NCBI
|
|
48
|
Huynh N, Shulkes A, Baldwin G and He H:
Up-regulation of stem cell markers by P21-activated kinase 1
contributes to 5-fluorouracil resistance of colorectal cancer.
Cancer Biol Ther. 17:813–823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thorne CA, Hanson AJ, Schneider J, Tahinci
E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson
AG, et al: Small-molecule inhibition of Wnt signaling through
activation of casein kinase 1α. Nat Chem Biol. 6:829–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Riffell JL, Lord CJ and Ashworth A:
Tankyrase-targeted therapeutics: Expanding opportunities in the
PARP family. Nat Rev Drug Discov. 11:923–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Polakis P: Drugging Wnt signalling in
cancer. EMBO J. 31:2737–2746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lau T, Chan E, Callow M, Waaler J, Boggs
J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, et
al: A novel tankyrase small-molecule inhibitor suppresses APC
mutation-driven colorectal tumor growth. Cancer Res. 73:3132–3144.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong Y, Katavolos P, Nguyen T, Lau T,
Boggs J, Sambrone A, Kan D, Merchant M, Harstad E, Diaz D, et al:
Tankyrase inhibition causes reversible intestinal toxicity in mice
with a therapeutic index <1. Toxicol Pathol. 44:267–278. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lenz HJ and Kahn M: Safely targeting
cancer stem cells via selective catenin coactivator antagonism.
Cancer Sci. 105:1087–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fang L, Zhu Q, Neuenschwander M, Specker
E, Wulf-Goldenberg A, Weis WI, von Kries JP and Birchmeier W: A
small-molecule antagonist of the β-catenin/TCF4 interaction blocks
the self-renewal of cancer stem cells and suppresses tumorigenesis.
Cancer Res. 76:891–901. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Aguilera Ó, González-Sancho JM, Zazo S,
Rincón R, Fernández AF, Tapia O, Canals F, Morte B, Calvanese V,
Orgaz JL, et al: Nuclear DICKKOPF-1 as a biomarker of
chemoresistance and poor clinical outcome in colorectal cancer.
Oncotarget. 6:5903–5917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
González-Sancho JM, Aguilera O, García JM,
Pendás-Franco N, Peña C, Cal S, García de Herreros A, Bonilla F and
Muñoz A: The Wnt antagonist DICKKOPF-1 gene is a downstream target
of beta-catenin/TCF and is downregulated in human colon cancer.
Oncogene. 24:1098–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qi L, Sun B, Liu Z, Li H, Gao J and Leng
X: Dickkopf-1 inhibits epithelial-mesenchymal transition of colon
cancer cells and contributes to colon cancer suppression. Cancer
Sci. 103:828–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu Z, Sun B, Qi L, Li Y, Zhao X, Zhang D
and Zhang Y: Dickkopf-1 expression is down-regulated during the
colorectal adenoma-carcinoma sequence and correlates with reduced
microvessel density and VEGF expression. Histopathology.
67:158–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rodilla V, Villanueva A, Obrador-Hevia A,
Robert-Moreno A, Fernández-Majada V, Grilli A, López-Bigas N,
Bellora N, Albà MM, Torres F, et al: Jagged1 is the pathological
link between Wnt and Notch pathways in colorectal cancer. Proc Natl
Acad Sci USA. 106:pp. 6315–6320. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Atashpour S, Fouladdel S, Movahhed TK,
Barzegar E, Ghahremani MH, Ostad SN and Azizi E: Quercetin induces
cell cycle arrest and apoptosis in CD133(+) cancer stem cells of
human colorectal HT29 cancer cell line and enhances anticancer
effects of doxorubicin. Iran J Basic Med Sci. 18:635–643.
2015.PubMed/NCBI
|
|
67
|
Temraz S, Mukherji D and Shamseddine A:
Potential targets for colorectal cancer prevention. Int J Mol Sci.
14:17279–17303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Johnson JL, Rupasinghe SG, Stefani F,
Schuler MA and Gonzalez de Mejia E: Citrus flavonoids luteolin,
apigenin, and quercetin inhibit glycogen synthase kinase-3β
enzymatic activity by lowering the interaction energy within the
binding cavity. J Med Food. 14:325–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ning Y, Zhang W, Hanna DL, Yang D, Okazaki
S, Berger MD, Miyamoto Y, Suenaga M, Schirripa M, El-Khoueiry A and
Lenz HJ: Clinical relevance of EMT and stem-like gene expression in
circulating tumor cells of metastatic colorectal cancer patients.
Pharmacogenomics J. Aug 9–2016.(Epub ahead of print). View Article : Google Scholar
|
|
70
|
Rajamanickam S, Velmurugan B, Kaur M,
Singh RP and Agarwal R: Chemoprevention of intestinal tumorigenesis
in APCmin/+ mice by silibinin. Cancer Res. 70:2368–2378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kaur M, Velmurugan B, Tyagi A, Agarwal C,
Singh RP and Agarwal R: Silibinin suppresses growth of human
colorectal carcinoma SW480 cells in culture and xenograft through
down-regulation of beta-catenin-dependent signaling. Neoplasia.
12:415–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hoh C, Boocock D, Marczylo T, Singh R,
Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, et
al: Pilot study of oral silibinin, a putative chemopreventive
agent, in colorectal cancer patients: Silibinin levels in plasma,
colorectum, and liver and their pharmacodynamic consequences. Clin
Cancer Res. 12:2944–2950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kumar S, Raina K, Agarwal C and Agarwal R:
Silibinin strongly inhibits the growth kinetics of colon cancer
stem cell-enriched spheroids by modulating interleukin 4/6-mediated
survival signals. Oncotarget. 5:4972–4989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang ML, Chiou SH and Wu CW: Targeting
cancer stem cells: Emerging role of Nanog transcription factor.
Onco Targets Ther. 6:1207–1220. 2013.PubMed/NCBI
|
|
75
|
Ibrahim EE, Babaei-Jadidi R, Saadeddin A,
Spencer-Dene B, Hossaini S, Abuzinadah M, Li N, Fadhil W, Ilyas M,
Bonnet D and Nateri AS: Embryonic NANOG activity defines colorectal
cancer stem cells and modulates through AP1- and TCF-dependent
mechanisms. Stem Cells. 30:2076–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang J, Espinoza LA, Kinders RJ, Lawrence
SM, Pfister TD, Zhou M, Veenstra TD, Thorgeirsson SS and Jessup JM:
NANOG modulates stemness in human colorectal cancer. Oncogene.
32:4397–4405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee TK, Castilho A, Cheung VC, Tang KH, Ma
S and Ng IO: CD24(+) liver tumor-initiating cells drive
self-renewal and tumor initiation through STAT3-mediated NANOG
regulation. Cell Stem Cell. 9:50–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Niu CS, Li DX, Liu YH, Fu XM, Tang SF and
Li J: Expression of NANOG in human gliomas and its relationship
with undifferentiated glioma cells. Oncol Rep. 26:593–601.
2011.PubMed/NCBI
|
|
79
|
Zhou X, Zhou YP, Huang GR, Gong BL, Yang
B, Zhang DX, Hu P and Xu SR: Expression of the stem cell marker,
Nanog, in human endometrial adenocarcinoma. Int J Gynecol Pathol.
30:262–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jeter CR, Liu B, Liu X, Chen X, Liu C,
Calhoun-Davis T, Repass J, Zaehres H, Shen JJ and Tang DG: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zbinden M, Duquet A, Lorente-Trigos A,
Ngwabyt SN, Borges I and Ruiz i Altaba A: NANOG regulates glioma
stem cells and is essential in vivo acting in a cross-functional
network with GLI1 and p53. EMBO J. 29:2659–2674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Meng HM, Zheng P, Wang XY, Liu C, Sui HM,
Wu SJ, Zhou J, Ding YQ and Li J: Over-expression of Nanog predicts
tumor progression and poor prognosis in colorectal cancer. Cancer
Biol Ther. 9:295–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Özgül Özdemir RB, Özdemir AT, Oltulu F,
Kurt K, Yiğittürk G and Kirmaz C: A comparison of cancer stem cell
markers and nonclassical major histocompatibility complex antigens
in colorectal tumor and noncancerous tissues. Ann Diagn Pathol.
25:60–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Han J, Zhang F, Yu M, Zhao P, Ji W, Zhang
H, Wu B, Wang Y and Niu R: RNA interference-mediated silencing of
NANOG reduces cell proliferation and induces G0/G1 cell cycle
arrest in breast cancer cells. Cancer Lett. 321:80–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Choi SC, Choi JH, Park CY, Ahn CM, Hong SJ
and Lim DS: Nanog regulates molecules involved in stemness and cell
cycle-signaling pathway for maintenance of pluripotency of P19
embryonal carcinoma stem cells. J Cell Physiol. 227:3678–3692.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mattoo AR, Zhang J, Espinoza LA and Jessup
JM: Inhibition of NANOG/NANOGP8 downregulates MCL-1 in colorectal
cancer cells and enhances the therapeutic efficacy of BH3 mimetics.
Clin Cancer Res. 20:5446–5455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pan Q, Meng L, Ye J, Wei X, Shang Y, Tian
Y, He Y, Peng Z, Chen L, Chen W, et al: Transcriptional repression
of miR-200 family members by Nanog in colon cancer cells induces
epithelial-mesenchymal transition (EMT). Cancer Lett. 392:26–38.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Abetov D, Mustapova Z, Saliev T and
Bulanin D: Biomarkers and signaling pathways of colorectal cancer
stem cells. Tumour Biol. 36:1339–1353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yokobori T and Nishiyama M: TGF-β
signaling in gastrointestinal cancers: Progress in basic and
clinical research. J Clin Med. 6:pii: E112017. View Article : Google Scholar
|
|
92
|
Gil-Guerrero L, Dotor J, Huibregtse IL,
Casares N, López-Vázquez AB, Rudilla F, Riezu-Boj JI,
López-Sagaseta J, Hermida J, Van Deventer S, et al: In vitro and in
vivo down-regulation of regulatory T cell activity with a peptide
inhibitor of TGF-beta1. J Immunol. 181:126–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Llopiz D, Dotor J, Casares N, Bezunartea
J, Díaz-Valdés N, Ruiz M, Aranda F, Berraondo P, Prieto J, Lasarte
JJ, et al: Peptide inhibitors of transforming growth factor-beta
enhance the efficacy of antitumor immunotherapy. Int J Cancer.
125:2614–2623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gonzalez-Zubeldia I, Dotor J, Redrado M,
Bleau AM, Manrique I, de Aberasturi AL, Villalba M and Calvo A:
Co-migration of colon cancer cells and CAFs induced by TGFβ1
enhances liver metastasis. Cell Tissue Res. 359:829–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhan J, Yang M, Zhang J, Guo Y, Liu W and
Zhang H: Kindler syndrome protein Kindlin-1 is mainly expressed in
adult tissues originating from ectoderm/endoderm. Sci China Life
Sci. 58:432–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kong J, Du J, Wang Y, Yang M, Gao J, Wei
X, Fang W, Zhan J and Zhang H: Focal adhesion molecule Kindlin-1
mediates activation of TGF-β signaling by interacting with TGF-βRI,
SARA and Smad3 in colorectal cancer cells. Oncotarget.
7:76224–76237. 2016.PubMed/NCBI
|
|
97
|
Karagiannis GS, Afaloniati H, Karamanavi
E, Poutahidis T and Angelopoulou K: BMP pathway suppression is an
early event in inflammation-driven colon neoplasmatogenesis of
uPA-deficient mice. Tumour Biol. 37:2243–2255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kim JS, Crooks H, Dracheva T, Nishanian
TG, Singh B, Jen J and Waldman T: Oncogenic beta-catenin is
required for bone morphogenetic protein 4 expression in human
cancer cells. Cancer Res. 62:2744–2748. 2002.PubMed/NCBI
|
|
99
|
Lubbe SJ, Pittman AM, Matijssen C, Twiss
P, Olver B, Lloyd A, Qureshi M, Brown N, Nye E, Stamp G, et al:
Evaluation of germline BMP4 mutation as a cause of colorectal
cancer. Hum Mutat. 32:E1928–E1938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kosinski C, Li VS, Chan AS, Zhang J, Ho C,
Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al: Gene
expression patterns of human colon tops and basal crypts and BMP
antagonists as intestinal stem cell niche factors. Proc Natl Acad
Sci USA. 104:pp. 15418–15423. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jin X, Chen Z, Xiang L, Luo Q, Guo Z, Ding
X and Jin X: Colorectal polyp model established by transplacental
BMP4 RNAi. Mol Med Rep. 10:33–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dou J and Gu N: Emerging strategies for
the identification and targeting of cancer stem cells. Tumour Biol.
31:243–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lombardo Y, Scopelliti A, Cammareri P,
Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R
and Stassi G: Bone morphogenetic protein 4 induces differentiation
of colorectal cancer stem cells and increases their response to
chemotherapy in mice. Gastroenterology. 140:297–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhou L, Xie J, Gu EL, Huang Y, Qu Y, Xu
AP, Zhu Y and Wang H: Common genetic variant on BMP4 contributes to
colorectal adenoma and cancer: A meta-analysis based on 15 studies.
Cytokine. 72:154–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shi Y, Chen GB, Huang XX, Xiao CX, Wang
HH, Li YS, Zhang JF, Li S, Xia Y, Ren JL and Guleng B: Dragon
(repulsive guidance molecule b, RGMb) is a novel gene that promotes
colorectal cancer growth. Oncotarget. 6:20540–20554. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Halbrooks PJ, Ding R, Wozney JM and Bain
G: Role of RGM coreceptors in bone morphogenetic protein signaling.
J Mol Signal. 2:42007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Catalano V, Dentice M, Ambrosio R, Luongo
C, Carollo R, Benfante A, Todaro M, Stassi G and Salvatore D:
Activated thyroid hormone promotes differentiation and
chemotherapeutic sensitization of colorectal cancer stem cells by
regulating Wnt and BMP4 signaling. Cancer Res. 76:1237–1244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Miyamoto S and Rosenberg DW: Role of Notch
signaling in colon homeostasis and carcinogenesis. Cancer Sci.
102:1938–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sikandar SS, Pate KT, Anderson S, Dizon D,
Edwards RA, Waterman ML and Lipkin SM: NOTCH signaling is required
for formation and self-renewal of tumor-initiating cells and for
repression of secretory cell differentiation in colon cancer.
Cancer Res. 70:1469–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Reedijk M, Odorcic S, Zhang H, Chetty R,
Tennert C, Dickson BC, Lockwood G, Gallinger S and Egan SE:
Activation of Notch signaling in human colon adenocarcinoma. Int J
Oncol. 33:1223–1229. 2008.PubMed/NCBI
|
|
111
|
Fre S, Pallavi SK, Huyghe M, Laé M,
Janssen KP, Robine S, Artavanis-Tsakonas S and Louvard D: Notch and
Wnt signals cooperatively control cell proliferation and
tumorigenesis in the intestine. Proc Natl Acad Sci USA. 106:pp.
6309–6314. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hoey T, Yen WC, Axelrod F, Basi J,
Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et
al: DLL4 blockade inhibits tumor growth and reduces
tumor-initiating cell frequency. Cell Stem Cell. 5:168–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu Z, Fan F, Wang A, Zheng S and Lu Y:
Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer
Res Clin Oncol. 140:525–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yan M, Callahan CA, Beyer JC, Allamneni
KP, Zhang G, Ridgway JB, Niessen K and Plowman GD: Chronic DLL4
blockade induces vascular neoplasms. Nature. 463:E6–E7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fischer M, Yen WC, Kapoun AM, Wang M,
O'Young G, Lewicki J, Gurney A and Hoey T: Anti-DLL4 inhibits
growth and reduces tumor-initiating cell frequency in colorectal
tumors with oncogenic KRAS mutations. Cancer Res. 71:1520–1525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He P, Liang J, Shao T, Guo Y, Hou Y and Li
Y: HDAC5 promotes colorectal cancer cell proliferation by
up-regulating DLL4 expression. Int J Clin Exp Med. 8:6510–6516.
2015.PubMed/NCBI
|
|
117
|
van Es JH, Sato T, van de Wetering M,
Lyubimova A, Gregorieff A, Zeinstra L, van den Born M, Korving J,
Martens ACM, van den Oudenaarden A and Clevers H: Dll1+ secretory
progenitor cells revert to stem cells upon crypt damage. Nat Cell
Biol. 14:1099–1104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fre S, Huyghe M, Mourikis P, Robine S,
Louvard D and Artavanis-Tsakonas S: Notch signals control the fate
of immature progenitor cells in the intestine. Nature. 435:964–968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Grochowski CM, Loomes KM and Spinner NB:
Jagged1 (JAG1): Structure, expression, and disease associations.
Gene. 576:381–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
van Es JH, van Gijn ME, Riccio O, van den
Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ,
Radtke F and Clevers H: Notch/gamma-secretase inhibition turns
proliferative cells in intestinal crypts and adenomas into goblet
cells. Nature. 435:959–963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Guilmeau S, Flandez M, Mariadason JM and
Augenlicht LH: Heterogeneity of Jagged1 expression in human and
mouse intestinal tumors: Implications for targeting Notch
signaling. Oncogene. 29:992–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lu J, Ye X, Fan F, Xia L, Bhattacharya R,
Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, et al:
Endothelial cells promote the colorectal cancer stem cell phenotype
through a soluble form of Jagged-1. Cancer Cell. 23:171–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang R, Wang G, Song Y, Tang Q, You Q,
Liu Z, Chen Y, Zhang Q, Li J, Muhammand S and Wang X: Colorectal
cancer stem cell and chemoresistant colorectal cancer cell
phenotypes and increased sensitivity to Notch pathway inhibitor.
Mol Med Rep. 12:2417–2424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Meng RD, Shelton CC, Li YM, Qin LX,
Notterman D, Paty PB and Schwartz GK: Gamma-secretase inhibitors
abrogate oxaliplatin-induced activation of the Notch-1 signaling
pathway in colon cancer cells resulting in enhanced
chemosensitivity. Cancer Res. 69:573–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Akao Y, Noguchi S, Iio A, Kojima K, Takagi
T and Naoe T: Dysregulation of microRNA-34a expression causes
drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer
Lett. 300:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Horio Y, Hayashi T, Kuno A and Kunimoto R:
Cellular and molecular effects of sirtuins in health and disease.
Clin Sci (Lond). 121:191–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zu G, Ji A, Zhou T and Che N:
Clinicopathological significance of SIRT1 expression in colorectal
cancer: A systematic review and meta analysis. Int J Surg.
26:32–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:pp. 13421–13426. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Stiegelbauer V, Perakis S, Deutsch A, Ling
H, Gerger A and Pichler M: MicroRNAs as novel predictive biomarkers
and therapeutic targets in colorectal cancer. World J
Gastroenterol. 20:11727–11735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen X, Sun K, Jiao S, Cai N, Zhao X, Zou
H, Xie Y, Wang Z, Zhong M and Wei L: High levels of SIRT1
expression enhance tumorigenesis and associate with a poor
prognosis of colorectal carcinoma patients. Sci Rep. 4:74812014.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gentric G, Mieulet V and Mechta-Grigoriou
F: Heterogeneity in cancer metabolism: New concepts in an old
field. Antioxid Redox Signal. 26:462–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Stubbs M and Griffiths JR: The altered
metabolism of tumors: HIF-1 and its role in the Warburg effect. Adv
Enzyme Regul. 50:44–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Park SH, Lee DH, Kim JL, Kim BR, Na YJ, Jo
MJ, Jeong YA, Lee SY, Lee SI, Lee YY and Oh SC: Metformin enhances
TRAIL-induced apoptosis by Mcl-1 degradation via Mule in colorectal
cancer cells. Oncotarget. 7:59503–59518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Menendez JA, Joven J, Cufi S,
Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Martin-Castillo B,
López-Bonet E, Alarcón T and Vazquez-Martin A: The Warburg effect
version 2.0: Metabolic reprogramming of cancer stem cells. Cell
Cycle. 12:1166–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ben Sahra I, Laurent K, Giuliano S,
Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y,
Giorgetti-Peraldi S, Cormont M, Bertolotto C, et al: Targeting
cancer cell metabolism: The combination of metformin and
2-deoxyglucose induces p53-dependent apoptosis in prostate cancer
cells. Cancer Res. 70:2465–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: A new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Miranda VC, Braghiroli MI, Faria LD,
Bariani G, Alex A, Bezerra Neto JE, Capareli FC, Sabbaga J, Lobo
Dos Santos JF, Hoff PM and Riechelmann RP: Phase 2 trial of
metformin combined with 5-fluorouracil in patients with refractory
metastatic colorectal cancer. Clin Colorectal Cancer.
15:321–328.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhang HH and Guo XL: Combinational
strategies of metformin and chemotherapy in cancers. Cancer
Chemother Pharmacol. 78:13–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hosono K, Endo H, Takahashi H, Sugiyama M,
Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, et al:
Metformin suppresses colorectal aberrant crypt foci in a short-term
clinical trial. Cancer Prev Res (Phila). 3:1077–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang Y, Guan M, Zheng Z, Zhang Q, Gao F
and Xue Y: Effects of metformin on CD133+ colorectal cancer cells
in diabetic patients. PLoS One. 8:e812642013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Montales MT, Simmen RC, Ferreira ES, Neves
VA and Simmen FA: Metformin and soybean-derived bioactive molecules
attenuate the expansion of stem cell-like epithelial subpopulation
and confer apoptotic sensitivity in human colon cancer cells. Genes
Nutr. 10:492015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhou XZ, Xue YM, Zhu B and Sha JP: Effects
of metformin on proliferation of human colon carcinoma cell line
SW-480. Nan Fang Yi Ke Da Xue Xue Bao. 30:1935–1938. 1942.2010 (In
Chinese).
|
|
144
|
Nie Z, Zhu H and Gu M: Reduced colorectal
cancer incidence in type 2 diabetic patients treated with
metformin: A meta-analysis. Pharm Biol. 54:2636–2642. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sehdev A, Shih YC, Vekhter B, Bissonnette
MB, Olopade OI and Polite BN: Metformin for primary colorectal
cancer prevention in patients with diabetes: A case-control study
in a US population. Cancer. 121:1071–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Cho YH, Ko BM, Kim SH, Myung YS, Choi JH,
Han JP, Hong SJ, Jeon SR, Kim HG, Kim JO and Lee MS: Does metformin
affect the incidence of colonic polyps and adenomas in patients
with type 2 diabetes mellitus? Intest Res. 12:139–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lonardo E, Cioffi M, Sancho P, Crusz S and
Heeschen C: Studying pancreatic cancer stem cell characteristics
for developing new treatment strategies. J Vis Exp.
e528012015.PubMed/NCBI
|
|
148
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hirsch HA, Iliopoulos D and Struhl K:
Metformin inhibits the inflammatory response associated with
cellular transformation and cancer stem cell growth. Proc Natl Acad
Sci USA. 110:pp. 972–977. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Vazquez-Martin A, Oliveras-Ferraros C,
Cufi S, Del Barco S, Martin-Castillo B and Menendez JA: Metformin
regulates breast cancer stem cell ontogeny by transcriptional
regulation of the epithelial-mesenchymal transition (EMT) status.
Cell Cycle. 9:3807–3814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Sui X, Xu Y, Yang J, Fang Y, Lou H, Han W,
Zhang M, Chen W, Wang K, Li D, et al: Use of metformin alone is not
associated with survival outcomes of colorectal cancer cell but
AMPK activator AICAR sensitizes anticancer effect of 5-fluorouracil
through AMPK activation. PLoS One. 9:e977812014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Burn J and Sheth H: The role of aspirin in
preventing colorectal cancer. Br Med Bull. 119:17–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich
CM, White E, Baron JA, Berndt SI, Brenner H, Butterbach K, et al:
Association of aspirin and NSAID use with risk of colorectal cancer
according to genetic variants. JAMA. 313:1133–1142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Sabatino L, Pancione M, Votino C,
Colangelo T, Lupo A, Novellino E, Lavecchia A and Colantuoni V:
Emerging role of the β-catenin-PPARγ axis in the pathogenesis of
colorectal cancer. World J Gastroenterol. 20:7137–7151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Dihlmann S, Siermann A and von Knebel
Doeberitz M: The nonsteroidal anti-inflammatory drugs aspirin and
indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene.
20:645–653. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hawcroft G, D'Amico M, Albanese C, Markham
AF, Pestell RG and Hull MA: Indomethacin induces differential
expression of beta-catenin, gamma-catenin and T-cell factor target
genes in human colorectal cancer cells. Carcinogenesis. 23:107–114.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Alberts SR, Sargent DJ, Nair S, Mahoney
MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S,
et al: Effect of oxaliplatin, fluorouracil, and leucovorin with or
without cetuximab on survival among patients with resected stage
III colon cancer: A randomized trial. JAMA. 307:1383–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Cao R, Zhang S, Ma D and Hu L: A
multi-center randomized phase II clinical study of bevacizumab plus
irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with
FOLFIRI alone as second-line treatment for Chinese patients with
metastatic colorectal cancer. Med Oncol. 32:3252015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yamada Y, Takahari D, Matsumoto H, Baba H,
Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y,
et al: Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab
versus S-1 and oxaliplatin plus bevacizumab in patients with
metastatic colorectal cancer (SOFT): An open-label,
non-inferiority, randomised phase 3 trial. Lancet Oncol.
14:1278–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Schmoll HJ, Tabernero J, Maroun J, de
Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K and
Haller DG: Capecitabine plus oxaliplatin compared with
fluorouracil/folinic acid as adjuvant therapy for stage iii colon
cancer: Final results of the NO16968 randomized controlled phase
III trial. J Clin Oncol. 33:3733–3740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Élez E, Kocáková I, Höhler T, Martens UM,
Bokemeyer C, Van Cutsem E, Melichar B, Smakal M, Csőszi T, Topuzov
E, et al: Abituzumab combined with cetuximab plus irinotecan versus
cetuximab plus irinotecan alone for patients with KRAS wild-type
metastatic colorectal cancer: The randomised phase I/II POSEIDON
trial. Ann Oncol. 26:132–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sclafani F, Kim TY, Cunningham D, Kim TW,
Tabernero J, Schmoll HJ, Roh JK, Kim SY, Park YS, Guren TK, et al:
A randomized phase II/III study of dalotuzumab in combination with
cetuximab and irinotecan in chemorefractory, KRAS wild-type,
metastatic colorectal cancer. J Natl Cancer Inst. 107:djv2582015.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Fujita K, Kubota Y, Ishida H and Sasaki Y:
Irinotecan, a key chemotherapeutic drug for metastatic colorectal
cancer. World J Gastroenterol. 21:12234–12248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
de Gramont A, Van Cutsem E, Schmoll HJ,
Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht
JR, Rivera F, et al: Bevacizumab plus oxaliplatin-based
chemotherapy as adjuvant treatment for colon cancer (AVANT): A
phase 3 randomised controlled trial. Lancet Oncol. 13:1225–1233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Bayoglu IV, Yildiz I, Varol U, Cokmert S,
Alacacıoğlu A, Kucukzeybek Y, Akyol M, Demir L, Dirican A and
Tarhan O: Uracil/tegafur as a possible salvage therapy in
chemo-refractory colorectal cancer patients: A single institutional
retrospective study. Contemp Oncol (Pozn). 19:385–390.
2015.PubMed/NCBI
|
|
168
|
Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC,
Zhu DX and Xu JM: Anti-EGFR and anti-VEGF agents: Important
targeted therapies of colorectal liver metastases. World J
Gastroenterol. 20:4263–4275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Strickler JH and Hurwitz HI:
Bevacizumab-based therapies in the first-line treatment of
metastatic colorectal cancer. Oncologist. 17:513–524. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Roviello G, Bachelot T, Hudis CA,
Curigliano G, Reynolds AR, Petrioli R and Generali D: The role of
bevacizumab in solid tumours: A literature based meta-analysis of
randomised trials. Eur J Cancer. 75:245–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Huang J, Nair SG, Mahoney MR, Nelson GD,
Shields AF, Chan E, Goldberg RM, Gill S, Kahlenberg MS, Quesenberry
JT, et al: Comparison of FOLFIRI with or without cetuximab in
patients with resected stage III colon cancer; NCCTG (Alliance)
intergroup trial N0147. Clin Colorectal Cancer. 13:100–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Terazawa T, Nishitani H, Kato K, Hashimoto
H, Akiyoshi K, Ito Y, Nakamoto A, Iwasa S, Nakajima TE, Hamaguchi
T, et al: Phase II study of cetuximab with irinotecan for KRAS
wild-type colorectal cancer in Japanese patients. Asia Pac J Clin
Oncol. 13:e132–e137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Tay RY, Wong R and Hawkes EA: Treatment of
metastatic colorectal cancer: Focus on panitumumab. Cancer Manag
Res. 7:189–198. 2015.PubMed/NCBI
|
|
174
|
Bahrami A, Hassanian SM, ShahidSales S,
Farjami Z, Hasanzadeh M, Anvari K, Aledavood A, Maftouh M, Ferns
GA, Khazaei M and Avan A: Targeting RAS signaling pathway as a
potential therapeutic target in the treatment of colorectal cancer.
J Cell Physiol. Mar 6–2017.(Epub ahead of print). View Article : Google Scholar
|
|
175
|
Françoso A and Simioni PU: Immunotherapy
for the treatment of colorectal tumors: Focus on approved and
in-clinical-trial monoclonal antibodies. Drug Des Devel Ther.
11:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ursem C, Van Loon K and Venook A: Adjuvant
therapy trials. Cancer J. 22:196–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Botchkina G: Colon cancer stem cells-from
basic to clinical application. Cancer Lett. 338:127–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Haraguchi N, Ohkuma M, Sakashita H,
Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H and Mori M:
CD133+CD44+ population efficiently enriches colon cancer initiating
cells. Ann Surg Oncol. 15:2927–2933. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zhu L, Gibson P, Currle DS, Tong Y,
Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW
and Gilbertson RJ: Prominin 1 marks intestinal stem cells that are
susceptible to neoplastic transformation. Nature. 457:603–607.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Botchkina IL, Rowehl RA, Rivadeneira DE,
Karpeh MS Jr, Crawford H, Dufour A, Ju J, Wang Y, Leyfman Y and
Botchkina GI: Phenotypic subpopulations of metastatic colon cancer
stem cells: Genomic analysis. Cancer Genomics Proteomics. 6:19–29.
2009.PubMed/NCBI
|
|
181
|
Zhou F, Mu YD, Liang J, Liu ZX, Zhou D,
Ning WL, Li YZ, Ding D and Zhang JF: Aldehyde dehydrogenase 1: A
specific cancer stem cell marker for human colorectal carcinoma.
Mol Med Rep. 11:3894–3899. 2015. View Article : Google Scholar : PubMed/NCBI
|