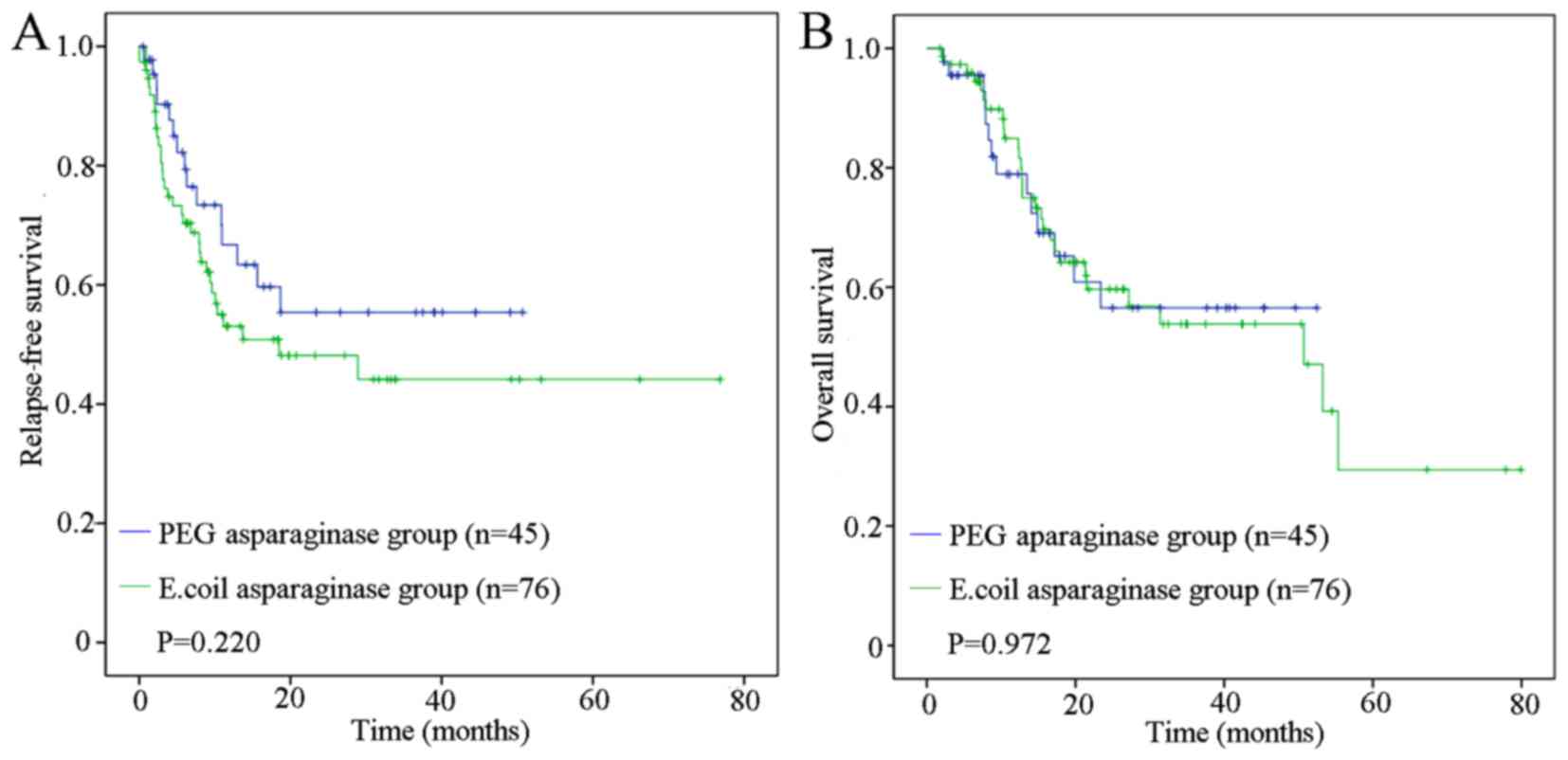
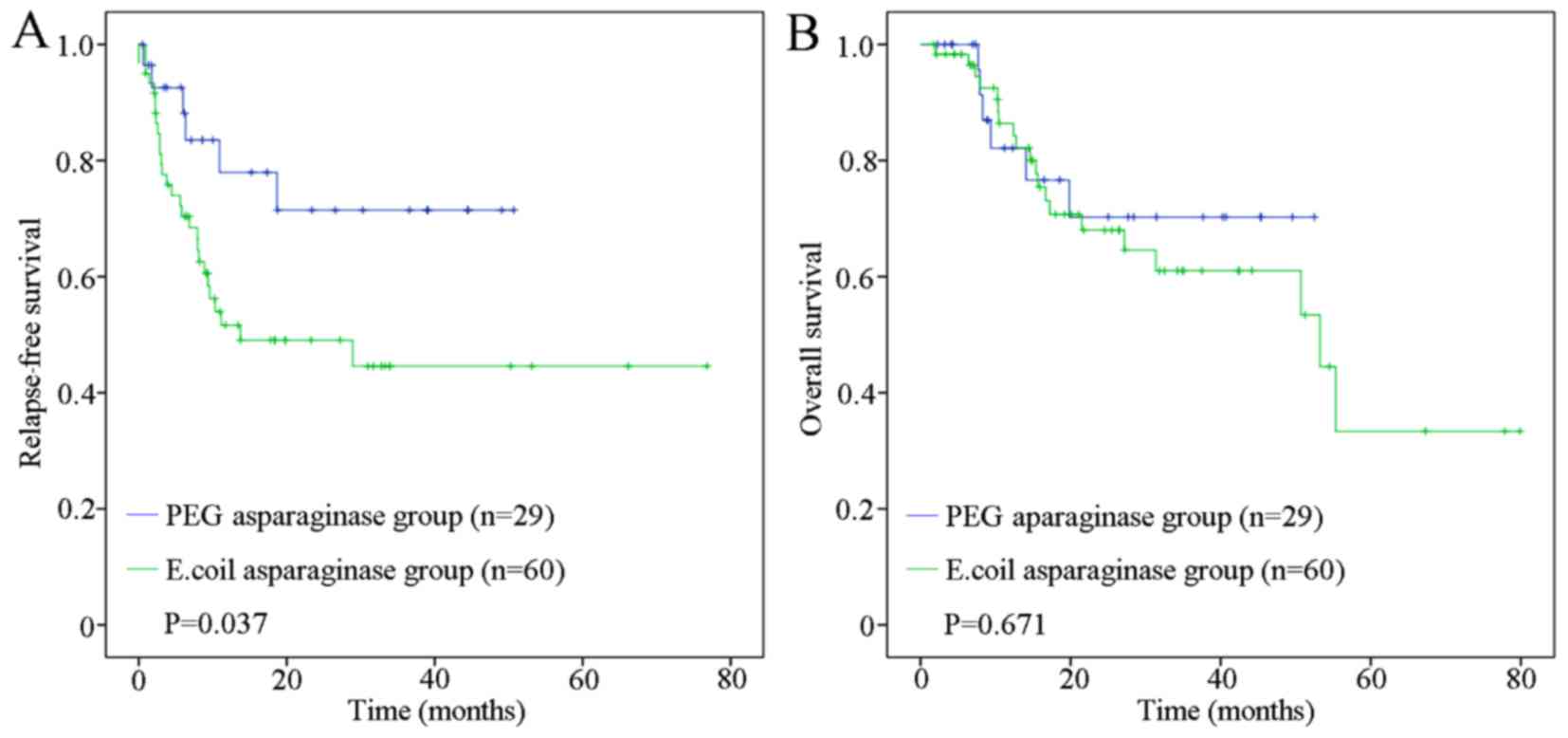
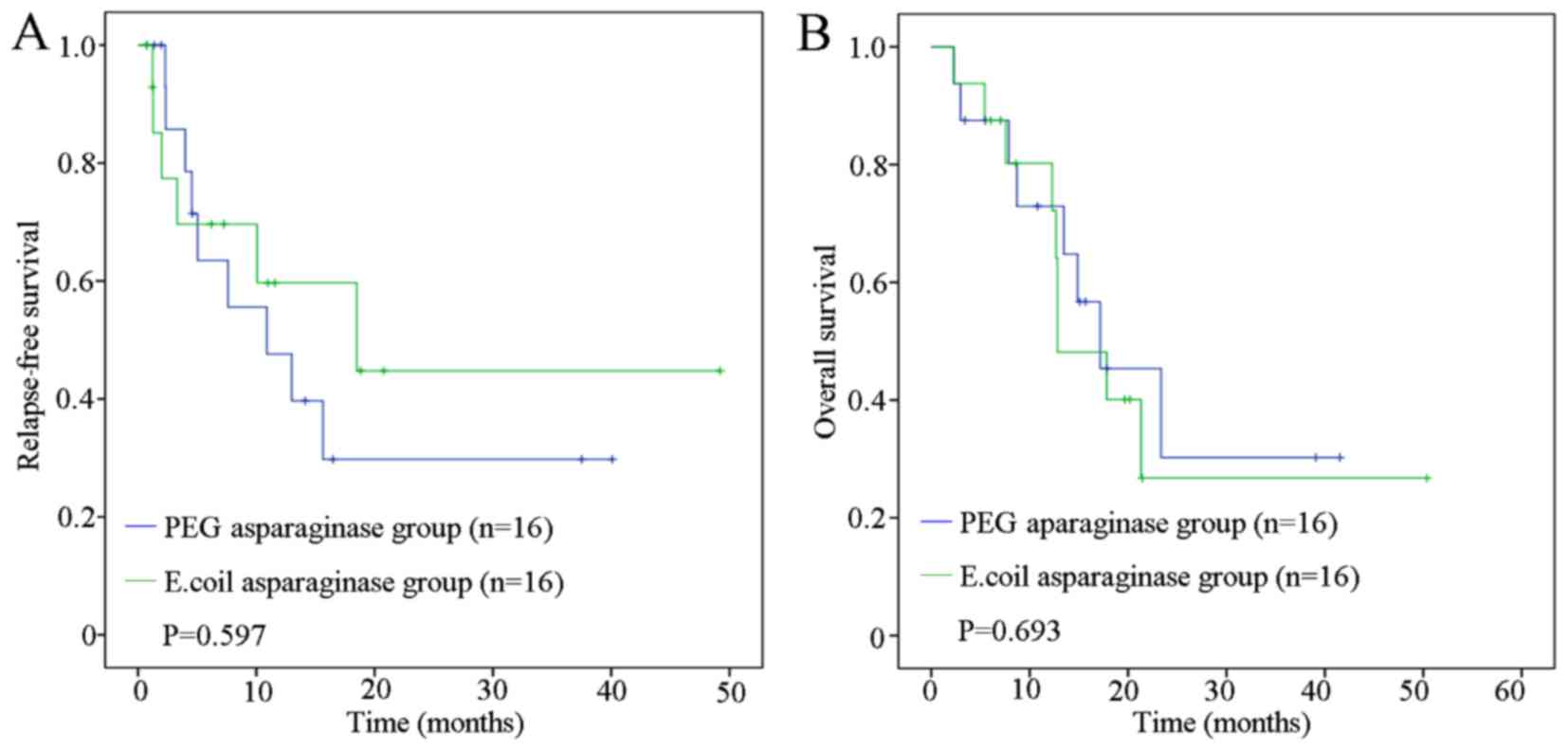
|
1
|
Kawedia JD and Rytting ME: Asparaginase in
Acute Lymphoblastic Leukemia. Clin Lymph Myelom Leukem. 14
Suppl:S14–S17. 2014. View Article : Google Scholar
|
|
2
|
Shrivastava A, Khan AA, Khurshid M, Kalam
MA, Jain SK and Singhal PK: Recent developments in L-asparaginase
discovery and its potential as anticancer agent. Crit Rev Oncol
Hematol. 100:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marinescu C, Vlădăreanu AM and Mihai F:
Acute lymphocytic leukemia in adults. Pathologic features and
prognosis. Rom J Intern Med. 53:31–36. 2015.PubMed/NCBI
|
|
4
|
Cooper SL and Brown PA: Treatment of
pediatric acute lymphoblastic leukemia. Pediatr Clin North Am.
62:61–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amylon MD, Shuster J, Pullen J, Berard C,
Link MP, Wharam M, Katz J, Yu A, Laver J, Ravindranath Y, et al:
Intensive high-dose asparaginase consolidation improves survival
for pediatric patients with T cell acute lymphoblastic leukemia and
advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group
study. Leukemia. 13:335–342. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silverman LB, Gelber RD, Dalton VK,
Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y,
Schorin MA, et al: Improved outcome for children with acute
lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol
91–01. Blood. 97:1211–1218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moghrabi A, Levy DE, Asselin B, Barr R,
Clavell L, Hurwitz C, Samson Y, Schorin M, Dalton VK, Lipshultz SE,
et al: Results of the Dana-Farber Cancer Institute ALL Consortium
Protocol 95–01 for children with acute lymphoblastic leukemia.
Blood. 109:896–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huguet F, Leguay T, Raffoux E, Thomas X,
Beldjord K, Delabesse E, Chevallier P, Buzyn A, Delannoy A,
Chalandon Y, et al: Pediatric-inspired therapy in adults with
Philadelphia chromosome-negative acute lymphoblastic leukemia: The
GRAALL-2003 study. J Clin Oncol. 27:911–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stock W, La M, Sanford B, Bloomfield CD,
Vardiman JW, Gaynon P, Larson RA and Nachman J; Children's Cancer
Group, : Cancer and Leukemia Group B studies: What determines the
outcomes for adolescents and young adults with acute lymphoblastic
leukemia treated on cooperative group protocols? A comparison of
Children's Cancer Group and Cancer and Leukemia Group B studies.
Blood. 112:1646–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salzer WL, Devidas M, Shuster JJ, Wang C,
Chauvenet A, Asselin BL, Camitta BM and Kurtzberg J; Children's
Oncology Group, : Intensified PEG-L-asparaginase and
antimetabolite-based therapy for treatment of higher risk
precursor-B acute lymphoblastic leukemia: A report from the
Children's Oncology Group. J Pediatr Hematol Oncol. 29:369–375.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stock W, Douer D, DeAngelo DJ, Arellano M,
Advani A, Damon L, Kovacsovics T, Litzow M, Rytting M, Borthakur G
and Bleyer A: Prevention and management of
asparaginase/pegasparaginase-associated toxicities in adults and
older adolescents: Recommendations of an expert panel. Leuk
Lymphoma. 52:2237–2253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kopeček J: Polymer-drug conjugates:
Origins, progress to date and future directions. Adv Drug Deliver
Rev. 65:49–59. 2013. View Article : Google Scholar
|
|
13
|
Pasut G, Sergi M and Veronese FM:
Anti-cancer PEG-enzymes: 30 years old, but still a current
approach. Adv Drug Deliv Rev. 60:69–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dinndorf PA, Gootenberg J, Cohen MH,
Keegan P and Pazdur R: FDA drug approval summary: Pegaspargase
(oncaspar) for the first-line treatment of children with acute
lymphoblastic leukemia (ALL). Oncologist. 12:991–998. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurre HA, Ettinger AG, Veenstra DL, Gaynon
PS, Franklin J, Sencer SF, Reaman GH, Lange BJ and Holcenberg JS: A
pharmacoeconomic analysis of pegaspargase versus native Escherichia
coli L-asparaginase for the treatment of children with
standard-risk, acute lymphoblastic leukemia: The Children's Cancer
Group study (CCG-1962). J Pediatr Hematol Oncol. 24:175–181. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines)[S/OL]. Acute Lymphoblastic Leukemia.
Version 1. 2015, http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
|
|
17
|
National Cancer Institute [S/OL], . Common
Terminology Criteria for Adverse Events (CTCAE) Mar 4. 2010,
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf
|
|
18
|
Vrooman LM, Stevenson KE, Supko JG,
O'Brien J, Dahlberg SE, Asselin BL, Athale UH, Clavell LA, Kelly
KM, Kutok JL, et al: Postinduction dexamethasone and individualized
dosing of Escherichia coli L-asparaginase each improve outcome of
children and adolescents with newly diagnosed acute lymphoblastic
leukemia: Results from a randomized study-Dana-Farber Cancer
Institute ALL Consortium Protocol 00–01. J Clin Oncol.
31:1202–1210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panosyan EH, Seibel NL, Martin-Aragon S,
Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger
LJ, La M, et al: Asparaginase antibody and asparaginase activity in
children with higher-risk acute lymphoblastic leukemia: Children's
Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 26:217–226.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rytting M: Peg-asparaginase for acute
lymphoblastic leukemia. Expert Opin Biol Ther. 10:833–839. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas X, Cannas G, Chelghoum Y and
Gougounon A: Therapeutic alternatives to native L-asparaginase in
the treatment of adult acute lymphoblastic leukemia. Bull Cancer.
97:1105–1117. 2010.(In French). PubMed/NCBI
|
|
22
|
Graham ML: Pegaspargase: A review of
clinical studies. Adv Drug Deliv Rev. 55:1293–1302. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avramis VI, Sencer S, Periclou AP, Sather
H, Bostrom BC, Cohen LJ, Ettinger AG, Ettinger LJ, Franklin J,
Gaynon PS, et al: A randomized comparison of native Escherichia
coli asparaginase and polyethylene glycol conjugated asparaginase
for treatment of children with newly diagnosed standard-risk acute
lymphoblastic leukemia: A Children's Cancer Group study. Blood.
99:1986–1994. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panetta JC, Gajjar A, Hijiya N, Hak LJ,
Cheng C, Liu W, Pui CH and Relling MV: Comparison of native E. coli
and PEG asparaginase pharmacokinetics and pharmacodynamics in
pediatric acute lymphoblastic leukemia. Clin Pharmacol Ther.
86:651–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sirvent N, Suciu S, Rialland X, Millot F,
Benoit Y, Plantaz D, Ferster A, Robert A, Lutz P, Nelken B, et al:
Prognostic significance of the initial cerebro-spinal fluid (CSF)
involvement of children with acute lymphoblastic leukaemia (ALL)
treated without cranial irradiation: Results of European
Organization for Research and Treatment of Cancer (EORTC) Children
Leukemia Group study 58881. Eur J Cancer. 47:239–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pieters R, Hunger SP, Boos J, Rizzari C,
Silverman L, Baruchel A, Goekbuget N, Schrappe M and Pui CH:
L-asparaginase treatment in acute lymphoblastic leukemia: A focus
on Erwinia asparaginase. Cancer. 117:238–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henriksen LT, Nersting J, Raja RA,
Frandsen TL, Rosthøj S, Schrøder H and Albertsen BK; Nordic Society
of Paediatric Haematology and Oncology (NOPHO) group, :
Cerebrospinal fluid asparagine depletion during pegylated
asparaginase therapy in children with acute lymphoblastic
leukaemia. Br J Haematol. 166:213–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawedia JD, Liu C, Pei D, Cheng C,
Fernandez CA, Howard SC, Campana D, Panetta JC, Bowman WP, Evans
WE, et al: Dexamethasone exposure and asparaginase antibodies
affect relapse risk in acute lymphoblastic leukemia. Blood.
119:1658–1664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merryman R, Stevenson KE, Gostic WN,
Neuberg D II, O'Brien J, Sallan SE and Silverman LB:
Asparaginase-associated myelosuppression and effects on dosing of
other chemotherapeutic agents in childhood acute lymphoblastic
leukemia. Pediatr Blood Cancer. 59:925–927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Avramis VI and Tiwari PN: Asparaginase
(native ASNase or pegylated ASNase) in the treatment of acute
lymphoblastic leukemia. Int J Nanomedicine. 1:241–254.
2006.PubMed/NCBI
|
|
31
|
Tong WH, Pieters R, Kaspers GJ, Te LD,
Bierings MB, van den Bos C, Kollen WJ, Hop WC, Lanvers-Kaminsky C,
Relling MV, et al: A prospective study on drug monitoring of
PEGasparaginase and Erwinia asparaginase and asparaginase
antibodies in pediatric acute lymphoblastic leukemia. Blood.
123:2026–2033. 2014. View Article : Google Scholar : PubMed/NCBI
|