Introduction
Gastric cancer is common and remains a major public
health problem around the world (1–3). The
incidence of gastric cancer has declined recently; however, it
remains the fifth most frequently diagnosed cancer and the second
leading cause of cancer-related death globally (4). Unfortunately, gastric cancer is often
diagnosed at an advanced stage in China, and this is associated
with poor survival. Radical surgery remains the primary potentially
curative therapy for patients with resectable gastric cancer.
It is known that the number of metastatic lymph
nodes (LNs) is one of the most important prognostic factors for
patients with gastric cancer. Both the International Union Against
Cancer (UICC)/American Joint Committee on Cancer (AJCC) and the
Japanese Gastric Cancer Association (JGCA) recommend a goal of ≥15
LNs examined for optimal staging (3,5). A more
extensive LN dissection helps to improve staging accuracy and
survival outcomes of patients with advanced gastric cancer
(6–8).
However, the beneficial survival outcome of more extensive LN
dissection may only be associated with stage migration or staging
accuracy; its direct contribution to improved survival remains
unclear (9,10). Moreover, more extensive LN dissection
may increase operation-related morbidity and mortality.
The efficacy of various types of LN dissection
remains controversial (11–14). In the West, D1 or a modified D2
lymphadenectomy (i.e., D1+) for gastrectomy has been identified as
the gold standard treatment for localized resectable gastric
cancer, and standard D2 lymphadenectomy is considered only a
recommended but not a required procedure (3,6,13,15,16). In
eastern Asia, especially in Japan and China, standard D2
lymphadenectomy has been the standard surgical therapy for curable
gastric cancer. However, D2 lymphadenectomy requires a significant
degree of surgical expertise and knowledge. In addition, D1+
lymphadenectomy helps to retrieve more LNs for optimal staging than
D1 lymphadenectomy, and with lower postoperative mortality and
morbidity than D2 lymphadenectomy. Thus, the efficacy of D1+
lymphadenectomy during gastrectomy, in comparison with D2
lymphadenectomy, in eastern Asia remains unclear. The incidence of
gastric cancer in China is the highest in the world (17).
The average lifespans of men and women in China are
74 and 77 years, respectively. Therefore, the long-term effect of
curative gastrectomy for gastric cancer may not be evaluable in
such elderly patients (18). In the
light of these considerations, we conducted this study to
investigate the prognosis and survival outcomes, comparing D1+ and
standard D2 lymphadenectomy in distal subtotal gastrectomy for
locally advanced patients under 70 years of age in China.
Patients and methods
Patients
Between May 1987 and February 2014, patients with
advanced gastric cancer, who underwent subtotal gastrectomy in the
Department of Gastrointestinal Surgery of the Fourth Affiliated
Hospital and Cancer Research Institute of China Medical University,
were entered into a retrospectively maintained database. In total,
397 patients with locally advanced gastric cancer underwent distal
subtotal gastrectomy with D1+ or D2 lymphadenectomy. All patients
achieved a potentially curative resection for histologically proven
gastric adenocarcinoma. This study was approved by the Ethics
Committee of the Fourth Affiliated Hospital, China Medical
University. All patient records and information were anonymized and
de-identified prior to analysis. Research was conducted in
accordance with the principals of the 1964 Declaration of Helsinki
and its later amendments.
Included and excluded standards
The inclusion criteria were as follows: Patients
under 70 years of age; histologically proven adenocarcinoma;
cancers in pT2-4aN0-3M0 stage; negative resection margins (R0);
potentially curable, and a curative operation was performed;
complete medical records were available; with D1+ or D2
lymphadenectomy. The exclusion criteria were as follows:
Preoperative adjuvant therapy; previous or concomitant other
cancer; emergency surgery; and patients lost to follow-up.
Follow-up
The follow-up of the entire study population was
complete until death or the cutoff date (October 2014). All the
patients gave a history and underwent physical examination, and
their carcino-embryonic antigen (CEA) levels were assessed every 3
to 6 months for the first postoperative year, and every 6 to 12
months thereafter. Seven patients were lost to follow-up and
therefore were excluded. The rate of follow-up was 98.2%.
Therefore, a total of 390 patients with locally advanced gastric
cancer were included in this study.
Clinicopathologic characteristics
The clinicopathologic features that were
investigated for prognostic significance included sex, age,
previous history, family history of carcinoma, tumor size, blood
loss, macroscopic type, histologic grade, lymphatic vessel invasion
(LVI), number of LNs retrieved, depth of invasion (pT stage),
number of regional LN metastases (pN stage), reconstruction type,
inadequate or adequate LNs retrieved, LN metastasis, locoregional
recurrence, distant recurrence, and chemotherapy. Among the 390
patients included, 114 (29.2%) patients underwent D1+
lymphadenectomy, with an average of 7.94±6.86 LNs retrieved and
2.85±4.15 LN metastases; 276 (70.8%) patients underwent D2
lymphadenectomy, with an average of 17.58±9.24 LNs retrieved and
4.43±4.91 LN metastases (Table
I).
 | Table I.Clinicopathologic features of patients
who underwent D1+ and D2 lymphadenectomy (n=390). |
Table I.
Clinicopathologic features of patients
who underwent D1+ and D2 lymphadenectomy (n=390).
| Variables | D1+ lymphadenectomy
n=114 (%) | D2 lymphadenectomy
n=276 (%) | P-value |
|---|
| Sex |
|
| 0.276 |
| Female | 30 (26.3) | 88 (31.9) |
|
| Male | 84 (73.7) | 188 (68.1) |
|
| Age (years) | 59.24±11.17 | 58.59±11.88 | 0.621 |
| Previous history |
|
| 0.799 |
| Gastritis and (or)
ulcer | 32 (28.1) | 74 (26.8) |
|
| None | 82 (71.9) | 202 (73.2) |
|
| Family history of
carcinoma |
|
| 0.058 |
| Yes | 26 (22.8) | 41 (14.9) |
|
| No | 88 (77.2) | 235 (85.1) |
|
| Tumor size (cm) | 5.89±3.78 | 4.87±2.17 | 0.001a |
| Blood loss |
|
| 0.048a |
| <200 ml | 57 (50.0) | 108 (39.1) |
|
| ≥200 ml | 57 (50.0) | 168 (60.9) |
|
| Macroscopic
type |
|
| 0.014a |
| Borrmann 1 | 6 (5.3) | 5 (1.8) |
|
| Borrmann 2 | 35 (30.7) | 56 (20.3) |
|
| Borrmann 3 | 65 (57.0) | 201
(72.8) |
|
| Borrmann 4 | 8 (7.0) | 14 (5.1) |
|
| Histologic
grade |
|
|
<0.001a |
| Well
differentiated | 32 (28.1) | 41 (14.9) |
|
| Moderately
differentiated | 23 (20.2) | 50 (18.1) |
|
| Poorly
differentiated | 50 (43.8) | 178 (64.5) |
|
|
Undifferentiated | 9 (7.9) | 7 (2.5) |
|
| Lymphatic vessels
invasion |
|
| 0.881 |
| Negative | 90 (78.9) | 216 (78.3) |
|
| Positive | 24 (21.1) | 60 (21.7) |
|
| Number of LNs
retrieved | 7.94±6.86 | 17.58±9.24 |
<0.001a |
| pT stage |
|
| 0.269 |
| pT2 | 15 (13.2) | 55 (19.9) |
|
| pT3 | 59 (51.7) | 136 (49.3) |
|
| pT4a | 40 (35.1) | 85 (30.8) |
|
| pN stage |
|
|
<0.001a |
| pN0 | 44 (38.6) | 53 (19.2) |
|
| pN1 | 36 (31.6) | 75 (27.2) |
|
| pN2 | 17 (14.9) | 85 (30.8) |
|
| pN3 | 17 (14.9) | 63 (22.8) |
|
| Number of
metastatic LNs | 2.85±4.15 | 4.43±4.91 |
0.003a |
| Reconstruction
type |
|
|
<0.001a |
| Billroth I | 64 (56.1) | 225 (81.5) |
|
| Billroth II | 50 (43.9) | 51
(18.5) |
|
| Number of LNs
retrieved |
|
|
<0.001a |
| Inadequate (n
<15) | 88 (77.2) | 99
(35.9) |
|
| Adequate (n
≥15) | 26 (22.8) | 177 (64.1) |
|
| LN metastasis |
|
|
<0.001a |
| No | 44 (38.6) | 53
(19.2) |
|
| Yes | 70 (61.4) | 223 (80.8) |
|
| Locoregional
recurrence |
|
| 0.072 |
| Absent | 87 (76.3) | 232 (84.1) |
|
| Present | 27 (23.7) | 44
(15.9) |
|
| Distant
recurrence |
|
| 0.208 |
| Absent | 82 (71.9) | 215 (77.9) |
|
| Present | 32 (28.1) | 61
(22.1) |
|
| Chemotherapy |
|
| 0.117 |
| No | 78 (68.4) | 210 (76.1) |
|
| Yes | 36 (31.6) | 66
(23.9) |
|
D1+ and D2 lymphadenectomy
According to the Japanese Gastric Cancer Treatment
Guidelines of the Japanese Gastric Cancer Association (JGCA), D1
lymphadenectomy for distal gastrectomy includes stations Nos. 1, 3,
4sb, 4d, 5, 6, 7; D1+ lymphadenectomy includes D1 and stations Nos.
8a, 9; D2 lymphadenectomy includes D1 and stations Nos. 8a, 9, 11p,
12a (5).
Pathology
Two pathologists independently examined the
histologic sections, and disagreements were resolved by discussion
to determine the final diagnosis. The carcinoma lesions together
with the surrounding gastric wall were fixed in formalin and cut
into multiple 5 mm slices, which were parallel to the lesser
curvature. As many LNs as possible were retrieved for adequate
staging. According to the current guidelines for gastric cancer,
examining at least 15 LNs is strongly recommended for adequate
staging (6,13). The 8th Edition of the AJCC TNM staging
classification for carcinoma of the stomach was applied to re-stage
the cancers of all patients in this study. The pathology report
generally included tumor size, pT, pN, status of margin, LVI,
status of mucosa, status of LNs, number of LNs retrieved,
macroscopic type, and histologic grade.
Statistical analysis
Five-year overall survival (OS) rates were
calculated using Kaplan-Meier survival analysis. The number at risk
was also shown in all Kaplan-Meier curves. Two-sided χ2
tests or two-tailed t-tests were performed for comparison of
clinicopathologic features between patients who underwent D1+ and
D2 lymphadenectomy. The log-rank (Mantel-Cox) test was conducted in
the univariate analysis to identify independently significant
prognostic factors and prognostic factors correlated with LN
metastasis. Multivariate analysis was applied to identify
significant factors correlated with prognosis, including
lymphadenectomy and all significant factors identified by
univariate analysis. Univariate analysis was firstly applied to
find the potential prognostic factors. Then multivariate analysis
was applied to identify significant factors correlated with
prognosis, including all significant factors identified by the
univariate analysis and the factor lymphadenectomy. Moreover,
scatter-plots and population pyramid figures were used to compare
the distribution of metastatic LNs and retrieved LNs between
patients who underwent D1+ and D2 lymphadenectomy. A P-value of
less than 0.05 was defined as statistically significant. IBM SPSS
v.22.0 statistical software was used for all statistical analyses
(SPSS Inc., Chicago, IL, USA).
Results
In total, 390 patients with locally advanced gastric
cancer who underwent distal subtotal gastrectomy were assessed for
eligibility in this study. The age of the entire population ranged
from 30 to 70 years. Of these patients, 114 patients underwent D1+
lymphadenectomy and 276 patients underwent D2 lymphadenectomy.
Among the patients who underwent D1+ lymphadenectomy, 30 (26.3%)
were female and 84 (73.7%) were male; among those who underwent D2
lymphadenectomy, 88 (31.9%) were female and 188 (68.1%) were
male.
Clinicopathologic features
The two groups (D1+ vs. D2 lymphadenectomy) were
well balanced in sex (P=0.276), age (P=0.621), previous history
(P=0.799), family history of carcinoma (P=0.058), and chemotherapy
(P=0.117) (Table I). The median
number of LNs retrieved was significantly higher with D2 than D1+
lymphadenectomy (17.58±9.24 vs. 7.94±6.86; P<0.001). A
significant difference could be found in the number of LN
metastases when comparing D2 and D1+ lymphadenectomy (4.43±4.91 vs.
2.85±4.15, P=0.003). Similarly, significant differences were found
with regard to tumor size (P=0.001), blood loss (P=0.048),
macroscopic type (P=0.014), histologic grade (P<0.001), pN stage
(P<0.001), reconstruction type (P<0.001), and LN metastasis
(P<0.001) when comparing D2 and D1+ lymphadenectomy. No
significant difference could be found in LVI (P=0.881), pT stage
(P=0.269), locoregional recurrence (P=0.072), and distant
recurrence (P=0.208) when comparing D2 and D1+ lymphadenectomy
(Table I).
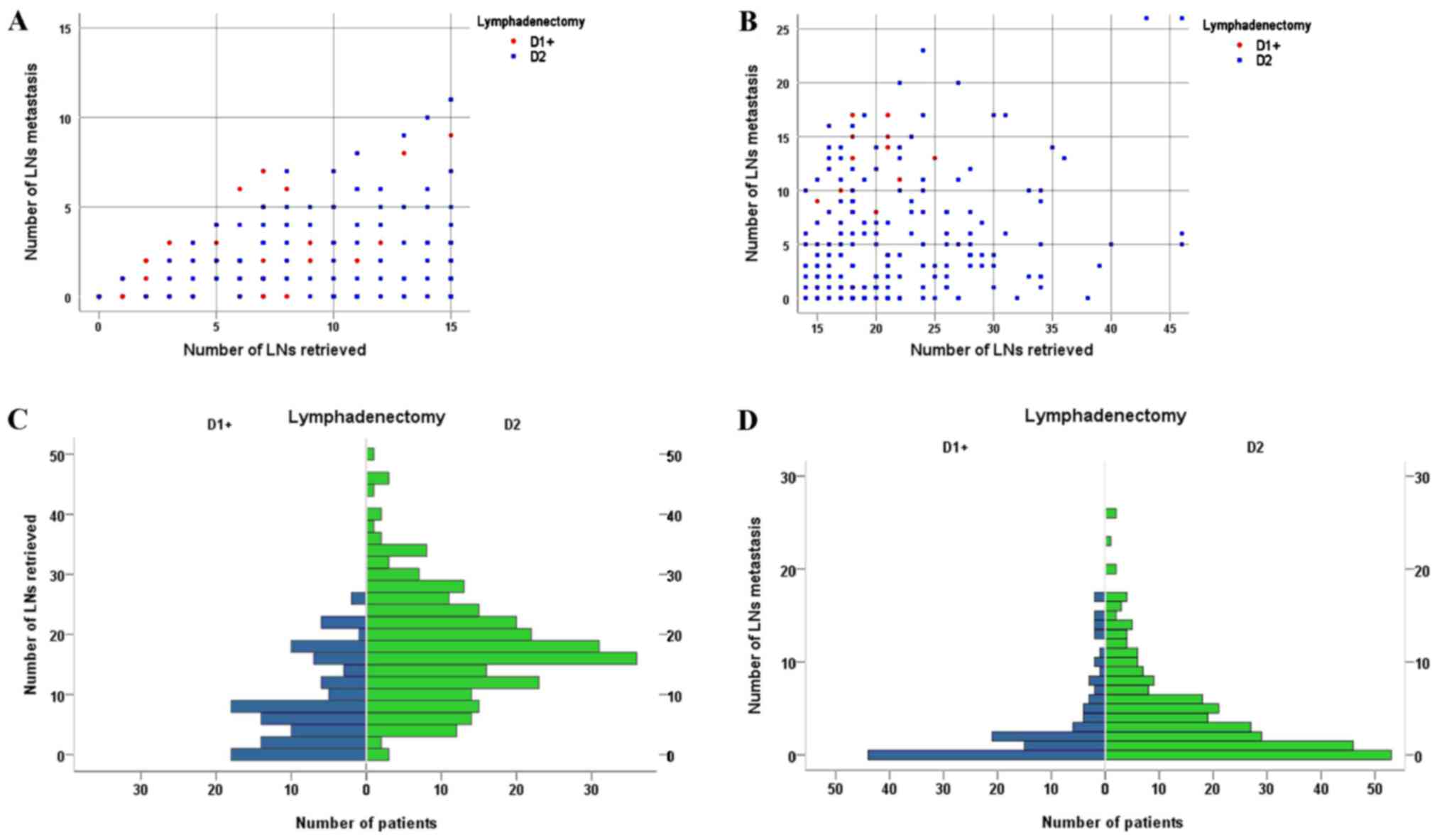
Fig. 1 shows the
distribution of the number of LN metastases according to the number
of LNs retrieved for patients with ≤15 LNs retrieved and >15 LNs
retrieved, comparing D1+ with D2 lymphadenectomy. Fig. 1 also shows the number of patients
distributed according to the number of LNs retrieved and the number
of LN metastases, comparing D1+ with D2 lymphadenectomy.
Outcomes
As far as we are concerned, lymphadenectomy is very
important for patients with gastric cancer surgery, which refers to
the removal of regional LNs. And lymphadenectomy may be classified
as D0, D1, D1+, or D2 depending on the extent of LNs removed at the
time of gastrectomy. More extensive lymph node dissection helps to
better accurate staging. Patients with accurate staging may receive
ideal postoperative treatments, which may contribute to survival
benefit. Therefore, identifying the best lymphadenectomy type for
every patient will be of great importance.
To identify which factors were correlated with
prognosis and were independent prognostic factors for the entire
study population. We firstly conducted univariate analysis to find
the potential prognostic factors and then multivariate analysis was
applied to identify significant factors correlated with prognosis,
including all significant factors identified by the univariate
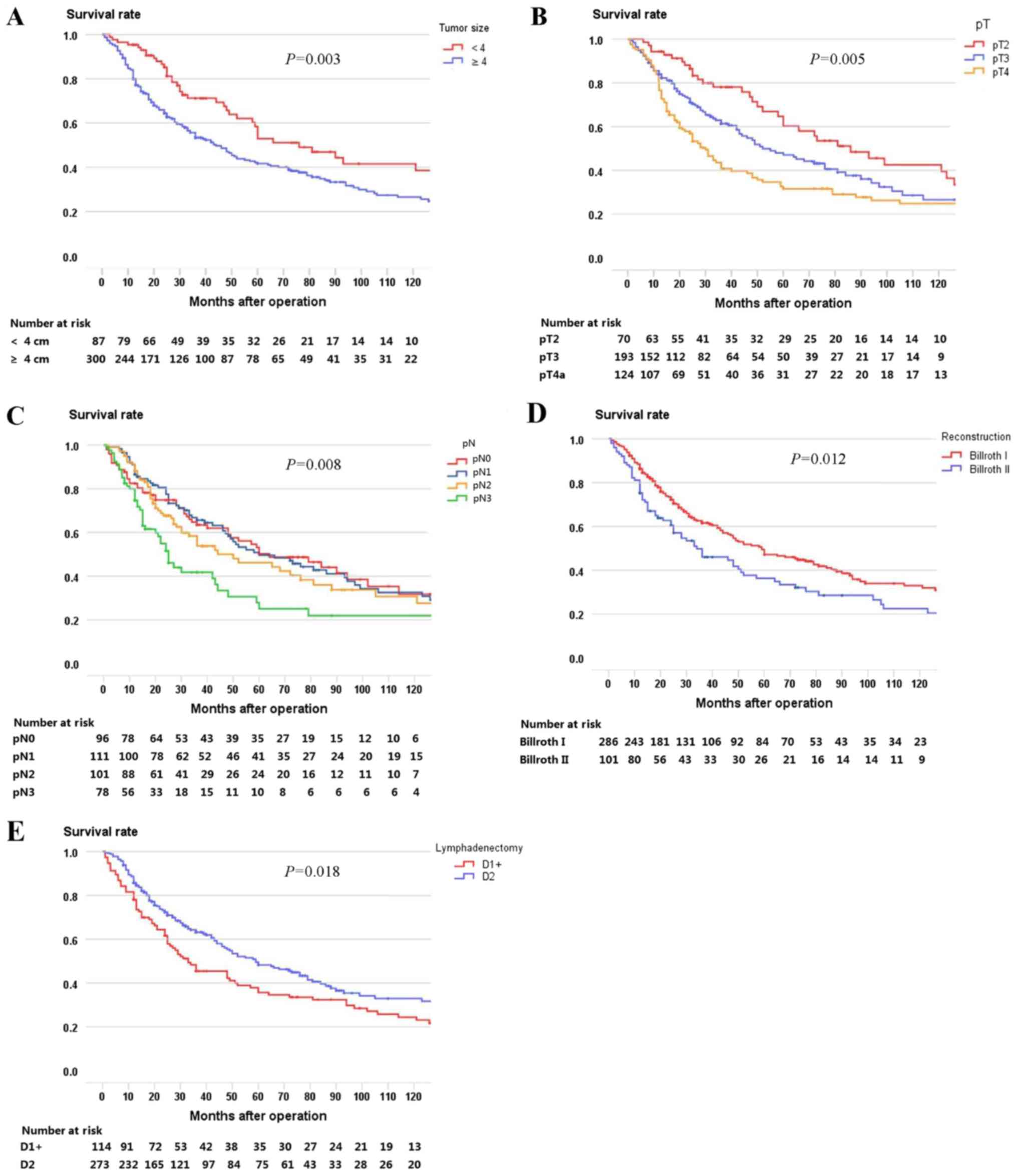
analysis and the factor lymphadenectomy. Firstly, univariate
analysis identified tumor size (P=0.003), pT stage (P=0.005), pN
stage (P=0.008), reconstruction type (P=0.012), and lymphadenectomy
(P=0.018) as potential factors correlated with prognosis for the
entire study population (Table II,
Fig. 2). Secondly, multivariate
analysis demonstrated that tumor size (RR 1.429, 95% CI
1.017–2.007, P=0.039), pT stage (RR 1.279, 95% CI 1.059–1.545,
P=0.011), pN stage (RR 1.302, 95% CI 1.139–1.487, P<0.001) and
lymphadenectomy (RR 0.653, 95% CI 0.490–0.870, P=0.004) were
independent prognostic factors for the entire study population
(Table II). Five-year overall
survival rates are also shown (Table
II).
 | Table II.Univariate and multivariable analysis
of prognostic factors for the entire study population
(n=390). |
Table II.
Univariate and multivariable analysis
of prognostic factors for the entire study population
(n=390).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | n (%) | 5-YSR (%) | P-value | RR | 95% CI | P-value |
|---|
| Sex |
|
| 0.706 |
|
|
|
|
Female | 118 (30.3) | 45.8 |
|
|
|
|
|
Male | 272 (69.7) | 40.6 |
|
|
|
|
| Age (years) |
|
| 0.308 |
|
|
|
|
<65 | 245 (62.8) | 40.9 |
|
|
|
|
|
≥65 | 145 (37.2) | 50.1 |
|
|
|
|
| Tumor size
(cm) |
|
| 0.003a | 1.429 | 1.017–2.007 | 0.039a |
|
<4 | 87
(22.3) | 52.9 |
|
|
|
|
| ≥4 | 303 (77.7) | 41.7 |
|
|
|
|
| Macroscopic
type |
|
| 0.279 |
|
|
|
|
Borrmann 1 | 11
(2.8) | 39.0 |
|
|
|
|
|
Borrmann 2 | 91
(23.3) | 52.7 |
|
|
|
|
|
Borrmann 3 | 266 (68.2) | 41.7 |
|
|
|
|
|
Borrmann 4 | 22
(5.7) | 37.3 |
|
|
|
|
| Histological
grade |
|
| 0.400 |
|
|
|
| Well
differentiated | 73
(18.7) | 55.9 |
|
|
|
|
|
Moderately differentiated | 73
(18.7) | 34.3 |
|
|
|
|
| Poorly
differentiated | 228 (58.5) | 44.8 |
|
|
|
|
|
Undifferentiated | 16
(4.1) | 34.7 |
|
|
|
|
| Lymphatic vessels
invasion |
|
| 0.068 |
|
|
|
|
Negative | 306 (78.5) | 46.0 |
|
|
|
|
|
Positive | 84
(21.5) | 37.8 |
|
|
|
|
| pT stage |
|
| 0.005a | 1.279 | 1.059–1.545 | 0.011a |
|
pT2 | 70
(17.9) | 60.2 |
|
|
|
|
|
pT3 | 195 (50.0) | 47.0 |
|
|
|
|
|
pT4a | 125 (32.1) | 31.6 |
|
|
|
|
| pN stage |
|
| 0.008a | 1.302 | 1.139–1.487 |
<0.001a |
|
pN0 | 97
(24.9) | 50.2 |
|
|
|
|
|
pN1 | 111 (28.5) | 49.5 |
|
|
|
|
|
pN2 | 102 (26.1) | 46.1 |
|
|
|
|
|
pN3 | 80
(20.5) | 25.0 |
|
|
|
|
| Reconstruction
type |
|
| 0.012a |
|
|
|
|
Billroth I | 289 (74.1) | 47.1 |
|
|
|
|
|
Billroth II | 101 (25.9) | 36.2 |
|
|
|
|
|
Lymphadenectomy |
|
| 0.018a | 0.653 | 0.490–0.870 | 0.004a |
|
D1+ | 114 (29.2) | 35.7 |
|
|
|
|
| D2 | 276 (70.8) | 48.2 |
|
|
|
|
| Number of LNs
retrieved |
|
| 0.057 |
|
|
|
|
Inadequate (n <15) | 187 (47.9) | 40.8 |
|
|
|
|
|
Adequate (n ≥15) | 203 (52.1) | 48.9 |
|
|
|
|
| LN metastasis |
|
| 0.170 |
|
|
|
| No | 97
(24.9) | 50.2 |
|
|
|
|
|
Yes | 293 (75.1) | 42.1 |
|
|
|
|
| Locoregional
recurrence |
|
| 0.274 |
|
|
|
|
Absent | 319 (81.8) | 45.1 |
|
|
|
|
|
Present | 71
(18.2) | 38.4 |
|
|
|
|
| Distant
recurrence |
|
| 0.238 |
|
|
|
|
Absent | 297 (76.2) | 45.3 |
|
|
|
|
|
Present | 93
(23.8) | 40.8 |
|
|
|
|
| Chemotherapy |
|
| 0.057 |
|
|
|
| No | 288 (73.8) | 50.3 |
|
|
|
|
|
Yes | 102 (26.2) | 36.1 |
|
|
|
|
To identify which factors were correlated with LN
metastasis, we firstly conducted univariate analysis to find the
potential factors correlated with LN metastasis and then
multivariate analysis was applied to identify significant factors
correlated with LN metastasis, including all significant factors
identified by the univariate analysis and the factor
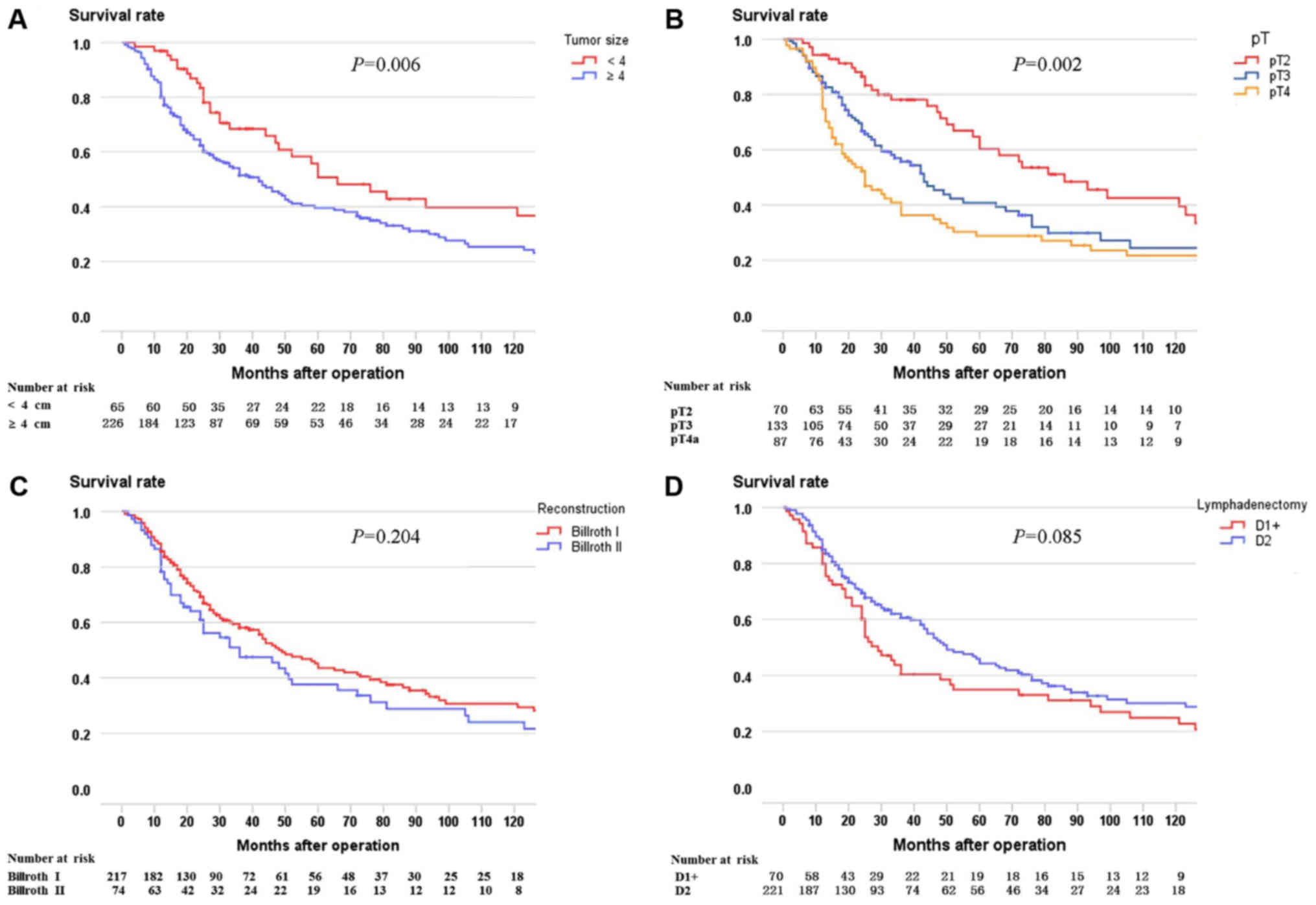
lymphadenectomy. Firstly, univariate analysis identified tumor size
(P=0.006) and pT stage (P=0.002) as potential factors correlated
with LN metastasis (Table III).
Secondly, multivariate analysis demonstrated that tumor size (RR
1.486, 95% CI 1.059–2.087, P=0.022), pT stage (RR 1.247, 95% CI
1.055–1.540, P=0.012), and lymphadenectomy (D1+ vs. D2, RR 0.740,
95% CI 0.565–0.969, P=0.028) were independent prognostic factors
predicting LN metastasis (Table
III). Survival curves comparing tumor size, pT stage,
reconstruction type, and lymphadenectomy are shown in Fig. 3.
 | Table III.Univariate and multivariate analysis
of factors predicting LN metastasis (n=293). |
Table III.
Univariate and multivariate analysis
of factors predicting LN metastasis (n=293).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | LN metastasis
(+) | 5-YSR (%) | P-value | RR | 95% CI | P-value |
|---|
| Sex |
|
| 0.475 |
|
|
|
|
Female | 93
(31.7) | 37.5 |
|
|
|
|
|
Male | 200 (68.3) | 44.1 |
|
|
|
|
| Age (years) |
|
| 0.527 |
|
|
|
|
<65 | 190 (64.8) | 39.1 |
|
|
|
|
|
≥65 | 103 (35.2) | 47.9 |
|
|
|
|
| Tumor size
(cm) |
|
| 0.006a | 1.486 | 1.059–2.087 | 0.022a |
|
<4 | 65
(22.2) | 50.7 |
|
|
|
|
| ≥4 | 228 (77.8) | 39.6 |
|
|
|
|
| Previous
history |
|
| 0.939 |
|
|
|
|
Gastritis and (or) ulcer | 76
(25.9) | 42.5 |
|
|
|
|
|
None | 217 (74.1) | 41.6 |
|
|
|
|
| Family history of
carcinoma |
|
| 0.432 |
|
|
|
| No | 246 (84.0) | 43.3 |
|
|
|
|
|
Yes | 47
(16.0) | 35.0 |
|
|
|
|
| Macroscopic
type |
|
| 0.197 |
|
|
|
|
Borrmann 1 | 8 (2.7) | 35.0 |
|
|
|
|
|
Borrmann 2 | 66
(22.5) | 50.5 |
|
|
|
|
|
Borrmann 3 | 201 (68.6) | 39.7 |
|
|
|
|
|
Borrmann 4 | 18
(6.2) | 34.4 |
|
|
|
|
| Histological
grade |
|
| 0.737 |
|
|
|
| Well
differentiated | 50
(17.1) | 54.1 |
|
|
|
|
|
Moderately differentiated | 48
(16.4) | 34.6 |
|
|
|
|
| Poorly
differentiated | 188 (64.1) | 41.4 |
|
|
|
|
|
Undifferentiated | 7 (2.4) | 28.6 |
|
|
|
|
| Lymphatic vessels
invasion |
|
| 0.228 |
|
|
|
|
Negative | 221 (75.4) | 43.1 |
|
|
|
|
|
Positive | 72
(24.6) | 39.9 |
|
|
|
|
| pT stage |
|
| 0.002a | 1.274 | 1.055–1.540 | 0.012a |
|
pT2 | 70
(23.9) | 60.2 |
|
|
|
|
|
pT3 | 135 (46.1) | 40.8 |
|
|
|
|
|
pT4a | 88
(30.0) | 28.8 |
|
|
|
|
| Reconstruction
type |
|
| 0.204 |
|
|
|
|
Billroth I | 219 (74.7) | 43.6 |
|
|
|
|
|
Billroth II | 74
(25.3) | 37.6 |
|
|
|
|
|
Lymphadenectomy |
|
| 0.085 | 0.740 | 0.565–0.969 | 0.028a |
|
D1+ | 70
(23.9) | 34.9 |
|
|
|
|
| D2 | 223 (76.1) | 44.3 |
|
|
|
|
| Number of LNs
retrieved |
|
| 0.351 |
|
|
|
|
Inadequate (n <15) | 122 (41.6) | 41.7 |
|
|
|
|
|
Adequate (n ≥15) | 171 (58.4) | 42.4 |
|
|
|
|
Comparisons of prognosis for patients who underwent
D1+ and D2 lymphadenectomy are shown in Table IV, as stratified by pT stage, pN
stage, and the number of LNs retrieved. As shown, D2
lymphadenectomy helped to achieve a higher 5-year OS rate, compared
with D1+ lymphadenectomy for the entire sample of patients (35.7%
for D1+, 48.2% for D2) and for patients in stage pT2 (51.9% for
D1+, 63.0% for D2), pT3 (38.3% for D1+, 51.8% for D2), pT4a (25.9%
for D1+, 34.3% for D2), pN0 (36.6% for D1+, 63.9% for D2), pN1
(42.7% for D1+, 52.7% for D2), pN2 (32.7% for D1+, 49.2% for D2),
and pN3 (17.7% for D1+, 27.7% for D2), as well as patients with
adequate (34.3% for D1+, 46.9% for D2) or inadequate LN retrieval
(44.7% for D1+, 49.6% for D2). Importantly, a statistically
significant difference in 5-year OS rate could be found in the
entire study population (35.7% for D1+, 48.2% for D2, log-rank
test, P=0.018), and especially for patients with pN0 cancer (36.6%
for D1+, 63.9% for D2, log-rank test, P=0.021).
 | Table IV.Comparison of prognosis for all
patients comparing D1+ and D2 lymphadenectomy (n=390). |
Table IV.
Comparison of prognosis for all
patients comparing D1+ and D2 lymphadenectomy (n=390).
|
| D1+
lymphadenectomy | D2
lymphadenectomy |
|
|---|
|
|
|
|
|
|---|
| Variables | n | 5-YSR (%) | n | 5-YSR (%) | P-value |
|---|
| For the entire
population | 114 | 35.7 | 276 | 48.2 | 0.018a |
| pT stage |
|
|
|
|
|
|
pT2 | 15 (13.2) | 51.9 | 55 (19.9) | 63.0 | 0.820 |
|
pT3 | 59 (51.7) | 38.3 | 136 (49.3) | 51.8 | 0.074 |
|
pT4a | 40 (35.1) | 25.9 | 5 (1.8) | 34.3 | 0.131 |
| pN stage |
|
|
|
|
|
|
pN0 | 44 (38.6) | 36.6 | 53 (19.2) | 63.9 | 0.021a |
|
pN1 | 36 (31.6) | 42.7 | 75 (27.2) | 52.7 | 0.166 |
|
pN2 | 17 (14.9) | 32.7 | 85 (30.8) | 49.2 | 0.642 |
|
pN3 | 17 (14.9) | 17.7 | 63 (22.8) | 27.7 | 0.138 |
| Number of LNs
retrieved |
|
|
|
|
|
|
Inadequate (n ≥15) | 26 (22.8) | 44.7 | 177 (64.1) | 49.6 | 0.403 |
|
Adequate (n <15) | 88 (77.2) | 34.3 | 99 (35.9) | 46.9 | 0.149 |
Discussion
Radical surgery is still the primary potentially
curable treatment for resectable gastric cancer, and R0 resection
is recommended as the gold standard. For patients with distal
gastric cancer, subtotal gastrectomy is preferred for its similar
outcomes and fewer complications, when compared with total
gastrectomy (19). Therefore, in this
study, only patients with locally advanced gastric cancer who
underwent subtotal gastrectomy were included. In addition, the
average lifespans of men and women in China are 74 and 77 years,
respectively. Therefore, if we include patients older than age of
70 years, the long-term effect of curative gastrectomy for gastric
cancer may not be evaluable; thus, we only included patients under
age of 70 years in this study.
Recently, D1 or D1+ lymphadenectomy for gastrectomy
has been identified as the gold standard treatment for localized
resectable gastric cancer in the West; however, D2 lymphadenectomy
is considered only a recommended but not a required procedure,
which may only contribute to accurate staging (3,6,13,15,16). In
addition, its contribution to survival benefit is under debate and
may be due to the effect of ‘stage migration’. D2 lymphadenectomy
has been a standard therapy for curable gastric cancer in eastern
Asia; however, it was reported to be associated with significantly
higher postoperative mortality and morbidity, when compared with D1
lymphadenectomy (11). As far as we
are concerned, D1+ lymphadenectomy helps to retrieve more LNs for
optimal staging than D1 lymphadenectomy, and D1+ lymphadenectomy
may be associated with lower postoperative mortality and morbidity
than D2 lymphadenectomy. Thus, the efficacy of D1+ lymphadenectomy
in eastern Asia is still under debate. D1+ lymphadenectomy in total
gastrectomy has been shown to be effective for gastric carcinoma
with LN metastasis, but this requires further validation (20,21). This
study was conducted to investigate survival outcomes, comparing D1+
and standard D2 lymphadenectomy in distal subtotal gastrectomy, for
patients with locally advanced gastric cancer.
Recurrences were classified as locoregional and
distant recurrence. Locoregional recurrence was identified as any
cancer recurrence in the gastric bed, anastomotic sites, and
regional LNs. Distant recurrence was identified as visceral
metastases, peritoneal metastases, and LN metastases beyond the
regional LNs. All recurrences were diagnosed clinically or
radio-graphically, with histopathologic testing or radiography,
including computer tomography (CT) scan (head, chest, abdomen, and
pelvis) and bone scans; positron emission tomography CT (PET/CT)
would be applied if necessary. According to the findings of these
examinations, the incidence of recurrence was comparable between
patients who underwent D1+ and D2 lymphadenectomy.
To investigate the independent prognostic factors
for the entire study population, both univariate and multivariate
analyses were performed. We finally identified that tumor size
(P=0.039), pT stage (P=0.011), pN stage (P<0.001), and
lymphadenectomy (P=0.004) as independent prognostic factors. Our
result is similar to those of many previous studies concerning
independent factors for locally advanced gastric cancer.
Both the UICC and JGCA recommend that a sufficient
number and level of LNs should be retrieved. A minimum of 15 LNs
retrieved is recommended for both the UICC and JGCA staging
systems. Insufficient LN retrieval may lead to residual positive
LNs. In our study, the median number of LNs retrieved for patients
with D1+ lymphadenectomy was significantly less than that of
patients with D2 lymphadenectomy (7.94±6.86 for D1+ lymphadenectomy
vs. 17.58±9.24 for D2 lymphadenectomy, P<0.001). The 5-year OS
rate of patients with D1+ lymphadenectomy was significantly lower
than that of patients with D2 lymphadenectomy (35.7% for D1+
lymphadenectomy vs. 48.2% for D2 lymphadenectomy, P=0.018). The
number of LNs retrieved for patients with D1+ lymphadenectomy is
inadequate (7.94±6.86), which is much fewer than the minimum of 15
LNs as recommended by the UICC and JGCA staging system; therefore,
down-staging may occur as a result of residual positive lymph
nodes. More extensive lymph node dissection helps to better
accurate staging. Thus, patients with accurate staging may receive
ideal postoperative treatments, which may contribute to survival
benefit. These results indicate that patients would benefit from D2
lymphadenectomy, which helps to retrieve adequate LNs for optimal
staging and to improve survival outcomes.
Lymph node metastasis is a poor prognostic factor
for gastric cancer and the number of regional LN metastases will
influences survival significantly (22). In this current cohort, tumor size
(P=0.022), pT stage (P=0.012), and lymphadenectomy (P=0.028) were
proved as independent prognostic factors predicting LN metastasis.
Accordingly, patients with larger tumor size (≥4 cm), higher pT
stage, and who underwent D1+ lymphadenectomy had a higher risk of
LN metastasis and shorter survival times. It is not surprising that
patients with larger tumor size, higher pT stage, and D1+
lymphadenectomy would have a worse survival outcome. Larger cancers
with higher pT stage are more locally advanced, and may have a
higher risk of LN metastasis; therefore, D2 lymphadenectomy is
strongly recommended, especially for larger cancers with higher pT
stage. As many LNs should be retrieved as possible to avoid
residual LNs (as least 15 LNs were recommended), especially for
patients with larger tumor size and higher pT stage. However, the
results of our study should be interpreted with caution and need to
be clarified in further studies.
To evaluate prognosis, 5-year OS rates for patients
who underwent D1+ and D2 lymphadenectomy were calculated. According
to our study, D2 lymphadenectomy helped to achieve higher 5-year OS
rates for the entire study population, patients in pT2-4a and pN0-3
stages, and patients with adequate or inadequate LNs retrieved.
Statistically significant differences in 5-year OS rate could be
found for the entire study population, and for patients in pN0
stage. Recent studies have shown that D2 lymphadenectomy is
associated with fewer postoperative complications and a trend
toward an improved OS rate when performed in high-volume centers
with sufficient experience of the operation and postoperative
management (23–25). Therefore, we believed that standard D2
lymphadenectomy helps to retrieve adequate LNs and improve staging
accuracy and survival outcomes; however, it should be performed by
experienced surgeons in high-volume centers.
However, limitations still exist in the present
study. First, this retrospective study was based on a follow-up
that varied from operation to operation and has changed during the
past 27 years. During this large time frame, the effects of
surgical progress, surgical techniques, surgical skill and adjuvant
therapy may have changed, which may have produced bias. Second, our
study lacked the investigation of safety outcomes, such as
operation-related morbidity, mortality, and so on, which are also
very important and need to be investigated in future studies.
Third, it is necessary to note that selection bias may exist
because this study was not a randomized controlled trial.
Therefore, our study still needs to be validated by future
prospective and randomized controlled studies.
In conclusion, cancers of larger size, higher pT
stage, and with D1+ lymphadenectomy have higher risk of LN
metastasis. This study demonstrated that standard D2
lymphadenectomy helps to retrieve adequate LNs to improve staging
accuracy and survival. Therefore, we recommend standard D2
lymphadenectomy in distal subtotal gastrectomy for locally advanced
gastric cancer, especially for cancers of larger size and higher pT
stage of patients younger than age of 70 years. However, standard
D2 lymphadenectomy requires surgeons to have undergone an
appropriate learning curve and needs to be performed by experienced
surgeons in high-volume centers.
Acknowledgements
This study was funded by the Natural Science
Foundation of Liaoning Province (no. 201602817).
Glossary
Abbreviations
Abbreviations:
|
UICC
|
International Union Against Cancer
|
|
AJCC
|
American Joint Committee on Cancer
|
|
JGCA
|
Japanese Gastric Cancer
Association
|
|
NCCN
|
national comprehensive cancer
network
|
|
R0
|
negative resection margins
|
|
OS
|
overall survival
|
|
RR
|
relative risk
|
|
SD
|
standard deviation
|
|
95% CI
|
95% confidence interval
|
|
LN
|
lymph node
|
|
LVI
|
lymphovascular invasion
|
|
D1+
|
modified D2 lymphadenectomy
|
|
D2
|
standard D2 lymphadenectomy
|
|
CEA
|
carcino-embryonic antigen
|
|
CT
|
computer tomography
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goggins WB and Wong GK: Poor survival for
US Pacific Islander cancer patients: Evidence from the
surveillance, epidemiology, and end results database: 1991 to 2004.
J Clin Oncol. 25:5738–5741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwarz RE and Smith DD: Clinical impact
of lymphadenectomy extent in resectable gastric cancer of advanced
stage. Ann Surg Oncol. 14:317–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith DD, Schwarz RR and Schwarz RE:
Impact of total lymph node count on staging and survival after
gastrectomy for gastric cancer: Data from a large US-population
database. J Clin Oncol. 23:7114–7124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eom BW, Joo J, Kim YW, Reim D, Park JY,
Yoon HM, Ryu KW, Lee JY and Kook MC: Improved survival after adding
dissection of the superior mesenteric vein lymph node (14v) to
standard D2 gastrectomy for advanced distal gastric cancer.
Surgery. 155:408–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feinstein AR, Sosin DM and Wells CK: The
Will Rogers phenomenon. Stage migration and new diagnostic
techniques as a source of misleading statistics for survival in
cancer. N Engl J Med. 312:1604–1608. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seevaratnam R, Bocicariu A, Cardoso R,
Yohanathan L, Dixon M, Law C, Helyer L and Coburn NG: How many
lymph nodes should be assessed in patients with gastric cancer? A
systematic review. Gastric Cancer. 15 Suppl 1:S70–S88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hartgrink HH, van de Velde CJ, Putter H,
Bonenkamp JJ, Kranenbarg E Klein, Songun I, Welvaart K, van Krieken
JH, Meijer S, Plukker JT, et al: Extended lymph node dissection for
gastric cancer: Who may benefit? Final results of the randomized
Dutch gastric cancer group trial. J Clin Oncol. 22:2069–2277. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuschieri A, Weeden S, Fielding J,
Bancewicz J, Craven J, Joypaul V, Sydes M and Fayers P: Patient
survival after D1 and D2 resections for gastric cancer: Long-term
results of the MRC randomized surgical trial. Surgical Co-operative
Group. Br J Cancer. 79:1522–1530. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJ: Surgical treatment of gastric cancer:
15-year follow-up results of the randomised nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sierra A, Regueira FM, Hernández-Lizoáin
JL, Pardo F, Martínez-Gonzalez MA and A-Cienfuegos J: Role of the
extended lymphadenectomy in gastric cancer surgery: Experience in a
single institution. Ann Surg Oncol. 10:219–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Degiuli M, Sasako M, Calgaro M, Garino M,
Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A and Calvo
F; Italian Gastric Cancer Study Group, : Morbidity and mortality
after D1 and D2 gastrectomy for cancer: Interim analysis of the
Italian Gastric Cancer Study Group (IGCSG) randomised surgical
trial. Eur J Surg Oncol. 30:303–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Degiuli M, Sasako M, Ponti A and Calvo F:
Survival results of a multicentre phase II study to evaluate D2
gastrectomy for gastric cancer. Br J Cancer. 90:1727–1732. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zong L, Abe M, Seto Y and Ji J: Randomized
controlled trial of laparoscopic versus open D2 distal gastrectomy
for advanced gastric cancer: How should we define the age of
included patents? J Clin Oncol. Aug 9–2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bozzetti F, Marubini E, Bonfanti G, Miceli
R, Piano C and Gennari L: Subtotal versus total gastrectomy for
gastric cancer: Five-year survival rates in a multicenter
randomized Italian trial. Italian Gastrointestinal Tumor Study
Group. Ann Surg. 230:170–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galizia G, Lieto E, De Vita F, Castellano
P, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, De Sena G, De
Stefano L, et al: Modified versus standard D2 lymphadenectomy in
total gastrectomy for nonjunctional gastric carcinoma with lymph
node metastasis. Surgery. 157:285–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galizia G, Lieto E, Zamboli A, Auricchio A
and Orditura M: Reply ‘Modified D2 lymphadenectomy is effective in
patients with node-positive gastric cancers undergoing potentially
curative total gastrectomy’. Surgery. 158:1447–1448. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karpeh MS, Leon L, Klimstra D and Brennan
MF: Lymph node staging in gastric cancer: Is location more
important than Number? An analysis of 1,038 patients. Ann Surg.
232:362–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enzinger PC, Benedetti JK, Meyerhardt JA,
McCoy S, Hundahl SA, Macdonald JS and Fuchs CS: Impact of hospital
volume on recurrence and survival after surgery for gastric cancer.
Ann Surg. 245:426–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Degiuli M, Sasako M and Ponti A; Italian
Gastric Cancer Study Group, : Morbidity and mortality in the
Italian Gastric Cancer Study Group randomized clinical trial of D1
versus D2 resection for gastric cancer. Br J Surg. 97:643–649.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Degiuli M, Sasako M, Ponti A, Vendrame A,
Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G and Morino M;
Italian Gastric Cancer Study Group, : Randomized clinical trial
comparing survival after D1 or D2 gastrectomy for gastric cancer.
Br J Surg. 101:23–31. 2014. View
Article : Google Scholar : PubMed/NCBI
|